Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(4); 2024 > Article
-
Review
Welcoming the new, revisiting the old: a brief glance at cytopathology reporting systems for lung, pancreas, and thyroid -
Rita Luis1,2,*
 , Balamurugan Thirunavukkarasu3,*
, Balamurugan Thirunavukkarasu3,* , Deepali Jain3,**
, Deepali Jain3,** , Sule Canberk,4,5,6,**
, Sule Canberk,4,5,6,**
-
Journal of Pathology and Translational Medicine 2024;58(4):165-173.
DOI: https://doi.org/10.4132/jptm.2024.06.11
Published online: July 15, 2024
1Department of Pathology, Unidade Local de Saúde São José, Lisbon, Portugal
2Pathology Institute, Lisbon School of Medicine, Lisbon, Portugal
3Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
4Instituto de Investigação e Inovação em Saúde (i3S), University of Porto, Porto, Portugal
5Institute of Molecular Pathology and Immunology of the University of Porto (Ipatimup), Porto, Portugal
6Abel Salazar Institute of Biomedical Sciences (ICBAS), University of Porto, Porto, Portugal
- Corresponding Author: Sule Canberk, MD, MIAC, Cancer Signaling and Metabolism Group, Instituto de Investigação e Inovação em Saúde (i3S), Rua Alfredo Allen, 208 4200-135, Porto, Portugal Tel: +351-225-570-700, Fax: +351-225-570-798, E-mail: scanberk@ipatimup.pt
- *Rita Luis and Balamurugan Thirunavukkarasu contributed equally to this work as first author.
**Deepali Jain and Sule Canberk contributed equally to this work as second author.
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- This review addresses new reporting systems for lung and pancreatobiliary cytopathology as well as the most recent edition of The Bethesda Reporting System for Thyroid Cytopathology. The review spans past, present, and future aspects within the context of the intricate interplay between traditional morphological assessments and cutting-edge molecular diagnostics. For lung and pancreas, the authors discuss the evolution of reporting systems, emphasizing the bridge between past directives and more recent collaborative efforts of the International Academy of Cytology and the World Health Organization in shaping universal reporting systems. The review offers a brief overview of the structure of these novel systems, highlighting their strengths and pinpointing areas that require further refinement. For thyroid, the authors primarily focus on the third edition of The Bethesda System for Reporting Thyroid Cytopathology, also considering the two preceding editions. This review serves as an invaluable resource for cytopathologists, offering a panoramic view of the evolving landscape of cytopathology reporting and pointing out the integrative role of the cytopathologist in an era of rapid diagnostic and therapeutic advancements.
- Pulmonary masses and nodules are increasingly recognized by imaging techniques and subsequently targeted for cytologic and/or small-volume biopsy evaluation, responsible for the diagnosis of an estimated 70% of pulmonary malignancies and allowing disease staging [3]. In fact, lung cancer is mostly a time-sensitive condition, as a significant fraction of patients present with or rapidly progress to advanced stages, hampering a surgical approach and relying on cytological and histological samples to further determine potential therapies.
- Rationale, historical background, and state of the art
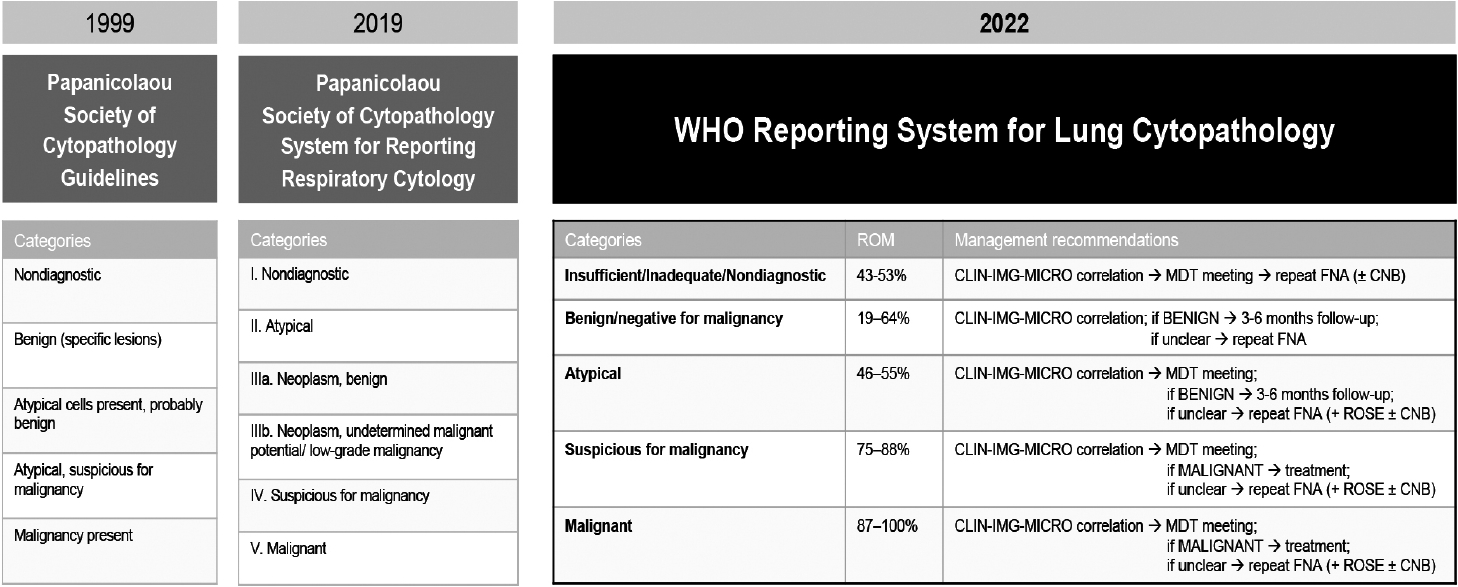
- In 1999, the PSC Task Force on Standards of Practice issued detailed guidelines for handling each type specimen obtained using lower-respiratory-exfoliative or FNA techniques and briefly outlined six recommended categories for reporting these samples (Fig. 1) [1,5,6]. The 1999 guidelines emphasized safety and efficiency and underlined that, despite the main use of diagnosis of malignancy, the guidelines could also be used to identify benign conditions [7].
- The 2016 update of the guidelines proposed a new standardized terminology, recognizing that the lack of homogeneity in reporting could be hindering the clinical decision-making process [1]. The diagnostic criteria were refined, specific diagnoses/ entities were listed, expected ROM rates were reported, and the categories were renamed and sequentially numbered (Fig. 1). The assessment of regional lymph nodes (mediastinal and hilar) was nested under the same classification system. Recommendations for ancillary studies were also included [1], primarily addressing the evaluation of predictive markers (such as programmed death-ligand 1 [PD-L1] and potential oncogenic genetic changes) using immunohistochemistry and molecular techniques. Institutional data [8] confirmed the overall ROM for each category and shed light on inconsistencies in overall diagnostic accuracy and ROM estimation across the various procedural approaches used to assess a given lesion.
- The PSC System for Reporting Respiratory Cytology [9] was subsequently published in 2019. This atlas expanded the morphologic criteria and was supported by an extensive assortment of photographs and explanatory notes, sample reports, and updated ROM rates (stratified by primary lesion and nodal assessment). The directives for ancillary studies were included but indicated that PD-L1 testing lacked comprehensive validation and did not provide specific recommendations.
- In 2022, the International Academy of Cytology and the IARC, which oversees the WHO Classification of Tumors, combined forces to develop an analogous series of WHO reporting systems for lung cytopathology. Indeed, the so-called WHO Blue Books and the WHO cytopathology system are directly linked and are both offered in print and online forms. The main goal was to define a universal lexicon and specify criteria by which robust cytopathological diagnoses could be achieved worldwide, even by laboratories with limited resources. Five categories applicable to all types of specimens were established (Fig. 1; see also below). Under the banners of “Benign” and “Malignant,” the authors explained specific lesions or entities through subsections that echo the WHO Blue Books structure. Additional sections described best practices for specimen collection and handling, provided recommendations for ancillary testing (formally including PDL1 determinations) and for management of each diagnostic category, emphasizing the role of multidisciplinary study and rapid onsite evaluation (ROSE) [3]. The five primary diagnostic categories are presented as follows.
- This category applies to specimens that cannot be reliably diagnosed due to inadequate cellularity, poor preparation, or obscuring factors. Each institution should resort to a single term to label this category and document the reasons for specimen insufficiency. The presence of any atypical cells upgrades the specimen to a higher category. The overall ROM for this category ranges from 40% to 60%; repeated sampling increases the sensitivity, especially of exfoliative specimens.
- Specimens in this category show clear cytopathologic signs of benign processes or neoplasms. It is essential to thoroughly compare these findings with imaging results; any disparities should be noted, with recommendations for further diagnostic steps, including a conservative approach, prompt reassessment of the morphological lesion, or (in such cases as an infection secondary to bronchial obstruction), surgical treatment. The ROM was projected to be 20% to 40%, and further assessment is necessary to refine this estimate.
- This category includes specimens displaying predominantly benign characteristics but featuring worrisome findings that raise the suspicion for malignancy, without sufficient evidence for conclusive diagnosis. These cases require correlation with clinical and imaging data and carry an ROM of 50% to 60%.
- Specimens that show features indicative of malignancy but lack conclusive evidence for a definitive diagnosis fall into this category. This category implies a degree of uncertainty while maintaining a high positive predictive value, with an ROM around 82%. Further investigation is typically warranted, and ancillary techniques can help refine the diagnosis.
- This definitive category is used when the specimen exhibits clear-cut features of malignancy without ambiguity; subclassification based on cytopathologic features and immunocytochemistry markers may also be undertaken. The ROM in this category exceeds 90%, and the diagnosis should be supported by clinical and imaging data to guide appropriate treatment.
- Cytopathological practice
- The WHO Reporting System for Lung Cytopathology reflects a convergence of principles from previous classifications and ongoing initiatives to address the challenges in lung cancer diagnosis. The framework is structured to document and interpret small-volume biopsies, standardize procedures, and stratify the ROM, the latter of which is particularly important given early detection can significantly impact treatment outcomes.
- Faced with continuing instrument development, pathologists in the field must make deliberate efforts to adhere to the directives to reduce subjectivity and variability. The ROM should be considered with a critical outlook, and professionals should assume responsibility for institutional cytohistological correlation series, on whose account the ROM will be periodically revisited.
- Future perspectives
- Like so many other fields in pathology, the scientific knowledge of lung cancer is constantly evolving, not only with respect to basic science, but also with respect to clinical trial data that may quickly alter the standard of care; the WHO Reporting System must remain adaptable to this dynamic landscape. In particular, the emergence of liquid biopsies has the potential to provide deeper insights into the molecular profile of each tumor, while likely relying on morphological correlation for validation.
- First, determination of PD-L1 in ethanol-fixed non-cellblock specimens should be validated through large-scale studies. Second, a more structured role could be carefully outlined for ROSE, potentially involving a dedicated and abbreviated classification, with consideration of the use of telecytopathology platforms, an invaluable resource for a growing number of institutions.
- Digital pathology, artificial intelligence, and machine learning could streamline workflows by pre-selecting samples warranting examination and possibly identifying viral cytopathic effects or microorganisms. These measures may reduce the need for supplementary investigations that can consume both the often limited sample material and economic resources. In addition, subtle morphological changes that could otherwise be overlooked may be detected, enhancing accuracy and promoting consistency across categories.
LUNG
Insufficient/inadequate/nondiagnostic
Benign
Atypical
Suspicious for malignancy
Malignant
- The WHO Reporting System for Pancreaticobiliary Cytopathology is part of a new series aligned with the fifth edition of the Classification of Digestive System Tumors [10,11]. This system standardizes reporting based on modifications of the 2015 PSC System [12]. The new system introduces seven categories, including “Pancreaticobiliary neoplasm: low-risk/grade” and “High-risk/ grade,” based on a two-tiered stratification of cytological atypia. Notably, neuroendocrine tumors and solid pseudopapillary neoplasms are now in the “Malignant” category, while benign tumors like serous cystadenoma are classified as “Benign/Negative for malignancy.” The following sections offer a concise overview of these changes, providing insights into diagnostic categories, rationale for updates, and management implications.
- Rationale, historical background, and state of the art
- The WHO Reporting System for Pancreaticobiliary Cytopathology updates the 2015 PSC system for reporting Pancreaticobiliary Cytopathology [12]. Many entities have been reclassified in other categories in alignment with the WHO Classification of Digestive System Tumors. Ancillary studies like fluid biochemical assays, immunocytochemistry, fluorescent in situ hybridization (FISH), and next-generation sequencing (NGS) are essential in the diagnosis of pancreatic cysts and cases with suspicious morphology. Pancreatic FNA specimens and bile duct cytology have different ROMs owing to the inherent nature of the lesion and sampling techniques.
- In the new system, there are seven categories compared to six in the PSC system. Tumors that were placed in the “Neoplastic: other” category like pancreatic mucinous neoplasm, ductal lesions, biliary and pancreatic intraepithelial neoplasia (PanIN) are placed in “Pancreaticobiliary neoplasm: low-risk/grade or high-risk/grade” based on the cytological atypia.
- The seven diagnostic categories are as follows.
- This category has three options depending on the context and institutional practice. The categorization of tissue requires clinical and radiological correlation. If native tissue is sampled, it is prudent to categorize it as “Inadequate” rather than “Benign.” In contrast, even when extracellular mucin is devoid of epithelial cells, if the cyst fluid shows increased carcinoembryonic antigen (CEA) and corroborating radiological findings, it can be diagnostic. The ROM range for this category is 5%–25% [13]. Bile-duct stricture brushings have a higher ROM of 28%–69% due to sampling bias.
- This category combines the nomenclature of the PSC system and the WHO Classification of Digestive System Tumors. Either terminology can be used. These terms include both non-neoplastic entities such as pancreatitis, pseudocyst, and lymphoepithelial cyst and benign neoplasms such as serous cystadenoma and, rarely, schwannoma, or lymphangioma. The ROM is 0%–15% [13,14]. For bile-duct brushings, the ROM is 55%. This increased risk is due to the high threshold for malignancy leading to false negative cases.
- This category applies to cases that have architectural and cytological features that suggest more than a reactive process but for which there is insufficient evidence for placement in definite categories such as “Pancreaticobiliary neoplasm, low-risk/grade (PaN-low),” “Pancreaticobiliary neoplasm, high-risk/grade (PaN-high),” or “Malignant” [15]. Such limitations could be due to do low cellularity, artifacts, or the inherent nature of the lesion. In cases of mass lesion in pancreas, the atypia can be due to reactive atypia in pancreatitis or poor sampling of malignant lesions. In cystic lesions, only a minority of the cases may judiciously be placed in this category, after utilization of integrated approach to place them in the specific “PaN-low” category. The ROM is 30%–40% for pancreatic FNA samples and 25%–61% for bile-duct brushings [13,16].
- Pancreaticobiliary neoplasm (low risk/grade) is a new category incorporating the remaining entities of PSC “Neoplastic: other” after exclusion of solid pseudopapillary neoplasm and neuroendocrine tumors. This category includes cystic neoplasms and intraductal neoplasms with low-grade epithelial atypia. The two-tiered stratification (i.e., low-grade and high-grade atypia) is similar to the histological classifications provided in the fifth edition of the WHO Classification of Digestive System Tumors [17]. The included entities are intraductal papillary mucinous neoplasm (IPMN) low-grade, mucinous cystic neoplasm low-grade, biliary intraepithelial neoplasia low-grade, PanIN low-grade, intraductal papillary neoplasm of bile duct low-grade, and low-grade spindle cell neoplasm.
- In cystic neoplasms with mucin, the cellularity may be sparse with low- to intermediate-grade atypia [18]. The cells can be arranged in sheets and papillae. The background may show thick colloid-like mucin. Testing the cyst fluid for elevated CEA (above 192 ng/mL) is useful to identify neoplastic mucinous cysts [19]. In addition, testing for KRAS, GNAS, and RNF43 mutations in suspected cases of IPMN is advisable [20,21]. The estimated ROM for this category is 5%–20% [13]. The ROM for bile-duct brushings is not available.
- This category includes cystic neoplasms and intraductal neoplasms, as described above, that also display high-grade epithelial atypia (HGEA), as well as intraductal oncocytic papillary neoplasm and intraductal tubulopapillary neoplasm. High-grade dysplasia and invasive carcinoma can be difficult to distinguish based on cytology alone. An HGEA is defined as a cell smaller than a duodenal enterocyte (12 μm) with high nucleus/cytoplasm ratio and chromatin abnormalities with or without necrosis [22]. Intermediate-grade dysplasia is placed in the histological “low-grade” group, creating a diagnostic dilemma. Mutation testing for TP53, CDKN2A (p16), and SMAD4 deletion may indicate progression to malignancy [23,24]; p53 immunostaining (overexpression or null type) and loss of SMAD4 may also aid in diagnosis. The estimated ROM for this category is 60%–95% [13].
- This category is used when the features are suspicious but not diagnostic of malignancy. This uncertainty could be due to low cellularity, difficulty in interpretation due to inflammation/stenting, or inadequate tissue for ancillary testing. This category may be used to reduce false-positive cases and when diagnostic features are seen in only a small number of fragments [25]. Consensus review and ancillary testing can help guide further management [26]. The ROM is 80%–100% for pancreatic FNAs and 74%–100% for bile-duct brushings [13].
- Pancreatic ductal adenocarcinoma is the most common pancreatic malignancy. Other tumors that may share overlapping morphology are acinar cell carcinoma, neuroendocrine carcinoma, and metastatic carcinoma, which must be distinguished by ancillary studies [27]. Neuroendocrine tumors and solid pseudopapillary neoplasms are included in this category in accordance with the fifth edition of the WHO Classification of Digestive System Tumors. False-positive results may occur due to florid reactive atypia in autoimmune pancreatitis and primary sclerosing cholangitis. Integrating ancillary studies such as FISH and NGS will help guide further management [28]. The ROM is 99%– 100% for positive pancreatic FNA and is 96%–100% for biliary tract cytology [13,16].
PANCREAS
Insufficient/inadequate/nondiagnostic
Benign/negative for malignancy
Atypical
Pancreaticobiliary neoplasm, low risk/grade
Pancreaticobiliary neoplasm, high risk/grade
Suspicious for malignancy
Malignant
- FNA cytology plays a pivotal role in the management of thyroid nodules, reducing the need for unnecessary surgery. In 2010, TBSRTC introduced a six-tiered system to categorize thyroid FNA findings and their associated ROM [29], based on the proceedings of the October 2007 National Cancer Institute Thyroid FNA State of the Science Conference in Bethesda, Maryland [30].
- The third edition of the TBSRTC, released in 2023, further refines these categories, emphasizing clarity in reporting using explicit category names. The 2023 TBSRTC updates the ROM based on recent large-scale studies and responds to the ambiguous ROM associated with indeterminate diagnoses, not distinguished despite molecular testing.
- Rationale, historical background, and state of the art
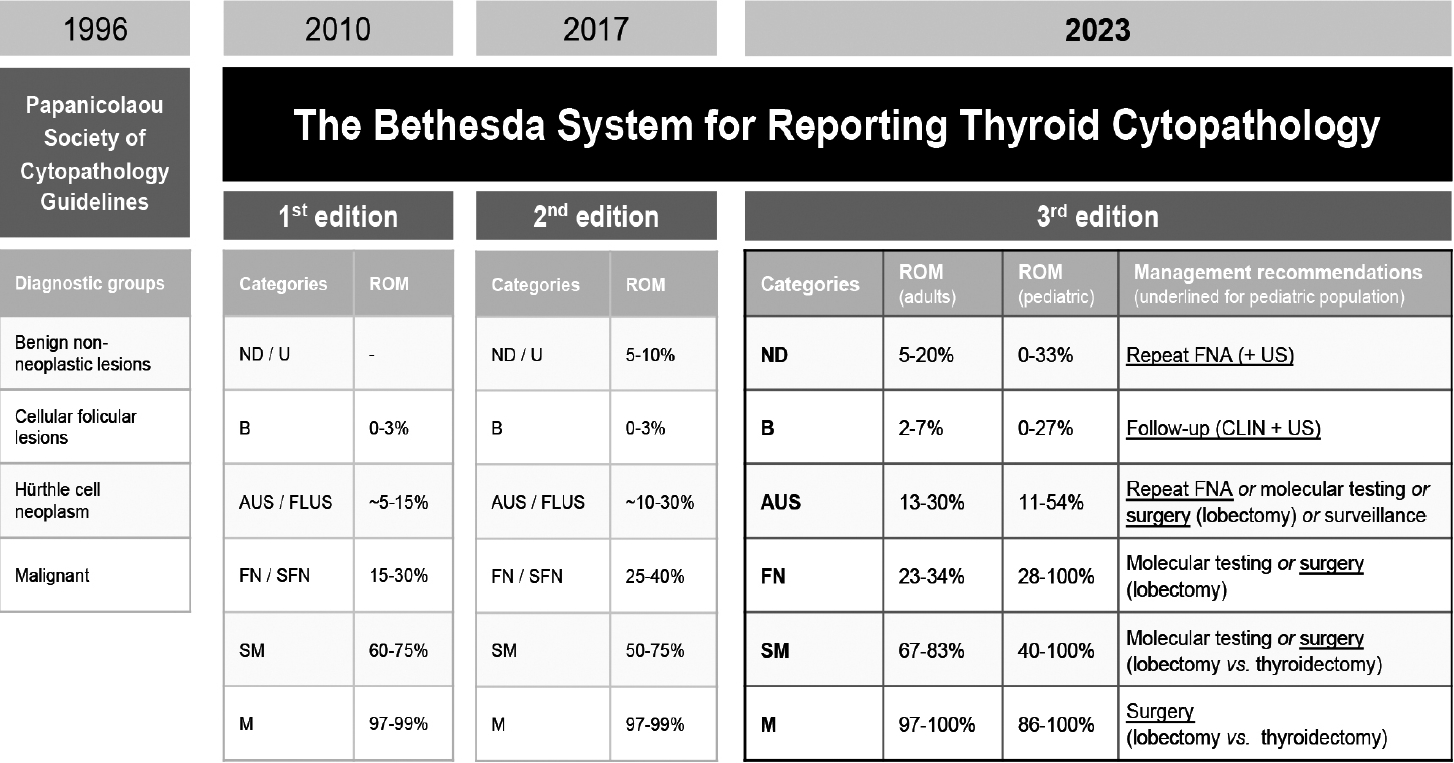
- In 1996, the PSC Task Force on Standards of Practice first released guidelines [30] pertaining to the evaluation of thyroid nodules by FNA. The document summarized technical matters, addressed interdisciplinary approaches, and proposed four tentative diagnostic groups (Fig. 2) [29,31-33].
- In 2010, the proposed TBSRTC (six-tiered system) (Fig. 2) was comprehensively explained and, regardless of being a first edition of the classification, attempted to establish the ROM of each category. The reporting system was globally acclaimed across medical specialties and readily adapted to the various national settings [34].
- In 2018, a second edition of TBSRTC was published [32], in which the nomenclature (Fig. 2) and general criteria remained largely unchanged while embracing a role for molecular pathology. The tiered ROMs were recalculated based on pooled data from multiple cyto-histological correlation series published after 2010, with an effort to forecast the ROM for the newly distinguished noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Additionally, characterization of the type of atypia observed in Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance (AUS/FLUS) was encouraged, loosely into classifiers of “cytologic,” “architectural,” “cytologic and architectural,” “Hürthle cell aspirates,” “not other specified,” and “atypical lymphoid cells.”
- In 2023, the third edition of the TBSRTC was released [33,35], reflecting the continuous effort to integrate clinical perspectives and data from imaging and genetic studies. The 2023 TBSRTC simplified the diagnostic criteria and terminology (Fig. 2) and aligned them with the 2022 WHO Thyroid Tumor Classification [36] by adopting updated histopathological nomenclature and discarding outdated terms like “Hürthle cell.” This 2023 edition continues to differentiate atypia on the basis of nuclear or architectural patterns, reflecting their heterogeneous implications for the ROM, and distinguishing the qualifiers of “atypia of undetermined significance” into “nuclear atypia” and “other.” The ROMs were revised and stratified by adult and pediatric age, and the bias introduced by NIFTP was acknowledged, with an estimated projected percent reduction of the ROM by category. New sections offer insights into radiologic correlations, molecular diagnostics, and pediatric-specific management.
- Cytopathology practice
- Given TBSRTC is among the most established cytopathology reporting systems, it is commonly referenced by the many disciplines encompassed by thyroidology. It provides a standardized language for communication clarity and consistency as pathologists can quickly craft a detailed report that conveys their reasoning without concern for potential interpretation bias, and intra- and interdepartmental datasets can be evaluated across institutions or even countries and continents. Use of the TBSRTC also contributes to a solid stratification and management, by serving as a blueprint to guide multidisciplinary decisions. The six tiers can serve as a helpful starting point for pathologists faced with challenging cases, allowing them to approach report drafting considering the clinical outcome first, rather than focusing solely on labeling a diagnosis.
- The system is not free from criticism. As expected for any non-dichotomic classification (benign versus malignant), categories that are less determinate (grey zones) can become mired in uncertainty; some of the uncertainty can be resolved by molecular pathology, but this technique is not available at many institutions. In addition, the overlap between some categories complicates a precise ROM calculation and selection of the most appropriate management. The ROM data stem from retrospective studies with a selection bias (i.e., lesions undergoing surgery), and tissue samples are obtained from very diverse populations and frequently from tertiary institutions, with significant variability in the criteria applied by cytopathologists and surgical pathologists, and questionable histological correlation with aspirated nodules [34].
- In this context, the contrast between Asian (namely Korean) and Western settings must be addressed. As reported in the literature, the former population is enriched for the conventional form of papillary thyroid carcinoma (PTC), allowing strict nuclear criteria assessment and targeted molecular BRAF V600E testing in cytology samples without loss of sensitivity. On the other hand, Western practices tend to be less conservative to not underdiagnose so-called RAS-like neoplasms and, in some instances, rely on broader molecular panels or even diagnostic lobectomy/thyroidectomy; this approach leads to surgical series filled with low-grade neoplasms that could be successfully managed through watchful monitoring, as established in Asian settings. These studies also underline the burden on healthcare systems stemming from the evaluation of minute nodules (<1 cm in diameter) without overt clinical or radiological malignant features, which cytopathology teams should strongly advise against in multidisciplinary settings [37-41].
- Future perspectives
- A consistent classification scheme should be revised periodically to address new information. In particular, the ROMs merit an update, especially with regard to stratification for adult and pediatric ages and the advent of entities like NIFTP. Moreover, several authors have noted that the “Follicular Neoplasm” category could be improved by further subdivision to account for the presence of nuclear features of PTC [42].
- Novel molecular data are frequently published and should be incorporated into cytology classification schemes, paving the way for tailored approaches. Special efforts should be devoted to developing inexpensive surrogate markers for actionable oncogenic variants and morphologic techniques with a high positive predictive value to identify high-risk lesions.
- Finally, digital pathology, artificial intelligence, and machine learning are poised to assume a more substantial—synergistic— role in the future. Potential applications may include automating triage tasks in centers with high workloads or limited staff potential, as well as identifying subtle nuclear features and performing overall pattern analysis to aid pathologists in faster and more accurate diagnoses, which may be particularly valuable in cases with artifacts or processing issues.
THYROID
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: SC, DJ. Writing—original draft: RL, BT. Writing—review & editing: SC, DJ. Approval of final manuscript: all authors.
Conflicts of Interest
D.J., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.


- 1. Layfield LJ, Baloch Z, Elsheikh T, et al. Standardized terminology and nomenclature for respiratory cytology: the Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol 2016; 44: 399-409. PubMed
- 2. Hiroshima K, Yoshizawa A, Takenaka A, et al. Cytology reporting system for lung cancer from the Japan Lung Cancer Society and Japanese Society of Clinical Cytology: an interobserver reproducibility study and risk of malignancy evaluation on cytology specimens. Acta Cytol 2020; 64: 452-62. ArticlePubMedPDF
- 3. Alves PM, Ferreira F, Oliveira T, Alves D, Canberk S, Schmitt FC. A new cytology staining method: a fast approach for rapid on-site evaluation on thyroid fine-needle aspiration cytology. Acta Cytol 2023; 67: 289-94. ArticlePubMedPDF
- 4. Ammanagi AS, Dombale VD, Patil SS. On-site toluidine blue staining and screening improves efficiency of fine-needle aspiration cytology reporting. Acta Cytol 2012; 56: 347-51. ArticlePubMedPDF
- 5. International Academy of Cytology - International Agency for Research on Cancer - World Health Organization Joint Editorial Board. IAC-IARC-WHO Cytopathology Reporting Systems. Vol. 1. WHO Reporting System for Lung Cytopathology. Lyon: International Agency for Research on Cancer, 2022.
- 6. Layfield LJ, Baloch Z. The Papanicolaou Society of Cytopathology System for Reporting Respiratory Cytology: definitions, criteria, explanatory notes, and recommendations for ancillary testing. Cham: Springer International Publishing, 2019.
- 7. Guidelines of the Papanicolaou Society of Cytopathology for the examination of cytologic specimens obtained from the respiratory tract. Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol 1999; 21: 61-9. PubMed
- 8. Canberk S, Montezuma D, Aydin O, et al. The new guidelines of Papanicolaou Society of Cytopathology for respiratory specimens: assessment of risk of malignancy and diagnostic yield in different cytological modalities. Diagn Cytopathol 2018; 46: 725-9. ArticlePubMedPDF
- 9. Hewer E, Schmitt AM. Ultrafast toluidine blue staining for rapid on-site evaluation of cytological smears. Acta Cytol 2020; 64: 375-7. ArticlePubMedPDF
- 10. WHO Classification of Tumours Editorial Board. Digestive system tumours. 5th ed. Vol. 1 [Internet]. Lyon: International Agency for Research on Cancer, 2019 [cited 2024 Apr 29]. Available from: https://publications.iarc.fr/579.
- 11. Pitman MB, Centeno BA, Reid MD, et al. The World Health Organization reporting system for pancreaticobiliary cytopathology. Acta Cytol 2023; 67: 304-20. ArticlePubMedPDF
- 12. Pitman MB, Layfield LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: a review. Cancer Cytopathol 2014; 122: 399-411. ArticlePubMed
- 13. Hoda RS, Arpin RN 3rd, Rosenbaum MW, Pitman MB. Risk of malignancy associated with diagnostic categories of the proposed World Health Organization International System for Reporting Pancreaticobiliary Cytopathology. Cancer Cytopathol 2022; 130: 195-201. ArticlePubMedPDF
- 14. Saieg M, Pitman MB. Experience and future perspectives on the use of the Papanicolaou Society of Cytopathology Terminology System for reporting pancreaticobiliary cytology. Diagn Cytopathol 2020; 48: 494-8. ArticlePubMedPDF
- 15. Ikemura K, Yan L, Park JW. Follow-up of indeterminate cytologic diagnoses of solid pancreatic lesions: atypia versus suspicious (one institution’s experience). J Am Soc Cytopathol 2018; 7: 160-5. ArticlePubMed
- 16. Sung S, Del Portillo A, Gonda TA, Kluger MD, Tiscornia-Wasserman PG. Update on risk stratification in the Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology categories: 3-year, prospective, single-institution experience. Cancer Cytopathol 2020; 128: 29-35. ArticlePubMedPDF
- 17. Pitman MB, Centeno BA, Reid MD, et al. A brief review of the WHO reporting system for pancreaticobiliary cytopathology. J Am Soc Cytopathol 2023; 12: 243-50. ArticlePubMed
- 18. Pitman MB, Centeno BA, Genevay M, Fonseca R, Mino-Kenudson M. Grading epithelial atypia in endoscopic ultrasound-guided fineneedle aspiration of intraductal papillary mucinous neoplasms: an international interobserver concordance study. Cancer Cytopathol 2013; 121: 729-36. ArticlePubMed
- 19. Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011; 40: 1024-8. ArticlePubMed
- 20. Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res 2014; 20: 4381-9. ArticlePubMedPDF
- 21. Lee JH, Kim Y, Choi JW, Kim YS. KRAS, GNAS, and RNF43 mutations in intraductal papillary mucinous neoplasm of the pancreas: a meta-analysis. Springerplus 2016; 5: 1172.ArticlePubMedPMCPDF
- 22. Goyal A, Abdul-Karim FW, Yang B, Patel JB, Brainard JA. Interobserver agreement in the cytologic grading of atypia in neoplastic pancreatic mucinous cysts with the 2-tiered approach. Cancer Cytopathol 2016; 124: 909-16. ArticlePubMedPDF
- 23. Laquiere AE, Lagarde A, Napoleon B, et al. Genomic profile concordance between pancreatic cyst fluid and neoplastic tissue. World J Gastroenterol 2019; 25: 5530-42. ArticlePubMedPMC
- 24. Visani M, Acquaviva G, De Leo A, et al. Molecular alterations in pancreatic tumors. World J Gastroenterol 2021; 27: 2710-26. ArticlePubMedPMC
- 25. Goyal A, Sharaiha RZ, Alperstein SA, Siddiqui MT. Cytologic diagnosis of adenocarcinoma on bile duct brushings in the presence of stent associated changes: a retrospective analysis. Diagn Cytopathol 2018; 46: 826-32. ArticlePubMedPDF
- 26. Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018; 67: 2131-41. ArticlePubMed
- 27. Younan G. Pancreas solid tumors. Surg Clin North Am 2020; 100: 565-80. ArticlePubMed
- 28. Singhi AD, Nikiforova MN, Chennat J, et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 2020; 69: 52-61. ArticlePubMed
- 29. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. New York: Springer, 2010.
- 30. Cibas ES, Ali SZ; NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol 2009; 132: 658-65. ArticlePubMed
- 31. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Guidelines of the Papanicolaou Society of Cytopathology for the examination of fine-needle aspiration specimens from thyroid nodules. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Diagn Cytopathol 1998; 15: 84-9. Article
- 32. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. Cham: Springer International Publishing, 2018; 2nd.
- 33. Ali SZ, VanderLaan PA. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. New York: Springer International Publishing, 2023; 3rd.
- 34. Baloch Z, LiVolsi VA. The Bethesda System for Reporting Thyroid Cytology (TBSRTC): from look-backs to look-ahead. Diagn Cytopathol 2020; 48: 862-6. ArticlePubMedPDF
- 35. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda system for reporting thyroid cytopathology. J Am Soc Cytopathol 2023; 12: 319-25. ArticlePubMed
- 36. Juhlin CC, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: novel concepts in nomenclature and grading. Endocr Relat Cancer 2023; 30: e220293.PubMed
- 37. Kakudo K, Higuchi M, Hirokawa M, Satoh S, Jung CK, Bychkov A. Thyroid FNA cytology in Asian practice: active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017; 28: 455-66. ArticlePubMedPDF
- 38. Kim M, Park HJ, Min HS, et al. The use of the Bethesda System for Reporting Thyroid Cytopathology in Korea: a nationwide multicenter survey by the Korean Society of Endocrine Pathologists. J Pathol Transl Med 2017; 51: 410-7. ArticlePubMedPMCPDF
- 39. Vuong HG, Ngo HT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: a systematic review and meta-analysis. Cancer Cytopathol 2020; 128: 238-49. ArticlePubMedPDF
- 40. Ooi LY, Nga ME. Atypia of undetermined significance/follicular lesion of undetermined significance: Asian vs. non-Asian practice, and the Singapore experience. Gland Surg 2020; 9: 1764-87. ArticlePubMedPMC
- 41. Kakudo K, Jung CK, Liu Z, et al. The Asian Thyroid Working Group, from 2017 to 2023. J Pathol Transl Med 2023; 57: 289-304. ArticlePubMedPMCPDF
- 42. Guerreiro SC, Tastekin E, Mourao M, et al. Impact of the 3rd edition of the Bethesda System for Reporting Thyroid Cytopathology on grey zone categories. Acta Cytol 2023; 67: 593-603. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- WHO Reporting System for Lung Cytopathology: Insights Into the Insufficient/Inadequate/Non‐Diagnostic, Atypical and Suspicious for Malignancy Categories and How to Use Them
Zahra Maleki, Sule Canberk, Andrew Field
Cytopathology.2025; 36(5): 434. CrossRef - Reproducibility of the Bethesda system for reporting thyroid cytopathology (TBSRTC): An observational study of 100 patients
Kishori Moni Panda, Reena Naik, Mohd Ghouse Mohiddin
Indian Journal of Pathology and Oncology.2024; 11(4): 385. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



 E-submission
E-submission