Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(2); 2024 > Article
-
Original Article
TRPS1 expression in non-melanocytic cutaneous neoplasms: an immunohistochemical analysis of 200 cases -
Yi A. Liu1
 , Phyu P. Aung1
, Phyu P. Aung1 , Yunyi Wang2
, Yunyi Wang2 , Jing Ning2
, Jing Ning2 , Priyadharsini Nagarajan1
, Priyadharsini Nagarajan1 , Jonathan L. Curry1
, Jonathan L. Curry1 , Carlos A. Torres-Cabala1
, Carlos A. Torres-Cabala1 , Doina Ivan1
, Doina Ivan1 , Victor G. Prieto1
, Victor G. Prieto1 , Qingqing Ding1
, Qingqing Ding1 , Woo Cheal Cho1
, Woo Cheal Cho1
-
Journal of Pathology and Translational Medicine 2024;58(2):72-80.
DOI: https://doi.org/10.4132/jptm.2024.01.23
Published online: February 26, 2024
1Department of Pathology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
2Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
- Corresponding Author: Woo Cheal Cho, MD, Department of Pathology, Section of Dermatopathology, University of Texas MD Anderson Cancer Center, B3.4607, Unit 085, 1515 Holcombe Blvd, Houston, TX 77030-4009, USA Tel: +1-877-632-6789, Fax: +1-713-745-0789, E-mail: wcho@mdanderson.org
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Although trichorhinophalangeal syndrome type 1 (TRPS1) was initially thought to be highly sensitive and specific for carcinomas and mesenchymal tumors of mammary origin, more recent data suggest its expression is not limited to breast neoplasms but also can be seen in other cutaneous neoplasms, such as extramammary Paget disease and squamous cell carcinoma (SCC) in situ.
-
Methods
- Two-hundred cases of non-melanocytic cutaneous neoplasm, including basal cell carcinomas (BCCs) (n = 41), SCCs (n = 35), Merkel cell carcinomas (MCCs) (n = 25), and adnexal neoplasms (n = 99), were tested for TRPS1 expression using a monoclonal anti- TRPS1 rabbit anti-human antibody.
-
Results
- TRPS1 expression was present in almost all cases of SCC (94%), with a median H-score of 200, while it was either absent or only focally present in most BCCs (90%), with a median H-score of 5. The difference between BCCs and SCCs in H-score was significant (p < .001). All MCCs (100%) lacked TRPS1 expression. TRPS1 expression was frequently seen in most adnexal neoplasms, benign and malignant, in variable intensity and proportion but was consistently absent in apocrine carcinomas. All endocrine mucin-producing sweat gland carcinomas (EMPSGCs) (100%, 6/6) showed diffuse and strong TRPS1 immunoreactivity, with a median H-score of 300, which was significantly different (p < .001) than that of BCCs.
-
Conclusions
- Our study shows that TRPS1 may be an effective discriminatory marker for BCCs and SCCs. It also has a role in distinguishing BCCs from EMPSGCs.
- Case selection
- After obtaining institutional review board (IRB) approval (IRB#: 2022-0662), with a waiver of informed consent, we searched for cases of non-melanocytic cutaneous epithelial neoplasms, including benign and malignant adnexal neoplasms, within our institutional pathology database. All hematoxylin and eosin (H&E)– stained slides and relevant immunohistochemical studies, if applicable, of candidate cases were reviewed by two board-certified pathologists (Y.A.L. and W.C.C.) to confirm the original diagnoses prior to retrieval of the corresponding formalin-fixed, paraffin-embedded (FFPE) tissue blocks. Those that lacked sufficient lesional cells of interest upon review of H&E-stained slides (microscopic 2-dimensional size being less than or equal to 1×1 mm) were excluded to avoid potential false-negativity or misinterpretation of TRPS1 expression. A total of 200 cases were included in the study, with their tumor type and frequency listed in Table 1. Of note, among the 41 selected cases of BCC, 21 cases exhibited squamous differentiation. Among the 35 selected cases of SCC, 18 were well-differentiated, 14 were moderately differentiated, and three were poorly differentiated.
- Immunohistochemical analysis
- A 4–5-μm-thick paraffin section was freshly cut from each FFPE tissue block of the selected cases. The unstained slides were subjected to immunohistochemical analysis with a monoclonal anti-TRPS1 rabbit anti-human antibody (1:2,000, EPR16171, Abcam, Cambridge, MA, USA) using a Leica Bond Max autostainer system (Leica Biosystems, GmbH, Nussloch, Germany) with standard automated protocols. Nuclear expression of TRPS1 in more than 5% of tumor cells was considered as positive immunoreactivity. The intensity of TRPS1 expression was classified into four categories (none, 0; weak, 1+; moderate, 2+; and strong, 3+), with the intensity of TRPS1 expression in innate eccrine glands being set as 3+ intensity (strong). H-scores were calculated by multiplying the percentage of positive tumor cells by the corresponding intensity of TRPS1 expression, with total scores ranging from 0 to 300. For practical purposes, the proportion of TRPS1 expression was also further classified into four categories: none, 0%–5%; focal, 6%–25%; patchy, 26%–75%; and diffuse, >75%. The IHC results of all tested cases were reviewed by two board-certified pathologists (Y.A.L. and W.C.C.).
- Statistical analysis
- Descriptive statistics were used to summarize the variables in the study. The range and median were reported for continuous variables, and the frequency and proportion (%) were reported for categorical variables. For multiple group comparison, the Kruskal-Wallis rank sum test was used for continuous variables and the Fisher exact test for categorical variables. The Wilcoxon rank sum test was applied for pairwise comparison. A p-value of less than .05 was considered statistically significant. All statistical analyses were performed using R ver. 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
MATERIALS AND METHODS
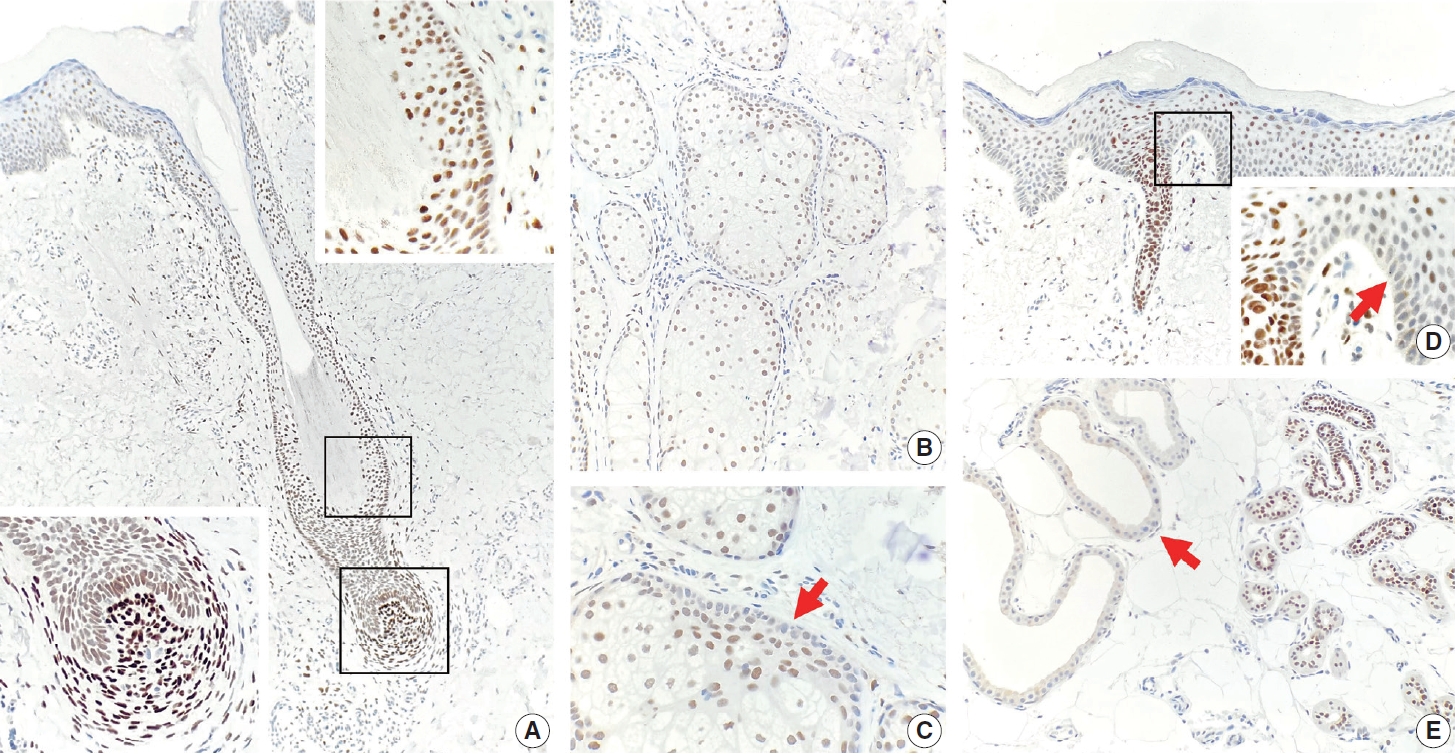
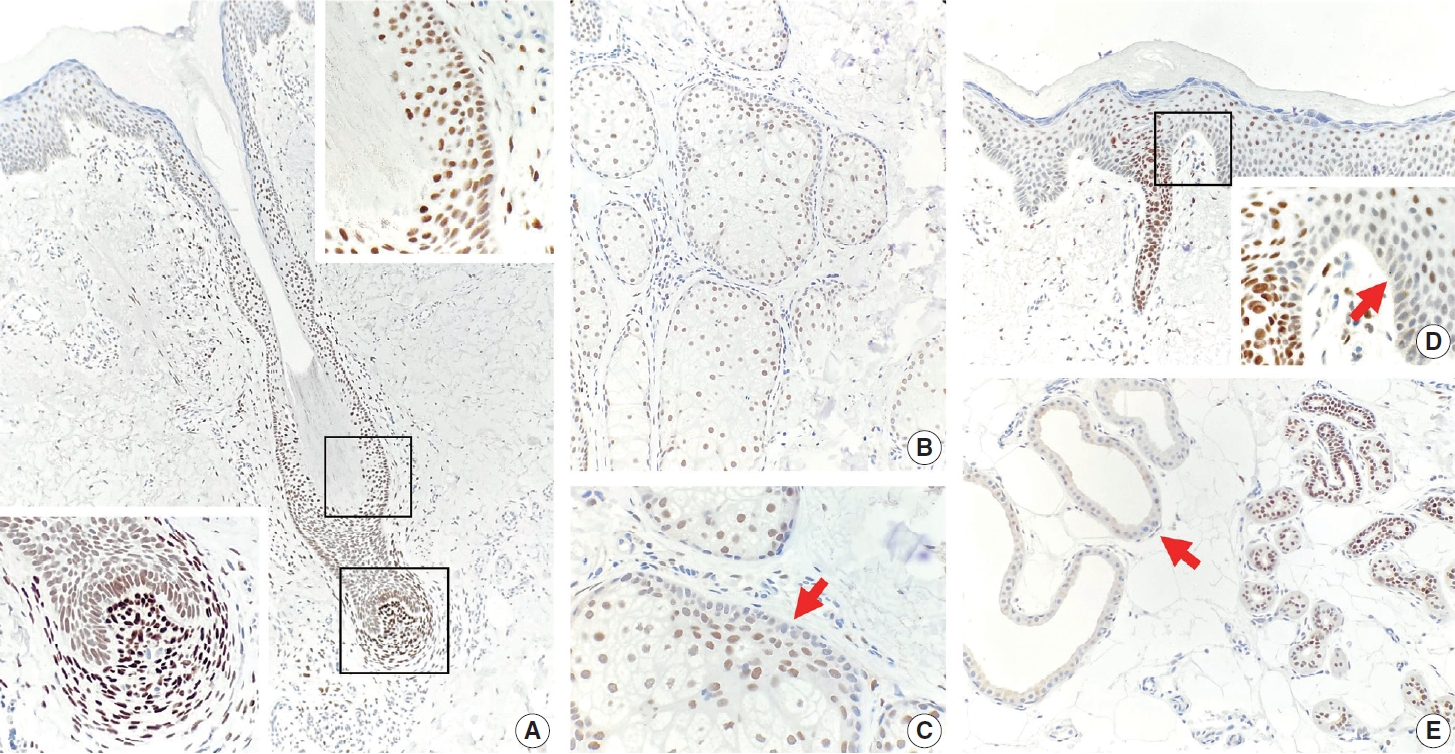
- TRPS1 expression in normal skin
- TRPS1 expression was present in most adnexal structures of normal skin (Fig. 1A–E), except for the apocrine glands (Fig. 1E), as previously reported [6]. The intensity of TRPS1 expression was variable, with the eccrine glands (Fig. 1E), acrosyringia (Fig. 1D), and mesenchymal cells of dermal papillae (Fig. 1A, bottom left inset) showing the strongest intensity (3+) of TRPS1 expression among all adnexal components in the skin. The epidermal keratinocytes, particularly in those with background actinic changes, showed weak-to-moderate TRPS1 expression (Fig. 1D), findings congruent with those seen in a recent study [6]. Notably, when TRPS1 expression was present in the normal epidermis, the basal keratinocytes, unlike those from the stratum spinosum layer, were completely devoid of TRPS1 expression (Fig. 1D, inset, red arrow).
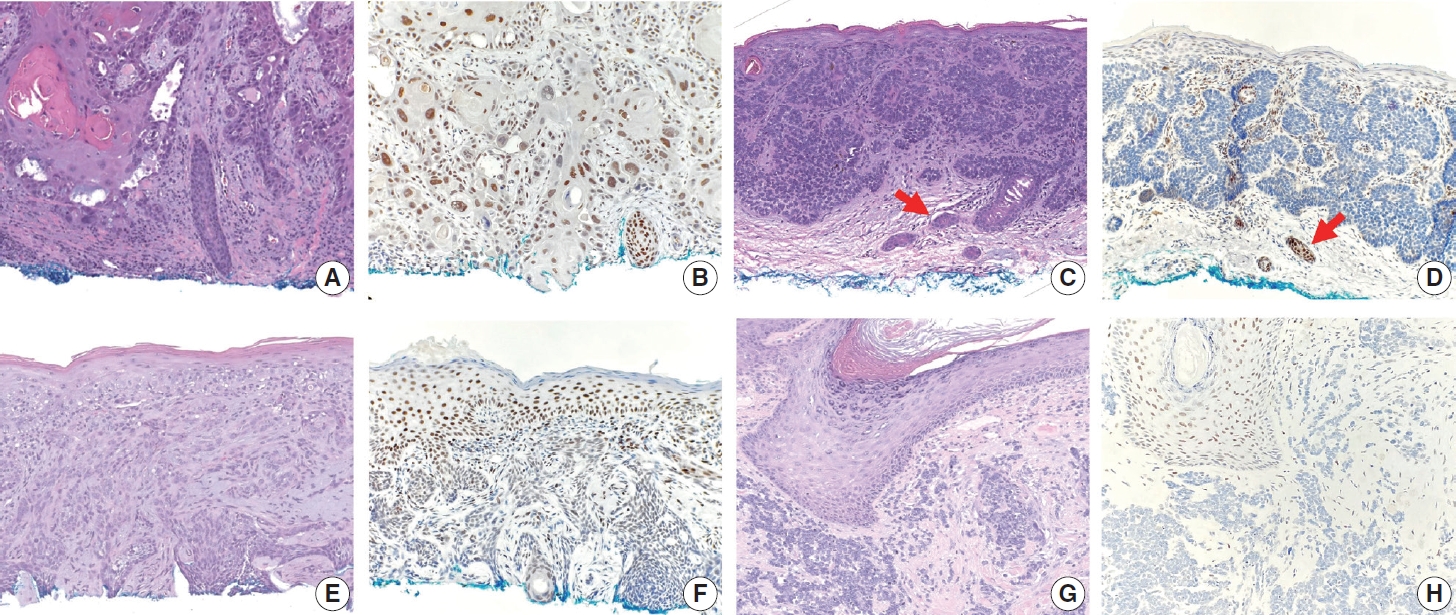
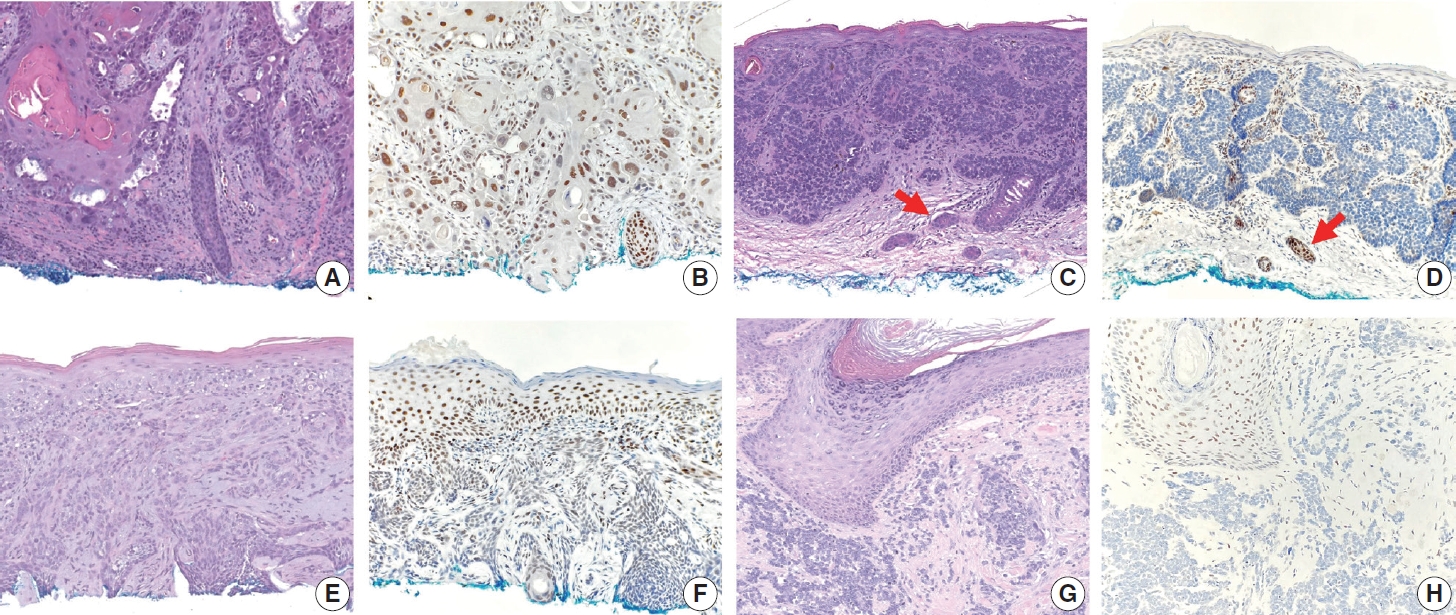
- TRPS1 expression in non-adnexal cutaneous carcinomas
- The frequencies, intensities, proportions (in four categories), and H-scores of TRPS1 expression in SCCs, BCCs, and MCCs are summarized in Table 1. TRPS1 expression was seen in almost all cases of SCC (94%, 33/35), the majority (82%, 27/33) of which exhibited at least moderate (2+ or 3+) expression intensity (Fig. 2A, B). The two cases of SCC that lacked TRPS1 expression (i.e., where less than or equal to 5% of tumor cells exhibited immunoreactivity) consisted of one moderately differentiated SCC and one poorly differentiated SCC. When present, TRPS1 expression in SCC was frequently (85%, 28/33) diffuse (Fig. 2B). The overall H-scores in SCCs ranged from 5 to 295, with a median of 200. In contrast, most BCCs (90%, 37/41) either completely lacked or only focally showed TRPS1 expression (Fig. 2C–F). Diffuse expression of TRPS1 was rare (5%, 2/41) in BCCs; these two cases with diffuse TRPS1 expression were hamartomatous (infundibulocystic) BCC and BCC with squamous differentiation, respectively. The histologic patterns of BCCs included in our study and their corresponding TRPS1 expression profiles are summarized in Supplementary Table S1. The overall H-scores in BCCs ranged from 0 to 160, with a median of 5. The difference between SCCs (regardless of the degree of differentiation) and BCCs (regardless of the presence or absence of squamous differentiation) in terms of H-score of TRPS1 expression was statistically significant (p<.001) (Tables 2, 3). There was no statistically significant difference in H-scores between BCCs without squamous differentiation (n=20) and those with squamous differentiation (n=21) (p=.086) (Table 4, Fig. 2E, F). All cases of MCC (100%, 25/25) consistently lacked TRPS1 expression (Fig. 2G, H).
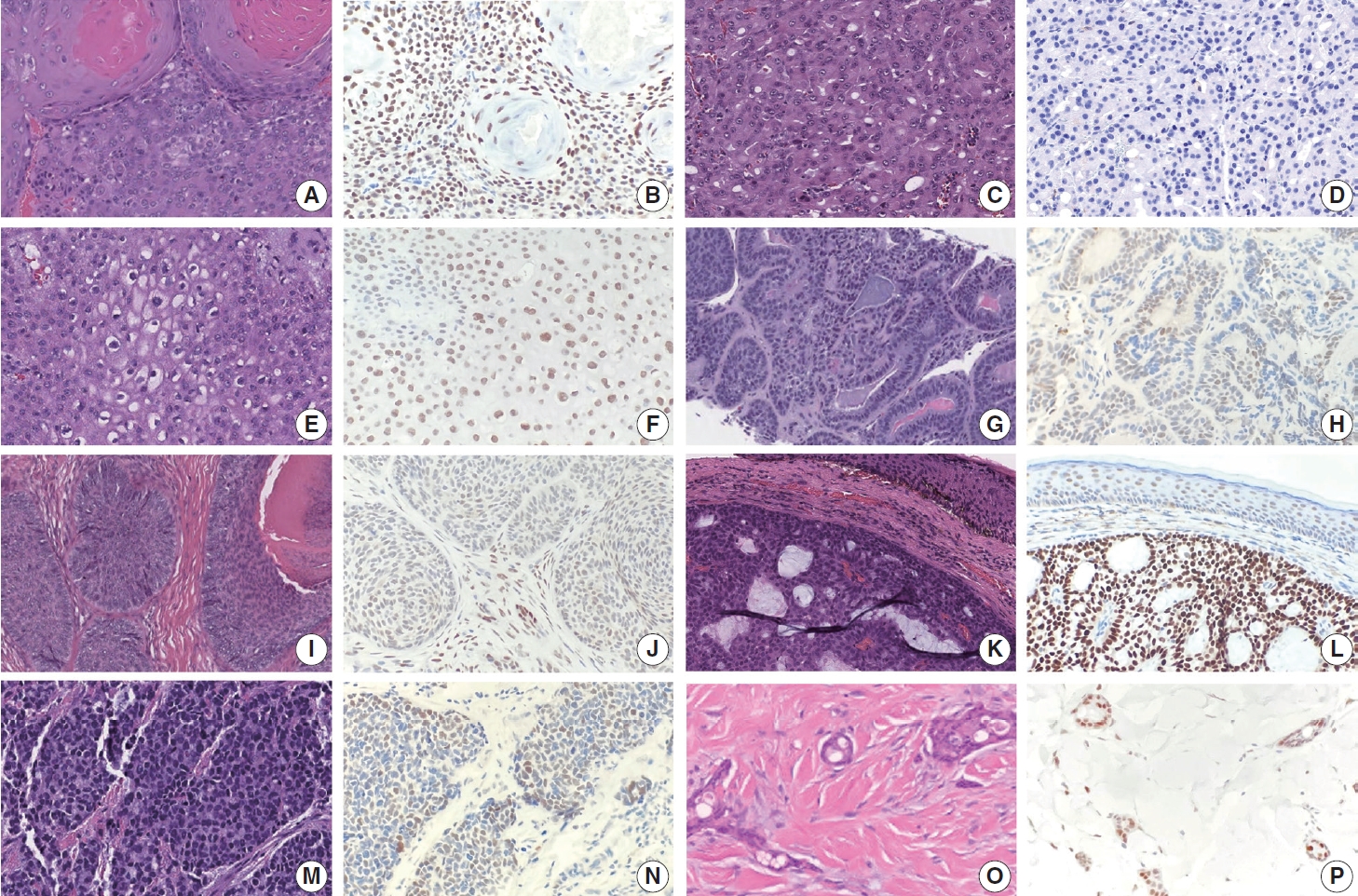
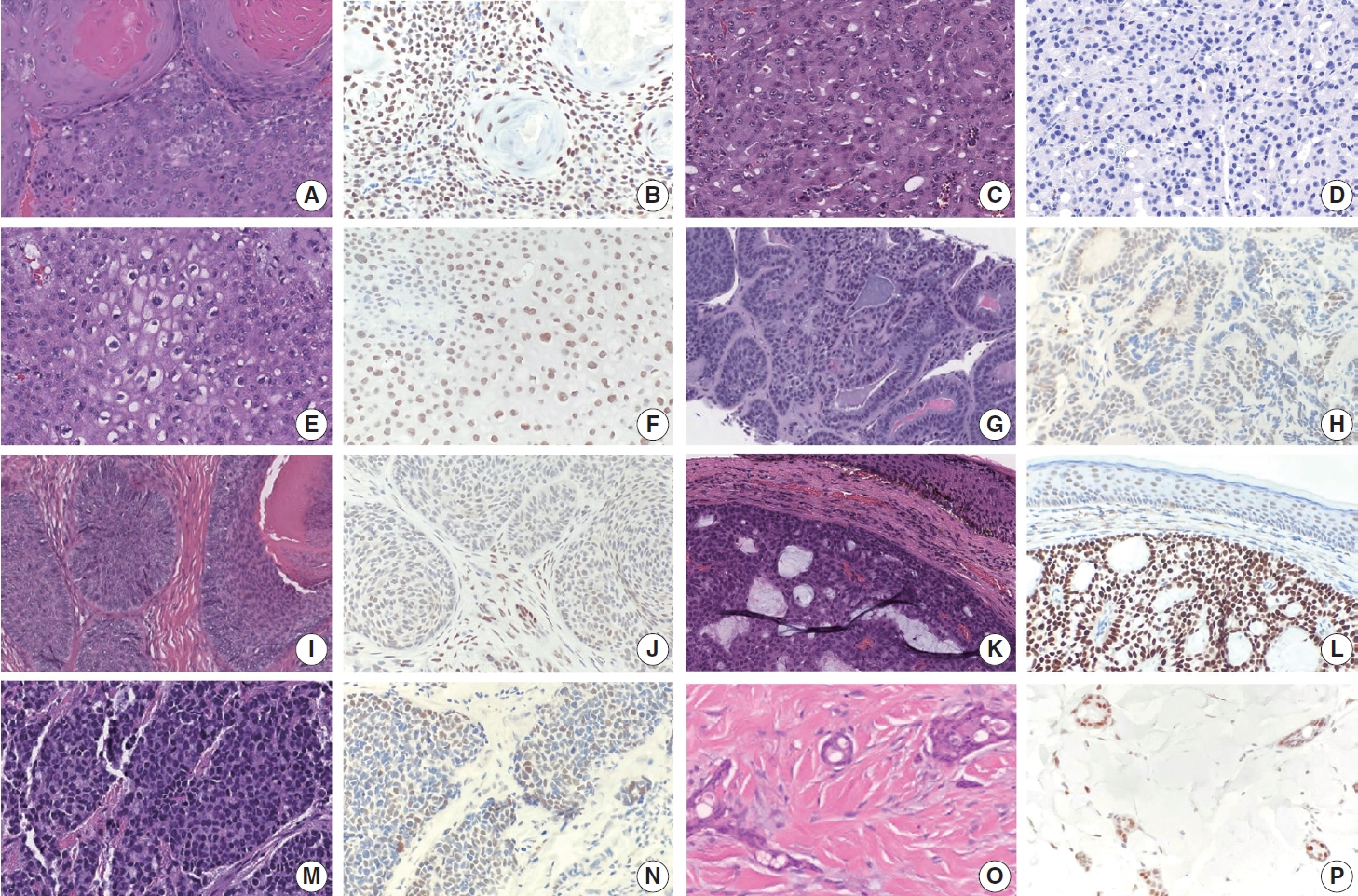
- TRPS1 expression in malignant adnexal neoplasms of pilosebaceous and sweat gland origin
- The frequencies, intensities, proportions (in four categories), and H-scores of TRPS1 expression in malignant adnexal carcinomas are summarized in Table 1. All adnexal carcinomas, including sebaceous carcinomas (n=8), trichilemmal carcinomas (n=6), endocrine mucin-producing sweat gland carcinomas (EMPSGCs) (n=6), malignant proliferating trichilemmal tumors (n=2), squamoid eccrine ductal carcinomas (n=2), digital papillary adenocarcinoma (DPA) (n=1), and trichoblastic carcinoma (n=1), showed at least focal TRPS1 immunoreactivity in variable intensity (Fig. 3A–P), except for apocrine carcinomas (n=3) (Fig. 3C, D). Of those expressing TRPS1, EMPSGCs consistently showed diffuse and strong expression of TRPS1 (100%, 6/6) (Fig. 3K, L). Overall, these cutaneous adnexal carcinomas showed statistically significant differences in terms of H-scores for TRPS1 expression when compared to that of BCCs (p<.001) (Supplementary Table S2).
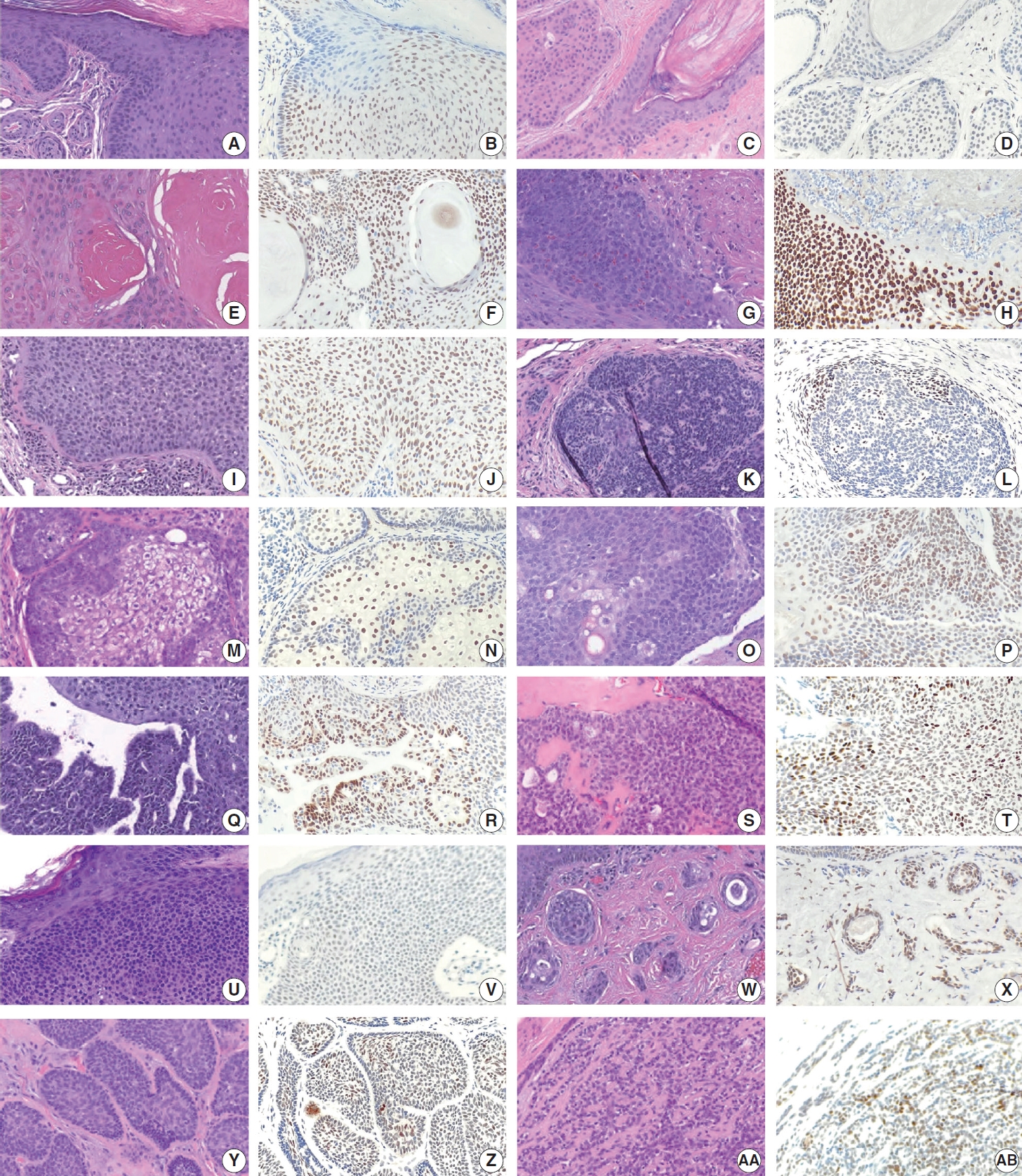
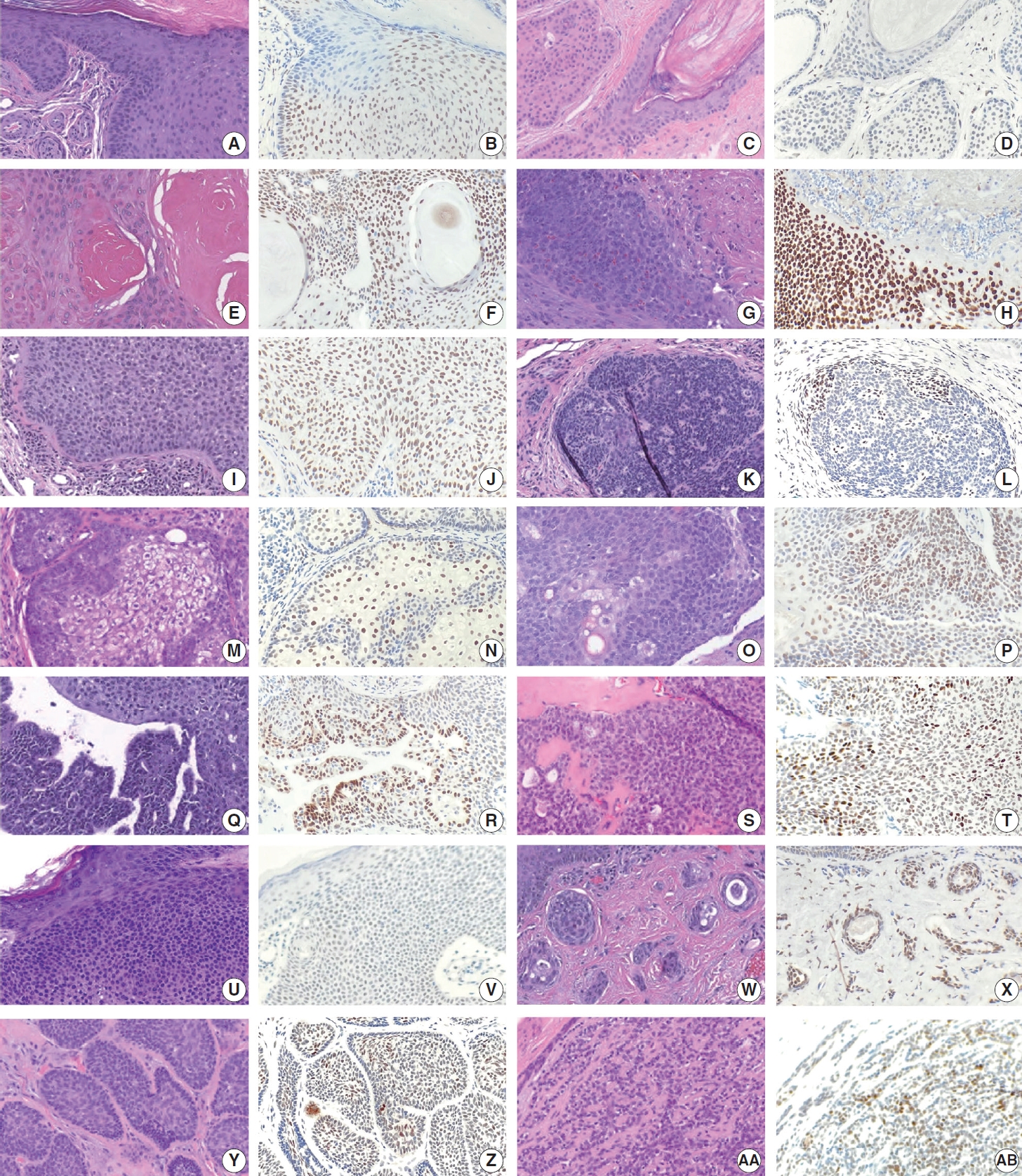
- TRPS1 expression in benign adnexal neoplasms of pilosebaceous and sweat gland origin
- The frequencies, intensities, proportions (in four categories), and H-scores of TRPS1 expression in benign adnexal neoplasms are summarized in Table 1. Similar to their malignant counterparts, almost all benign adnexal tumors, including poromas (n= 12), trichilemmomas (n=12), hidradenomas (n=9), trichoepitheliomas (n=9) (with 3 cases being desmoplastic trichoepitheliomas [DTEs]), pilomatricomas (n=6), spiradenomas/cylindromas (n=5), sebaceous adenomas (n=5), syringocystadenomas papilliferum (n=4), pilar sheath acanthomas (n=2), proliferating pilar tumors (n=2), sebaceomas (n=2), trichofolliculoma (n=1), and syringoma (n=1), exhibited TRPS1 expression in variable proportion and intensity (Fig. 4A–AB).
RESULTS
- The reported sensitivity and specificity of most immunohistochemical studies declines over time as additional data and publications accumulate. The same is also true for TRPS1, a marker initially reported to be highly sensitive and specific for carcinomas [4] and mesenchymal tumors [5] of mammary origin. As of this writing, TRPS1 expression by IHC has been additionally documented in several other tumor types of non-mammary origin, including salivary duct carcinomas [4], ovarian serous and non-serous carcinomas [4], pulmonary SCCs [4], synovial sarcomas [9], EMPDs [6,7], and cutaneous squamous cell carcinomas in situ [6]. Nonetheless, the results from our study indicate that there is potential diagnostic utility of TRPS1 IHC in skin specimens (besides the situations in which cutaneous metastasis of mammary carcinoma is suspected), further expanding our knowledge on the sensitivity and specificity of TRPS1 for various non-melanocytic cutaneous neoplasms.
- While immunohistochemical studies are typically unnecessary to differentiate between BCCs and SCCs, as their distinction can be established through morphologic assessment of H&E-stained sections alone, our findings indicate the potential utility of TRPS1 in certain scenarios. For instance, in cases where extensive squamous differentiation occurs within a BCC, mimicking an SCC, thereby posing challenges in the morphologic differentiation between BCC and SCC. The idea of testing BCCs for TRPS1 expression stemmed from our initial observation on the lack of TRPS1 immunoreactivity in the basal layer of epidermis of severely sun-damaged skin, which would normally show some degree of TRPS1 expression mostly limited to the strata spinosum and granulosum. Assuming most BCCs originate from the basal layer of the interfollicular epidermis [10] and the neoplastic cells of BCCs would likely retain similar immunophenotypic patterns to those of the innate cells of origin residing in the stratum basale, we anticipated that most BCCs would lack TRPS1 expression. A majority of BCCs (90%, 37/41) tested in our study indeed showed no or only focal expression of TRPS1, most of which (78%, 29/37) completely lacked immunoreactivity, supporting our speculation. In contrast, nearly all cases of SCC (94%, 33/35) regardless of their degree of differentiation showed TRPS1 expression, most of which (85%, 28/33) exhibited diffuse immunoreactivity. The difference between BCCs and SCCs in TRPS1 expression and corresponding H-score was significant (p<.001). This differential pattern of expression was also preserved in BCCs with squamous differentiation. When comparing BCCs without squamous differentiation with those with squamous differentiation, there was no significant difference in their H-scores (a median of 0 for the former vs. a median of 5 for the latter) for TRPS1 expression (p=.086) (Table 4). We additionally compared BCCs with squamous differentiation with SCCs to see whether the presence of squamous differentiation in BCCs affects the discriminatory ability of TRPS1 in this setting and found that TRPS1 expression was still significantly more frequent in SCCs (with a median H-score of 200) than BCCs with squamous differentiation (with a median H-score of 5) (p<.001) (Table 3). Thus, TRPS1 may be helpful in distinguishing SCCs from BCCs with squamous differentiation in those cases in which only the superficial portion of the lesion is available (e.g., thin shave biopsies).
- We also identified the potential diagnostic utility of TRPS1 in distinguishing BCCs from adnexal carcinomas, particularly EMPSGCs. Almost all adnexal carcinomas—albeit not entirely inclusive of all types of cutaneous adnexal carcinomas—tested in our study exhibited some level of TRPS1 immunoreactivity, except for apocrine carcinomas. Overall, there were significant differences in H-scores for TRPS1 expression between adnexal carcinomas (with a median H-score of 160) and BCCs (with a median H-score of 5) (p<.001) (Supplementary Table S2). Given the consistent presence of diffuse and strong (3+) TRPS1 expression in EMPSGCs, we additionally compared them with BCCs, a morphologic differential diagnosis of EMPSGC, in terms of H-score for TRPS1 expression and found a significant difference between the two groups (p<.001) (Supplementary Table S3). BCCs can exhibit neuroendocrine differentiation [11], albeit rarely, and thus, mimic EMPSGCs both morphologically and immunophenotypically. The distinction between the two entities can especially be challenging if the lesion arises in the eyelid or periorbital region, a common anatomic site for EMPSGCs, and only a small fraction of the lesion has been sampled in a superficially shaved biopsy specimen. TRPS1 IHC will be practically useful in this setting in that the presence of diffuse and strong TRPS1 expression will strongly favor a diagnosis of EMPSGC over BCC. A recent pilot study reinforces this concept, suggesting that in cases where neuroendocrine markers like INSM1 show immunoreactivity, concurrent strong and diffuse TRPS1 expression is likely to support an EMPSGC diagnosis over BCC [12]. Notably, besides EMPSGC, no primary cutaneous adnexal carcinoma known to date exhibits strong and diffuse TRPS1 expression while concurrently displaying neuroendocrine differentiation [12].
- In view of the relatively high frequency of TRPS1 expression in various adnexal carcinomas, TRPS1 IHC should be used with caution when the differential diagnosis includes cutaneous metastasis of breast carcinoma. Additional immunohistochemical studies, such as p63, D2-40, and calretinin [13-15], may still be needed along with thorough clinical and radiologic correlation to differentiate primary cutaneous adnexal carcinoma from metastatic breast carcinoma to the skin. Furthermore, the utility of TRPS1 IHC in differentiating an adnexal neoplasm from another type of adnexal neoplasm appears to be limited. The relatively small sample size of adnexal neoplasms (n=99) and lack of data on certain tumor types, such as microcystic adnexal carcinomas (MACs), in our study also further hinders the accurate assessment of the value of TRPS1 IHC in adnexal neoplasms and their nonadnexal morphologic mimics. The data from a recent study that investigated the frequency of TRPS1 expression in 51 cases of adnexal neoplasm, including 21 cases of adnexal carcinomas [16], may provide additional insights into the utility of TRPS1 IHC if combined with the results from our study. For instance, that recent study included five MACs, which were found to be consistently negative for TRPS1 expression [16]. The morphologic differential diagnosis of MAC typically includes infiltrative/morpheaform BCC and DTE, and less commonly, syringoma. Based on the recently published report and our study, it seems that TRPS1 IHC cannot distinguish MACs from BCCs or DTEs, as all MACs (n=5) [16] and a majority of BCCs and DTEs (in our study) lacked TRPS1 expression. Similarly, TRPS1 IHC does not seem to have a role in distinguishing DPA from hidradenomas, a common mimicker of DPA, especially if the lesion arises from acral sites. In our study, one case of DPA and 89% (8/9) of hidradenomas showed TRPS1 expression. A similar rate of expression frequency (82%, 9/11) was found in hidradenomas in the recent study by Zengin et al. [16] but no case of DPA was included in their study.
- In summary, we have herein characterized the expression pattern and frequency of TRPS1 in various non-melanocytic cutaneous neoplasms, including 41 cases of BCC and 35 cases of SCC. Our results indicate that TRPS1 IHC may be a useful discriminatory marker for BCCs and SCCs, the two cutaneous carcinomas most frequently encountered during routine dermatopathology practice, in certain contexts. While p40 and p63 IHCs are commonly utilized as sensitive markers for SCCs, their lack of specificity—being expressed in both SCCs and BCCs—can pose challenges. Hence, in situations where differentiation between BCCs and SCCs proves challenging due to extensive squamous differentiation within a BCC, augmenting the analysis with a panel of supplementary IHC markers—such as BerEP4 [17] and epithelial membrane antigen [17] alongside TRPS1— could substantially bolster the discriminatory capability, aiding in the precise distinction between BCCs and SCCs. This marker will also be useful in distinguishing BCCs from EMPSGCs, particularly when the lesion arises from the eyelid or periorbital region. As with all immunohistochemical studies, however, the interpretation of TRPS1 IHC results in skin specimens should be considered within the appropriate histopathologic context. Sole reliance on TRPS1 IHC is discouraged to prevent potential misdiagnosis. Furthermore, one should acknowledge that statistical significance (such as the statistically significant H-score difference in TPRS1 expression between SCCs and BCCs) does not always translate into clinical significance or practicality in everyday practice. Our data on the utility of TRPS1 IHC on most other malignant adnexal neoplasms are still limited due to the small sample size, although the currently available data seem to suggest that TRPS1 IHC lacks a discriminatory power for most adnexal carcinomas. Thus, further studies with a larger cohort are warranted before a clear conclusion can be drawn.
DISCUSSION
Supplementary Information
Ethics Statement
All procedures performed in the current study were approved by the IRB of the University of Texas MD Anderson Cancer Center (IRB#: 2022-0662) in accordance with the 1964 Helsinki declaration and its later amendments. Formal written informed consent was not required with a waiver by the appropriate IRB.
Availability of Data and Material
The data of this study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: WCC. Data curation: YAL. Formal analysis: YW, JN. Funding acquisition: YAL, WCC. Investigation: YAL, WCC. Methodology: WCC. Project administration: WCC. Resources: PPA, PN, JLC, CATC, DI, VGP, QD, WCC. Supervision: WCC. Validation: YAL, WCC. Visualization: YAL, WCC. Writing—original draft preparation: YAL, WCC. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
The Divisional Research Award/Fund from the University of Texas MD Anderson Cancer Center awarded to YAL and WCC was used to support the costs of preparation of unstained slides and immunohistochemical studies. This study was also supported in part by the Institutional Start-up Funds from the University of Texas MD Anderson Cancer Center awarded to WCC.
- 1. Momeni P, Glockner G, Schmidt O, et al. Mutations in a new gene, encoding a zinc-finger protein, cause tricho-rhino-phalangeal syndrome type I. Nat Genet 2000; 24: 71-4. ArticlePubMedPDF
- 2. Malik TH, Von Stechow D, Bronson RT, Shivdasani RA. Deletion of the GATA domain of TRPS1 causes an absence of facial hair and provides new insights into the bone disorder in inherited trichorhino-phalangeal syndromes. Mol Cell Biol 2002; 22: 8592-600. ArticlePubMedPMCPDF
- 3. Ludecke HJ, Schaper J, Meinecke P, et al. Genotypic and phenotypic spectrum in tricho-rhino-phalangeal syndrome types I and III. Am J Hum Genet 2001; 68: 81-91. PubMed
- 4. Ai D, Yao J, Yang F, et al. TRPS1: a highly sensitive and specific marker for breast carcinoma, especially for triple-negative breast cancer. Mod Pathol 2021; 34: 710-9. ArticlePubMedPDF
- 5. Wang J, Wang WL, Sun H, et al. Expression of TRPS1 in phyllodes tumor and sarcoma of the breast. Hum Pathol 2022; 121: 73-80. ArticlePubMed
- 6. Cho WC, Ding Q, Wang WL, et al. Immunohistochemical expression of TRPS1 in mammary Paget disease, extramammary Paget disease, and their close histopathologic mimics. J Cutan Pathol 2023; 50: 434-40. ArticlePubMedPDF
- 7. Liu YA, Collins K, Aung PP, et al. TRPS1 expression in primary and secondary extramammary Paget diseases: an immunohistochemical analysis of 93 cases. Hum Pathol 2024; 143: 5-9. ArticlePubMed
- 8. Cho WC, Nagarajan P, Ding Q, Prieto VG, Torres-Cabala CA. Trichorhinophalangeal syndrome type 1-positive cells in breast dermal granulation tissues and scars: a potential diagnostic pitfall. Am J Dermatopathol 2022; 44: 964-7. ArticlePubMed
- 9. Cloutier JM, Ingram DR, Wani K, Lazar AJ, Wang WL. Frequent TRPS1 expression in synovial sarcoma is associated with SS18-SSX fusion oncoprotein activity. Hum Pathol 2022; 130: 88-94. ArticlePubMed
- 10. Youssef KK, Van Keymeulen A, Lapouge G, et al. Identification of the cell lineage at the origin of basal cell carcinoma. Nat Cell Biol 2010; 12: 299-305. ArticlePubMedPDF
- 11. George E, Swanson PE, Wick MR. Neuroendocrine differentiation in basal cell carcinoma: an immunohistochemical study. Am J Dermatopathol 1989; 11: 131-5. PubMed
- 12. Liu YA, Cho WC. TRPS1 expression in endocrine mucin-producing sweat gland carcinoma: diagnostic utility and pitfalls. Am J Dermatopathol 2024; 46: 133-5. ArticlePubMed
- 13. Ivan D, Nash JW, Prieto VG, et al. Use of p63 expression in distinguishing primary and metastatic cutaneous adnexal neoplasms from metastatic adenocarcinoma to skin. J Cutan Pathol 2007; 34: 474-80. ArticlePubMed
- 14. Plaza JA, Ortega PF, Stockman DL, Suster S. Value of p63 and podoplanin (D2-40) immunoreactivity in the distinction between primary cutaneous tumors and adenocarcinomas metastatic to the skin: a clinicopathologic and immunohistochemical study of 79 cases. J Cutan Pathol 2010; 37: 403-10. ArticlePubMed
- 15. Mahalingam M, Nguyen LP, Richards JE, Muzikansky A, Hoang MP. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol 2010; 23: 713-9. ArticlePubMedPDF
- 16. Zengin HB, Bui CM, Rybski K, Pukhalskaya T, Yildiz B, Smoller BR. TRPS1 is differentially expressed in a variety of malignant and benign cutaneous sweat gland neoplasms. Dermatopathology (Basel) 2023; 10: 75-85. ArticlePubMedPMC
- 17. Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology 2000; 37: 218-23. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Trichorhinophalangeal syndrome type 1 (TRPS1) in breast pathology: diagnostic utility and pitfalls
Atif Ali Hashmi, Edi Brogi, Hannah Y. Wen
Diagnostic Pathology.2025;[Epub] CrossRef - Refining NTRK Fusion Detection in Papillary Thyroid Carcinoma Through Pan-TRK Immunohistochemistry and Histopathologic Features
Hyun Lee, Sue Youn Kim, Ji Min Park, Seung-Hyun Jung, Ozgur Mete, Chan Kwon Jung
Endocrine Pathology.2025;[Epub] CrossRef - Endocrine mucin-producing sweat gland carcinoma: Case report and literature review
Nan Guo, Zhenlin Fan, Yitong Chen, Qian Li, Limin Guo
European Journal of Ophthalmology.2025;[Epub] CrossRef - Updates on utility of immunohistochemistry in diagnosis of metastatic breast cancer
Hongxia Sun, Aysegul A. Sahin, Qingqing Ding
Human Pathology.2025; 162: 105821. CrossRef - Primary Cutaneous NUT Adnexal Carcinoma With BRD4::NUTM1 Fusion: A 19-Year Follow-Up
Elsayed Ibrahim, Richard K. Yang, Maria A. Gubbiotti, Victor G. Prieto, Woo Cheal Cho
The American Journal of Dermatopathology.2025; 47(9): 731. CrossRef - Primary mucinous carcinoma of the skin with co-expression of TRPS1 and GATA3: a case report
Liling Song, Ning Zhu, Lei Jiang, Dong Gao, Guohua Yu
Frontiers in Oncology.2025;[Epub] CrossRef - Metastatic Vulvar Paget's Disease Presenting in a Supraclavicular Lymph Node: A Diagnostic Challenge on Fine Needle Aspiration Cytology
Thiri Htoo Aung, Neha Seth, Anam Khan, Kasturi Das
Diagnostic Cytopathology.2025;[Epub] CrossRef - Diagnostic Algorithm for Secondary Extramammary Paget Disease from Institutional Cases and Literature Review
Salin Kiratikanon, Ayaka Fukui, Masahiro Hirata, Jakob M. T. Moran, Masakazu Fujimoto, Mai P. Hoang
Cancers.2025; 17(24): 4014. CrossRef - TRPS1 Expression Is Frequently Seen in a Subset of Cutaneous Mesenchymal Neoplasms and Tumors of Uncertain Differentiation: A Potential Diagnostic Pitfall
Moon Joo Kim, Yi A. Liu, Yunyi Wang, Jing Ning, Woo Cheal Cho
Dermatopathology.2024; 11(3): 200. CrossRef - TRPS1 expression in MPNST is correlated with PRC2 inactivation and loss of H3K27me3
Rossana Lazcano, Davis R. Ingram, Gauri Panse, Alexander J. Lazar, Wei-Lien Wang, Jeffrey M. Cloutier
Human Pathology.2024; 151: 105632. CrossRef - Syringocystadenoma Papilliferum-Like Features in Poroma: An Unusual Morphologic Pattern of Poroma or True Synchronous Occurrence of 2 Distinct Neoplasms?
Mouaz Alsawas, Fiorinda F. Muhaj, Phyu P. Aung, Priyadharsini Nagarajan, Woo Cheal Cho
The American Journal of Dermatopathology.2024; 46(12): 871. CrossRef - A Comprehensive Review of TRPS1 as a Diagnostic Immunohistochemical Marker for Primary Breast Carcinoma: Latest Insights and Diagnostic Pitfalls
Antonia-Carmen Georgescu, Tiberiu-Augustin Georgescu, Simona-Alina Duca-Barbu, Lucian Gheorghe Pop, Daniela Oana Toader, Nicolae Suciu, Dragos Cretoiu
Cancers.2024; 16(21): 3568. CrossRef - Expression of TRPS1 in Metastatic Tumors of the Skin: An Immunohistochemical Study of 72 Cases
Kassiani Boulogeorgou, Christos Topalidis, Triantafyllia Koletsa, Georgia Karayannopoulou, Jean Kanitakis
Dermatopathology.2024; 11(4): 293. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| No. | Proportion of TRPS1 expression |
Intensity of TRPS1 expression |
H-score |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Focal | Patchy | Diffuse | None | 1+ | 2+ | 3+ | Median | Range | ||
| Squamous cell carcinoma | 35 | 2 (5.7) | 2 (5.7) | 3 (8.6) | 28 (80.0) | 2 (5.7) | 6 (17.1) | 13 (37.1) | 14 (40.0) | 200 | 5–295 |
| Basal cell carcinoma | 41 | 29 (70.7) | 8 (19.5) | 2 (4.9) | 2 (4.9) | 29 (70.7) | 9 (22.0) | 2 (4.9) | 1 (2.4) | 5 | 0–160 |
| Merkel cell carcinoma | 25 | 25 (100) | 0 | 0 | 0 | 25 (100) | 0 | 0 | 0 | 0 | N/A |
| Apocrine carcinoma | 3 | 3 (100) | 0 | 0 | 0 | 3 (100) | 0 | 0 | 0 | 0 | N/A |
| Digital papillary adenocarcinoma | 1 | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 | 1 (100) | 210 | N/A |
| Endocrine mucin-producing sweat gland carcinoma | 6 | 0 | 0 | 0 | 6 (100) | 0 | 0 | 0 | 6 (100) | 300 | 265–300 |
| Malignant proliferating trichilemmal tumor | 2 | 0 | 0 | 0 | 2 (100) | 0 | 0 | 0 | 2 (100) | 245 | 220–270 |
| Sebaceous carcinoma | 8 | 0 | 2 (25.0) | 3 (37.5) | 3 (37.5) | 0 | 6 (75.0) | 1 (12.5) | 1 (12.5) | 92.5 | 10–270 |
| Squamoid eccrine ductal carcinoma | 2 | 0 | 0 | 1 (50.0) | 1 (50.0) | 0 | 1 (50.0) | 1 (50.0) | 0 | 120 | 70–170 |
| Trichilemmal carcinoma | 6 | 0 | 0 | 1 (16.7) | 5 (83.3) | 0 | 0 | 3 (50.0) | 3 (50.0) | 212.5 | 110–290 |
| Trichoblastic carcinoma | 1 | 0 | 1 (100) | 0 | 0 | 0 | 1 (100) | 0 | 0 | 20 | 20–20 |
| Hidradenoma | 9 | 1 (11.1) | 1 (11.1) | 0 | 7 (77.8) | 1 (11.1) | 1 (11.1) | 4 (44.4) | 3 (33.3) | 210 | 0–300 |
| Pilar sheath acanthoma | 2 | 0 | 0 | 0 | 2 (100) | 0 | 0 | 2 (100) | 0 | 190 | 180–200 |
| Pilomatricoma | 6 | 1 (16.7) | 0 | 0 | 5 (83.3) | 1 (16.7) | 0 | 1 (16.7) | 4 (66.7) | 267.5 | 5–290 |
| Poroma | 12 | 2 (16.7) | 4 (33.3) | 3 (25.0) | 3 (25.0) | 2 (16.7) | 7 (58.3) | 1 (8.3) | 2 (16.7) | 52.5 | 5–290 |
| Proliferating pilar cyst | 2 | 0 | 0 | 0 | 2 (100) | 0 | 0 | 1 (50.0) | 1 (50.0) | 222.5 | 150–295 |
| Syringocystadenoma papilliferum | 4 | 0 | 1 (25.0) | 0 | 3 (75.0) | 0 | 1 (25.0) | 1 (25.0) | 2 (50.0) | 255 | 35–300 |
| Sebaceoma | 2 | 0 | 0 | 0 | 2 (100) | 0 | 0 | 2 (100) | 0 | 185 | 180–190 |
| Sebaceous adenoma | 5 | 0 | 0 | 2 (40.0) | 3 (60.0) | 0 | 3 (60.0) | 0 | 2 (40.0) | 80 | 40–250 |
| Spiradenoma/Cylindroma | 5 | 0 | 0 | 0 | 5 (100) | 0 | 0 | 3 (60.0) | 2 (40.0) | 150 | 150–290 |
| Syringoma | 1 | 0 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | 0 | 205 | 205–205 |
| Trichilemmoma | 12 | 0 | 0 | 1 (8.3) | 11 (91.7) | 0 | 0 | 4 (33.3) | 8 (66.7) | 260 | 120–300 |
| Trichoepithelioma | 9 | 6 (66.7) | 2 (22.2) | 0 | 1 (11.1) | 6 (66.7) | 2 (22.2) | 0 | 1 (11.1) | 0 | 0–230 |
| Trichofolliculoma | 1 | 0 | 0 | 0 | 1 (100) | 0 | 0 | 1 (100) | 0 | 200 | N/A |
| BCC (n = 41) | SCC (n = 35) | p-value | |
|---|---|---|---|
| H-score | |||
| Median | 5 | 200 | < .001 |
| Range | 0–160 | 5–295 | |
| Proportion | < .001 | ||
| Absent | 29 (70.7) | 2 (5.7) | |
| Focal | 8 (19.5) | 2 (5.7) | |
| Patchy | 2 (4.9) | 3 (8.6) | |
| Diffuse | 2 (4.9) | 28 (80.0) | |
| Intensity | < .001 | ||
| None | 29 (70.7) | 2 (5.7) | |
| 1+ | 9 (22.0) | 6 (17.1) | |
| 2+ | 2 (4.9) | 13 (37.1) | |
| 3+ | 1 (2.4) | 14 (40.0) |
| BCCSq (n = 21) | SCC (n = 35) | p-value | |
|---|---|---|---|
| H-score | |||
| Median | 5 | 200 | < .001 |
| Range | 0–150 | 5–295 | |
| Proportion | < .001 | ||
| Absent | 13 (61.9) | 2 (5.7) | |
| Focal | 5 (23.8) | 2 (5.7) | |
| Patchy | 2 (9.5) | 3 (8.6) | |
| Diffuse | 1 (4.8) | 28 (80.0) | |
| Intensity | < .001 | ||
| None | 13 (61.9) | 2 (5.7) | |
| 1+ | 6 (28.6) | 6 (17.1) | |
| 2+ | 1 (4.8) | 13 (37.1) | |
| 3+ | 1 (4.8) | 14 (40.0) |
| BCC (n = 20) | BCCSq (n = 21) | p-value | |
|---|---|---|---|
| H-score | |||
| Median | 0 | 5 | .086 |
| Range | 0–160 | 0–150 | |
| Proportion | .208 | ||
| Absent | 16 (80.0) | 13 (61.9) | |
| Focal | 3 (15.0) | 5 (23.8) | |
| Patchy | 0 (0) | 2 (9.5) | |
| Diffuse | 1 (5.0) | 1 (4.8) | |
| Intensity | .239 | ||
| None | 16 (80.0) | 13 (61.9) | |
| 1+ | 3 (15.0) | 6 (28.6) | |
| 2+ | 1 (5.0) | 1 (4.8) | |
| 3+ | 0 (0) | 1 (4.8) |
Values are presented as number (%). TRPS1, trichorhinophalangeal syndrome type 1; N/A, not applicable.
Values are presented as number (%) unless otherwise indicated. TRPS1, trichorhinophalangeal syndrome type 1; BCC, basal cell carcinoma; SCC, squamous cell carcinoma.
Values are presented as number (%) unless otherwise indicated. TRPS1, trichorhinophalangeal syndrome type 1; BCCSq, basal cell carcinoma with squamous differentiation; SCC, squamous cell carcinoma.
Values are presented as number (%) unless otherwise indicated. TRPS1, trichorhinophalangeal syndrome type 1; BCC, basal cell carcinoma; BCCSq, basal cell carcinoma with squamous differentiation.

 E-submission
E-submission