Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(6); 2024 > Article
-
Original Article
Fine needle aspiration cytology diagnoses of follicular thyroid carcinoma: results from a multicenter study in Asia -
Hee Young Na1
 , Miyoko Higuchi2
, Miyoko Higuchi2 , Shinya Satoh3
, Shinya Satoh3 , Kaori Kameyama4,5
, Kaori Kameyama4,5 , Chan Kwon Jung6
, Chan Kwon Jung6 , Su-Jin Shin7
, Su-Jin Shin7 , Shipra Agarwal8
, Shipra Agarwal8 , Jen-Fan Hang9
, Jen-Fan Hang9 , Yun Zhu10
, Yun Zhu10 , Zhiyan Liu11
, Zhiyan Liu11 , Andrey Bychkov12
, Andrey Bychkov12 , Kennichi Kakudo13
, Kennichi Kakudo13 , So Yeon Park1
, So Yeon Park1
-
Journal of Pathology and Translational Medicine 2024;58(6):331-340.
DOI: https://doi.org/10.4132/jptm.2024.10.12
Published online: November 7, 2024
1Department of Pathology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
2Department of Diagnostic Pathology and Cytology, Kuma Hospital, Kobe, Japan
3Department of Endocrine Surgery, Yamashita Thyroid Hospital, Fukuoka, Japan
4Department of Pathology, Showa University Northern Yokohama Hospital, Yokohama, Japan
5Department of Pathology, Ito Hospital, Tokyo, Japan
6Department of Hospital Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
7Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
8Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
9Department of Pathology and Laboratory Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
10Department of Pathology, Jiangyuan Hospital Affiliated to Jiangsu Institute of Nuclear Medicine, Wuxi, China
11Department of Pathology, Shanghai Sixth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
12Department of Pathology, Kameda Medical Center, Kamogawa, Japan
13Department of Pathology and Thyroid Disease Center, Izumi City General Hospital, Izumi, Japan
- Corresponding Author: So Yeon Park, MD, PhD, Department of Pathology, Seoul National University Bundang Hospital, 82, Gumi-ro 173 Beon-gil, Bundang-gu, Seongnam 13620, Korea Tel: +82-31-787-7712, Fax: +82-31-787-4012, E-mail: sypmd@snu.ac.kr
© 2024The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,725 Views
- 265 Download
- 1 Crossref
Abstract
-
Background
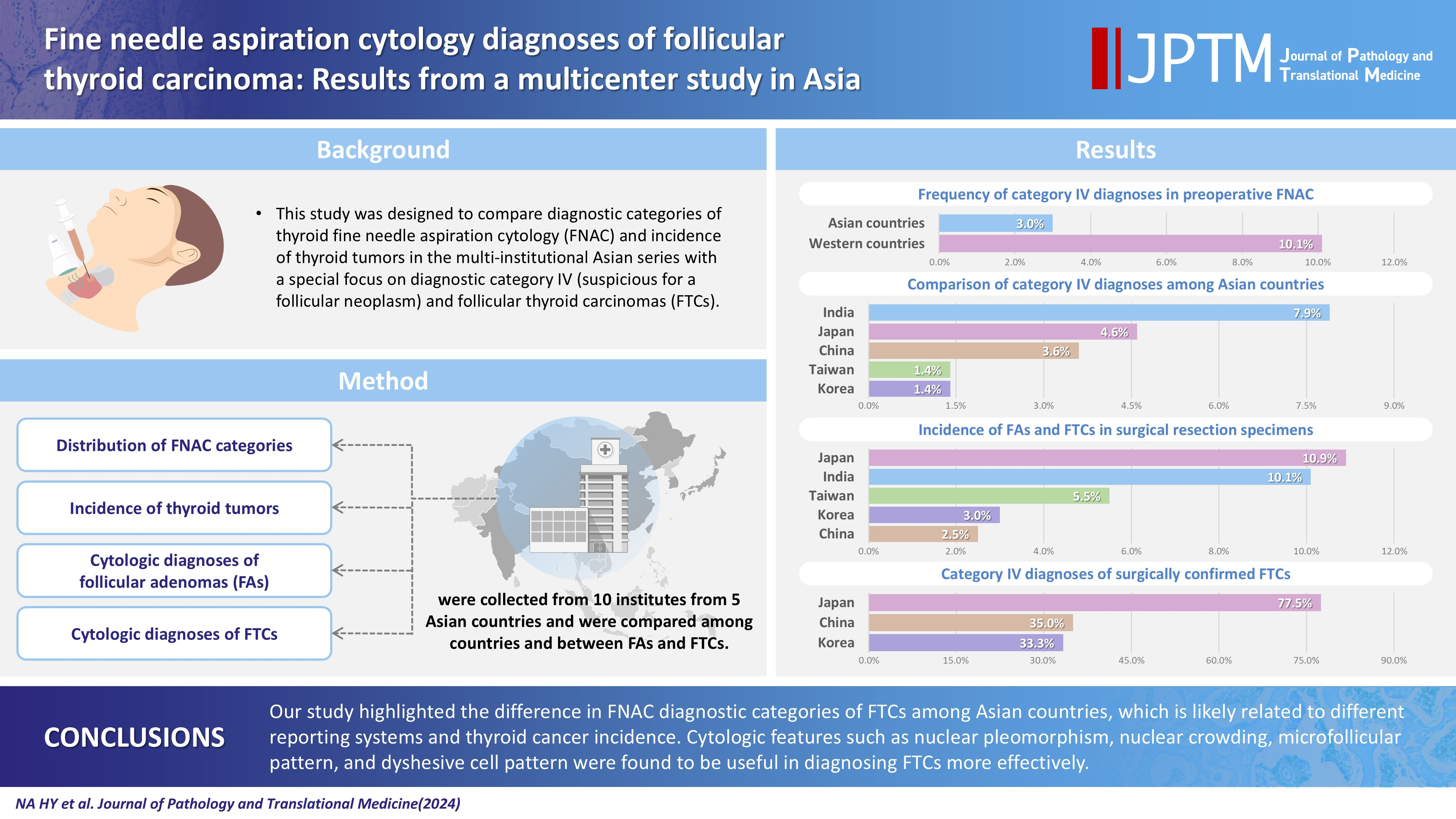
- This study was designed to compare diagnostic categories of thyroid fine needle aspiration cytology (FNAC) and incidence of thyroid tumors in the multi-institutional Asian series with a special focus on diagnostic category IV (suspicious for a follicular neoplasm) and follicular thyroid carcinomas (FTCs).
-
Methods
- Distribution of FNAC categories, incidence of thyroid tumors in resection specimens and cytologic diagnoses of surgically confirmed follicular adenomas (FAs) and FTCs were collected from 10 institutes from five Asian countries and were compared among countries and between FAs and FTCs.
-
Results
- The frequency of category IV diagnoses (3.0%) in preoperative FNAC were significantly lower compared to those in Western countries (10.1%). When comparing diagnostic categories among Asian countries, category IV was more frequent in Japan (4.6%) and India (7.9%) than in Taiwan (1.4%), Korea (1.4%), and China (3.6%). Similarly, incidence of FAs and FTCs in surgical resection specimens was significantly higher in Japan (10.9%) and India (10.1%) than in Taiwan (5.5%), Korea (3.0%), and China (2.5%). FTCs were more commonly diagnosed as category IV in Japan (77.5%) than in Korea (33.3%) and China (35.0%). Nuclear pleomorphism, nuclear crowding, microfollicular pattern, and dyshesive cell pattern were more common in FTCs compared with FAs.
-
Conclusions
- Our study highlighted the difference in FNAC diagnostic categories of FTCs among Asian countries, which is likely related to different reporting systems and thyroid cancer incidence. Cytologic features such as nuclear pleomorphism, nuclear crowding, microfollicular pattern, and dyshesive cell pattern were found to be useful in diagnosing FTCs more effectively.
- Ultrasonography-guided fine needle aspiration cytology (FNAC) is a standard diagnostic tool for evaluating thyroid nodules. However, preoperative diagnosis of follicular adenomas (FAs) and follicular thyroid carcinomas (FTCs) remains challenging because certain cytologic features are commonly shared by other follicular-patterned thyroid lesions, including nodular hyperplasia, follicular variant of papillary thyroid carcinoma (FVPTC), and noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) [1,2]. Moreover, a definitive diagnosis of FA or FTC can only be confirmed after thorough pathologic examination of capsular or vascular invasion in surgically resected specimens.
- Although the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) proposed detailed criteria for diagnostic category IV (suspicious for a follicular neoplasm [SFN]) [3], a significant number of cases showing concordant cytologic findings for category IV (SFN) turn out to be non-neoplastic lesions, and some FAs and FTCs are classified as category II (benign) or III (atypia of undetermined significance [AUS]) in FNAC [4-6]. However, it is important not to underdiagnose FTCs, because minority of FTCs can invade into blood vessels and develop distant metastasis, which significantly affects disease course. It has been reported that underdiagnosis of FTC in FNAC is associated with various clinicopathological factors, including macrofollicular histologic variant [7,8], and low cellularity in FNAC specimen [9].
- The proportion of category IV (SFN) in TBSRTC varies among studies and there may be geographic differences. According to meta-analysis performed by Vuong et al. [10], the proportion of category IV (SFN) among all TBSRTC categories in Western and Asian countries was 7.9% (range, 5.7% to 10.1%) and 3.5% (range, 1.9% to 5.1%), respectively. A nationwide survey in Korea revealed average rate of 0.9% (range, 0% to 2.1%) for category IV (SFN) [11], which is much lower than in Western and other Asian countries. One possible explanation for this discrepancy might be the difference in the prevalence of thyroid cancer subtypes among countries. Interobserver variability, mis- or underdiagnosis, and differences in clinical management among countries [12,13] could be other reasons.
- Although these differences between Asian and Western countries have been well known, variability among Asian countries has been rarely addressed. In the present study, we investigated the distribution of thyroid FNAC diagnostic categories and the incidence of thyroid tumors during a study period in five Asian countries with a special focus on the differences in the proportion of TBSRTC category IV and the incidence of follicular neoplasms among countries. In addition, we analyzed the preoperative FNAC diagnostic categories of surgically confirmed FAs and FTCs and compared their differences between countries. Finally, we compared cytologic features of FAs and FTCs to discover malignancy-associated cytomorphological features that can lead to improved FNAC diagnosis.
- Study design and data collection
- This study was performed within the network of the Asian Thyroid Working Group [12,14,15]. A total of 13 cytopathologists in 10 institutes from five Asian countries participated in this study. To standardize data collection from laboratories with different caseloads, in each institute, study period was determined to include at least 10 surgically resected FTC cases of which the preoperative FNAC slides were available. During the study period, distribution of thyroid FNAC diagnostic categories, incidence of histological types of thyroid tumors in surgical specimens, and preoperative FNAC categories of FAs and FTCs were collected by searching electronic medical records. Clinical features including ultrasonographic findings of FTCs and FAs were also retrieved from the medical records. In Korea, Taiwan, China, and India, thyroid FNACs were categorized according to the 2017 TBSRTC [3]. In Japan, FNAC diagnoses were originally based on the 2013 Japan Thyroid Association (JTA) guidelines [16] but they were converted to TBSRTC during data collection as depicted in Supplementary Fig. S1.
- Cytologic evaluation
- FNAC slides of a total of 110 FTC and 112 FA cases from 10 institutes were retrieved from the archives. First, the original diagnostic categories were recorded from the electronic medical records. Then the slides were reviewed by 10 participating thyroid cytopathologists who were blinded to the final diagnoses (FA or FTC) and clinical data. The slides of each institute were reviewed by the representative cytopathologists of the same institute. The diagnoses were based on the criteria proposed by the 2017 TBSRTC [3]. The diagnostic criteria for TBSRTC category IV (SFN) are summarized as follows: moderate to marked cellularity, significant alteration in architecture characterized by cell crowding, microfollicles and dispersed isolated cells, normal or enlarged cells, and scant or absent colloid [3]. After reviewing the slides, the diagnostic category was revised where needed, and the revised categories were recorded. In addition, various cytologic features were scored in each case, including predominant microfollicular pattern (>50%), presence of microfollicles in trabecular arrangement, predominant macrofollicular pattern (> 50%), solid or trabecular pattern, extensive dyshesive cells, nuclear crowding, nuclear enlargement, nuclear pleomorphism, nuclear hyperchromatism, coarse chromatin pattern, prominent nucleoli, the quantity and quality of colloid, and cystic change. For nuclear enlargement, increased size of nuclei compared to the normal follicular cells (7–9 μm) was considered positive [17].
- Surgical diagnosis of thyroid tumors
- Total number of thyroidectomy specimens and the number of resected thyroid tumors were collected for comparison of thyroid tumor incidence among the countries. Thyroid tumors recorded were FA, Hürthle cell (oncocytic) adenoma (HCA), NIFTP, papillary thyroid carcinoma (PTC), minimally invasive FTC (MIFTC), encapsulated angioinvasive FTC (AIFTC), widely invasive FTC (WIFTC), Hürthle cell carcinoma (HCC), and other rare tumors. Surgical diagnosis of thyroid tumors was based on the 2017 World Health Organization (WHO) classification [18]. In particular, FTC with only capsular invasion was diagnosed as MIFTC, whereas encapsulated FTC harboring vascular invasion was diagnosed as AIFTC [18]. WIFTC was diagnosed when extensive invasion to the adjacent thyroid parenchyma, extrathyroidal soft tissues, and vascular invasion were evident [18].
- Survey regarding the diagnosis of follicular neoplasms
- We also conducted a survey regarding the diagnostic practice of the participants. A total of nine pathologists from five Asian countries (3 from Japan, 3 from Korea, and 1 from Taiwan, China, and India, respectively) took part in the survey. A total of four questions were asked and participants were allowed to select multiple answers.
- Statistical analysis
- Distribution of FNAC diagnostic categories, incidence of thyroid tumors, and preoperative FNAC categories or clinico-cytologic features of FAs and FTCs were compared using Pearson chi-square test or Fisher’s exact test. Corrections for multiple testing were performed with Bonferroni method and adjusted p-values are presented. All p-values were two-sided, and a p-value of less than .05 was considered statistically significant. Data analyses were conducted using SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA).
MATERIALS AND METHODS
- Distribution of FNAC diagnostic categories
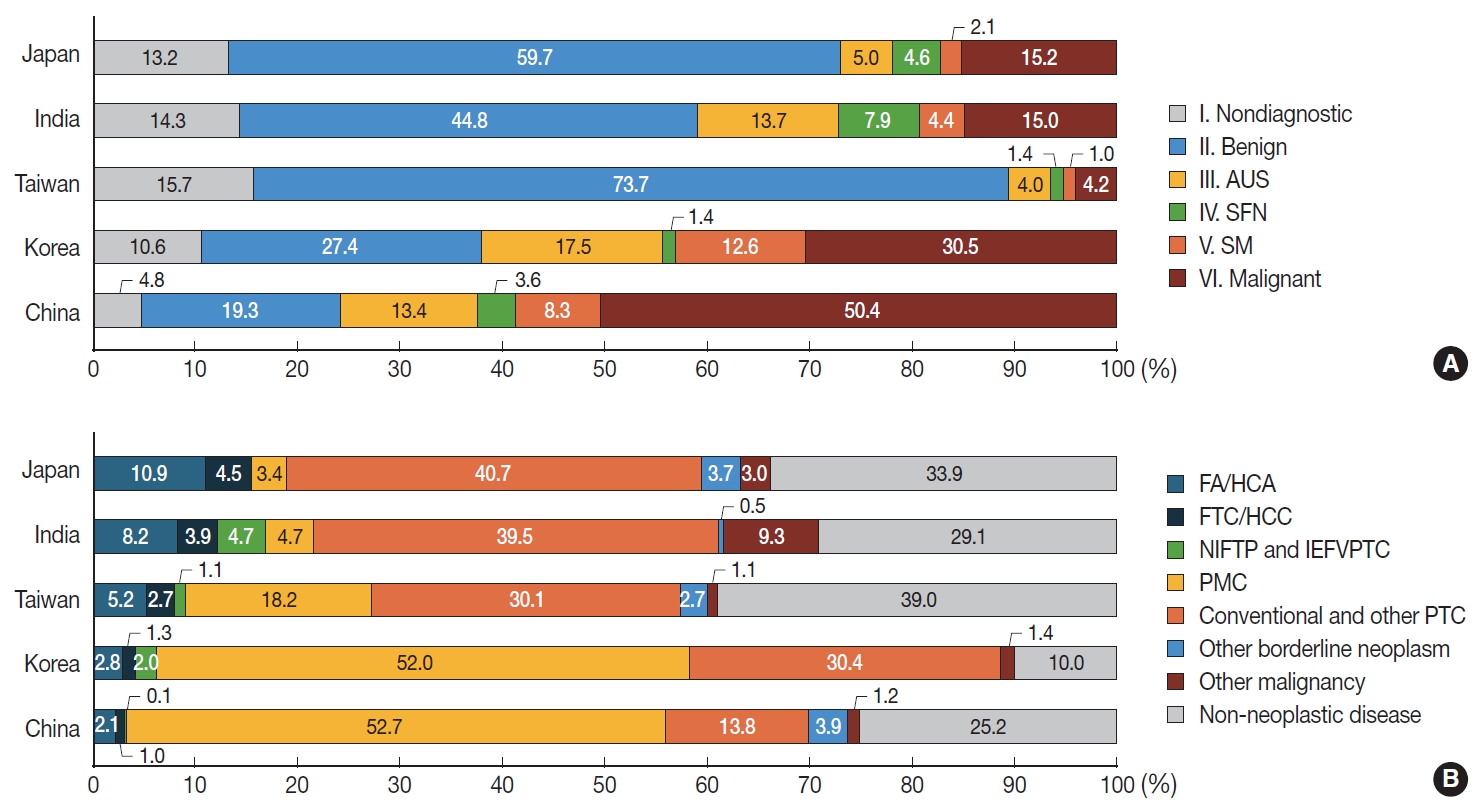
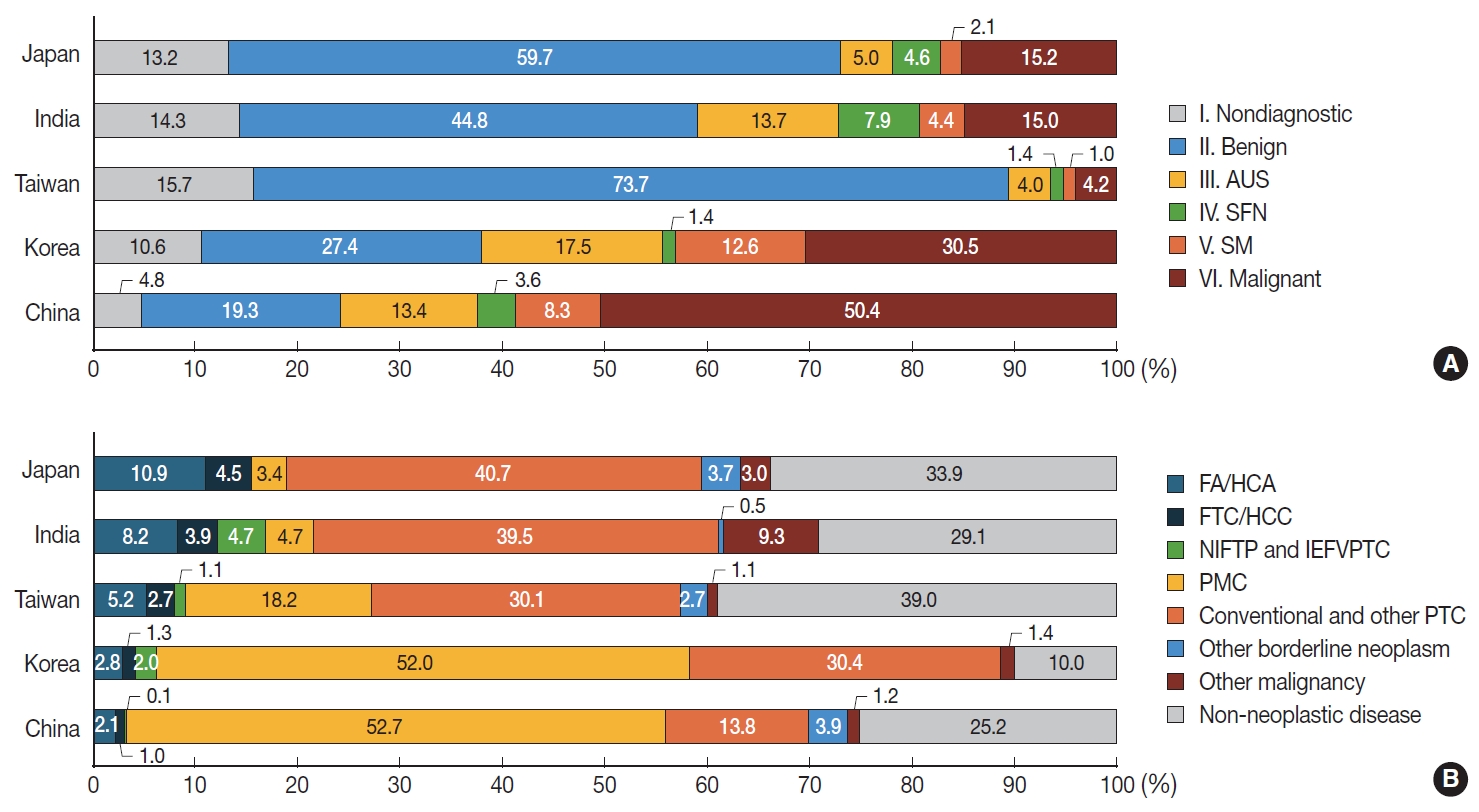
- The study period defined by at least 10 consecutive FTC cases ranged from 3 to 35 months (median, 20.0 months). A total of 38,771 FNAC samples from 10 institutes were included in this study (Supplementary Table S1). Among them, 3,931 (10.1%), 14,533 (37.5%), 4,680 (12.1%), 1,163 (3.0%), 2,921 (7.5%), and 11,543 (29.8%) cases were classified as diagnostic category I (non-diagnostic), II (benign), III (AUS), IV (SFN), V (suspicious for malignancy [SM]), and VI (malignant), respectively (Supplementary Table S2). Compared to data reported in two meta-analyses [8,10], our series showed higher incidence of category III (AUS), V (SM), and VI (malignant), and lower incidence of category I (non-diagnostic), II (benign), and IV (SFN). Countrywise, the frequency of category IV (SFN) was higher in Japan and India than in other countries. The frequency of category III (AUS), V (SM), and VI (malignant) was higher in Korea and China (Fig. 1A, Supplementary Table S2).
- Distribution of surgical diagnoses of thyroid lesions
- During the study period, a total of 18,068 thyroid lesions were surgically resected (Supplementary Table S3). Of them, 2,994 (16.6%) were diagnosed as non-neoplastic thyroid lesions and 15,074 (83.4%) were diagnosed as thyroid tumors. Specific histologic diagnoses of thyroid tumors were as follows: FAs (2.8% of all thyroid lesions), HCAs (0.8%), NIFTPs (0.4%), PTCs (75.5%), FTCs (1.1%), and HCCs (0.3%) (Supplementary Table S3). In PTC, papillary microcarcinoma, which is 1 cm or less in size, constituted 45.9 % (8,292/18,068), while tumors larger than 1 cm accounted for 29.6% (5,355/18,068) (Supplementary Table S3). Overall, benign and borderline thyroid tumors comprised 4.8% (867/18,068). FA and FTC accounted for 4.0% (715/18,068), while Hürthle cell neoplasms (HCA and HCC) constituted 1.1% (206/18,068).
- When comparing a total of eight institutes which further provided PTC subtypes, surgical diagnoses of follicular-patterned neoplasm including FA, HCA, FTC, HCC, NIFTP, and invasive encapsulated FVPTC, were significantly more frequent in Japan and India than in other Asian countries (Fig. 1B). In Korea and China, PTCs, including all subtypes, were more prevalent than in other countries. Of note, the incidence of microcarcinoma in these two countries was significantly higher than that in other countries (Fig. 1B).
- Preoperative FNAC diagnoses of FAs and FTCs
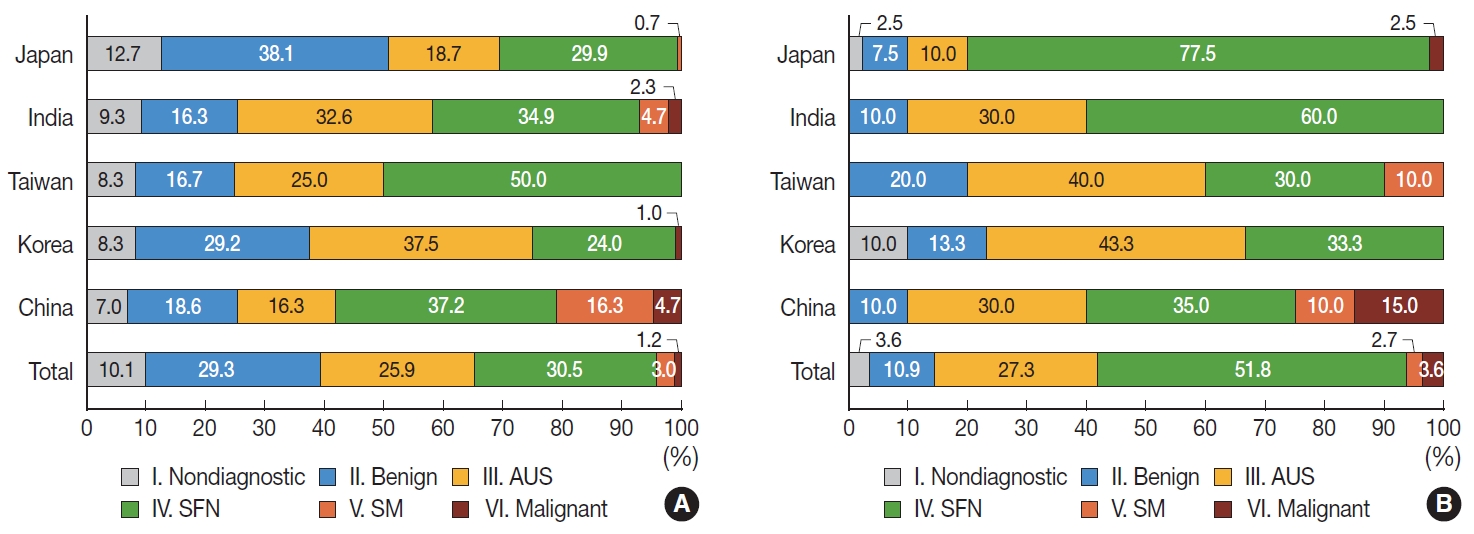
- Surgically resected FAs were preoperatively classified as diagnostic category I (non-diagnostic) (10.1%), II (benign) (29.3%), III (AUS) (25.9%), IV (SFN) (30.5%), V (SM) (3.0%), and VI (malignant) (1.2%), respectively, in thyroid FNAC (Supplementary Table S4). FAs were more frequently diagnosed as category III (AUS) in Korea than in Japan (Fig. 2A, Supplementary Table S4).
- Preoperative FNAC diagnoses of FTCs were diagnostic category I (non-diagnostic) (3.6%), II (benign) (10.9%), III (AUS) (27.3%), IV (SFN) (51.8%), V (SM) (2.7%), and VI (malignant) (3.6%), respectively (Supplementary Table S5). FTCs were more frequently diagnosed as category IV (SFN) in Japan than in Korea and China. On the other hand, they were more frequently diagnosed as category III (AUS) in Korea than in Japan (Fig. 2B, Supplementary Table S5).
- Comparison of FNAC diagnostic categories between FAs and FTCs
- Compared with FTCs, FAs were more frequently classified as category II (benign) both in original and revised diagnoses (p= .002 and p=.003, respectively) (Table 1) FTCs were more frequently classified as category IV (SFN) compared to FAs in original diagnoses (p=.032). However, the difference was not statistically significant upon review of the diagnoses (p=.107) (Table 1). Among subtypes of FTCs, AIFTCs, and WIFTCs were more frequently diagnosed as diagnostic category IV (SFN) than MIFTCs in revised diagnoses (p=.023) (Table 1).
- Comparison of clinico-cytologic features between FAs and FTCs
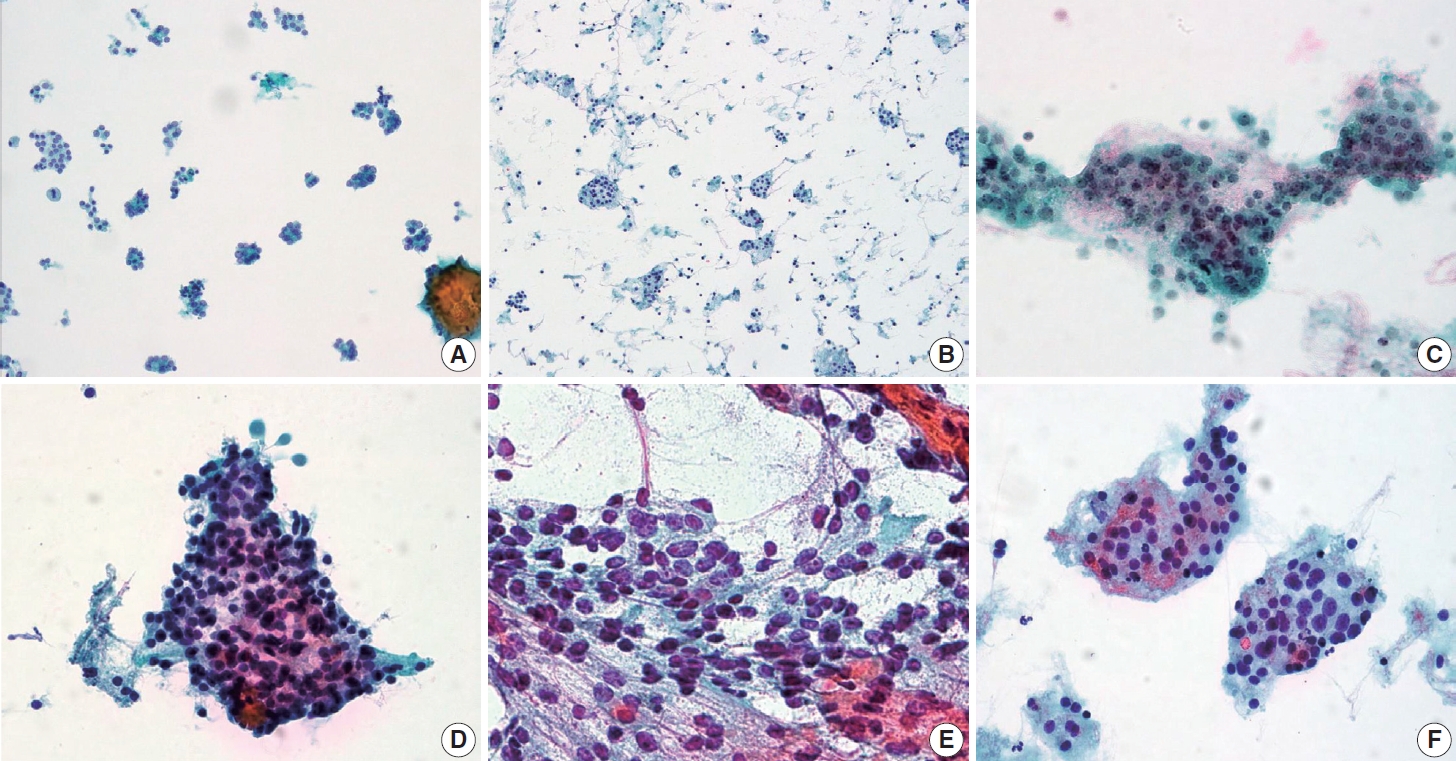
- After excluding cases with revised diagnostic category I (non-diagnostic), clinico-cytologic features were reviewed and compared between FAs and FTCs (Table 2). Compared with FAs, FTCs more frequently showed intermediate to high suspicious ultrasonography findings (solid hypoechoic nodule, partially cystic isoechoic nodule with any of the following features: microcalcification, nonparallel orientation, spiculated/microlobulated margin [19]) (p=.001) (Table 2). In FTCs, predominant microfollicular pattern, extensive dyshesive cells, marked nuclear crowding, and nuclear pleomorphism were more commonly observed than in FAs (all p<.05) (Table 2, Fig. 3). Among FTC subtypes, AIFTCs (mean, 3.8 cm) and WIFTCs (mean, 4.0 cm) presented with larger tumor size than MIFTCs (mean, 3.3 cm) (p= .022). In addition, AIFTCs and WIFTCs more frequently showed nuclear enlargement and hyperchromatism than MIFTCs (all p< .05) (Table 2, Fig. 3).
- Survey regarding diagnosis of follicular neoplasms in FNAC
- The results of the survey are summarized in Supplementary Table S6. Among various cytologic features, participants of our study regarded predominantly microfollicular architecture as the most important cytologic feature when diagnosing follicular neoplasms, followed by cellular crowding and moderately to markedly increased cellularity. The most common reason for underdiagnosing follicular neoplasms was low cellularity. Mixed micro- and macrofollicular pattern, and abundant thin colloid along with cystic change were other reasons. Nuclear enlargement, nuclear groove, and intranuclear pseudoinclusion were common reasons of misdiagnosing follicular neoplasms as PTCs. As for management for thyroid nodules diagnosed as diagnostic category IV (SFN), diagnostic lobectomy was performed after considering other clinical features in six institutes, and without any special consideration for clinical features in three Korean institutes.
RESULTS
- In the present study, we analyzed the distribution of thyroid FNAC diagnostic categories and the incidence of thyroid tumors in surgical resection specimens among five Asian countries with a special focus on diagnostic category IV (SFN) and FTCs. In addition, we compared the preoperative diagnostic categories between surgically confirmed FAs and FTCs and attempted to find out cytologic parameters associated with FTCs, especially those harboring vascular invasion or widely invasive pattern, which can lead to improved diagnostic accuracy.
- In total, the proportion of diagnostic category II (benign) and IV (SFN) was lower in our study than in the largest meta-analysis by Vuong which included both Asian and Western data [10]. In contrast, category V (SM) and VI (malignant) were more frequently diagnosed in our cohort. The low frequency of category II (benign) and high frequency of category V (SM) and VI (malignant) might arise from a selection bias, because all 10 institutes involved in the current study were tertiary hospitals. Moreover, high frequency of category V (SM) and VI (malignant), and low frequency of category IV (SFN) seems to be related to distinct pattern of thyroid cancer incidence in Asian countries. It was reported that age-standardized incidence of PTC and FTC in women were 143.3 and 2.1/100,000 person-years in Korea between 2008 and 2012 with PTC to FTC incidence rate ratio of 68.2, while their incidence were 25.6 and 1.87/100,000 person-years in the United States with the incidence rate ratio of 13.7 [20]. This is also evident in our study. PTC and FTC constituted 96.1% and 1.4% of thyroid cancers in our cohort, while they comprised 86.4% and 10.2% of all thyroid cancers diagnosed from 1975 to 2016, based on Surveillance, Epidemiology, and End Results data from the United States [21].
- Another notable finding based from the analysis of the pooled surgical pathology cohort collected for our study was a low incidence of benign and borderline thyroid tumors (5.8% of all thyroid tumors) in our Asian cohort, represented by FA with HCA (collectively 3.6%), NIFTP, and other borderline neoplasms. A very low incidence of NIFTP (0.4% of all thyroid resections) is consistent with the previous studies which addressed NIFTP rate in Asia [22-24]. Interestingly, the diagnosis of tumors of uncertain malignant potential was not extremely uncommon, which reflects a difficulty in diagnosing encapsulated follicular neoplasms [25]. Another finding was that Hürthle cell neoplasms were very rare, accounting for 1.4% of all thyroid tumors in our cohort. Nevertheless, Hürthle cell neoplasms were not uncommon among FA and FTC, with the ratio FA:HCA = 3.3:1 and FTC:HCC = 3.9:1. Considering that the reported incidence of HCCs is around 3%–4% of all thyroid carcinomas [26,27], the incidence of Hürthle cell neoplasms appears to be lower in Asian countries [28].
- We collected the data from individual institutes of each country, and thus, it may not be representative of the country. Nevertheless, there were some differences in the distribution of thyroid FNAC diagnostic categories among Asian countries: category IV (SFN) was less frequent in Korea and China compared to Japan, and category V (SM) and VI (malignant) were more frequent in the former two countries. This might be associated with the distribution of histologic subtypes of thyroid cancer: PTC constituted more than 80% of all thyroid tumors in Korea and China, while it accounted for around 50% of all tumors in Japan and India. These findings are similar with a recent population-based study, which reported significantly higher PTC incidence compared to that of FTC especially in Korea and China than other Asian countries [20]. Another possible explanation is the presence of interobserver variability in interpreting nuclear features of PTC. Although some researchers demonstrated moderate agreement in diagnosing nuclear features by implementing 3-point nuclear score [29], others still reported contradictory results [30]. Increased national thyroid screening program using high-resolution ultrasonography, which effectively detects smaller nodules [31,32], can be another explanation, considering papillary microcarcinoma accounted for 51.7% and 56.1% of all thyroid tumors in Korea and China, respectively. This might be also attributed to the varying clinical practices across Asian countries. While active surveillance is a common approach for managing papillary microcarcinomas in Japan, both China and Korea seem to favor surgical treatment for these cases. Lastly, as suggested in the survey we performed, diagnostic lobectomy without any special consideration for clinical features was generally favored in Korea for thyroid nodules diagnosed as category IV (SFN), which can lead to underdiagnosis in thyroid FNAC and thus could explain the lower incidence of category IV (SFN) in Korea.
- Preoperative FNAC diagnostic categories of FTC also varied among countries. FTCs were more frequently categorized as category IV (SFN) in Japan compared to Korea and China, suggesting a relative underdiagnosis in the latter two countries. This might be caused by different thyroid FNAC diagnosing guidelines used among the countries. In Japan, thyroid FNAC was originally diagnosed according to the 2013 JTA guidelines [16] and then converted to TBSRTC during the study. The presence of three subcategories in Indeterminate A (follicular neoplasm) in 2013 JTA guidelines is the most striking difference compared with TBSRTC [16]. Cases with absent or scant colloid, microfollicular growth, enlarged nuclei, three-dimensional clusters, and high cellularity, are recommended to be classified as Indeterminate A (follicular neoplasm) [16], which therefore ideally corresponds to TBSRTC diagnostic category IV (SFN) [3]. However, Indeterminate A (follicular neoplasm) is then subdivided into A-1 (favor benign), A-2 (borderline), and A-3 (favor malignant), based on the degree of nuclear and structural atypia [16]. Although Indeterminate A (follicular neoplasm) virtually corresponds to TBSRTC diagnostic category IV (SFN), previous studies conducted by Japanese cytopathologists demonstrated that the risk of malignancy of A-1 (favor benign), A-2 (borderline), and A-3 (favor malignant) is 5%–15%, 15%–30%, and 40%–60%, respectively [33-35]. Therefore, A-1 might contain some but not all cases that can be classified as TBSRTC diagnostic category III (especially AUS with architectural atypia). In addition, interobserver variability and different sample quality, including cellularity, in each institute might have contributed to our findings.
- Overall, FAs were more frequently classified as diagnostic category II (benign) than FTCs, and AIFTCs and WIFTCs were more commonly diagnosed as diagnostic category IV (SFN) than MIFTCs. When comparing various cytomorphologic features, FTCs more commonly presented with dominant microfollicular pattern, dyshesive cells in the background, nuclear crowding, and pleomorphism than FAs. Our results are in line with previous studies, which demonstrated increased nuclear size, prominent nucleoli, and nuclear crowding are more frequently observed in FTCs than in FAs [9,36-39]. More recent studies using computational pathology also confirmed that not only traditional cytomorphological features including cellularity, nuclear size, pleomorphism, quality of colloid, but also chromatin textural features can be helpful in distinguishing FTCs from FAs [40,41]. Although absence of thin colloid was generally suggestive of malignancy over benign lesion [17], there was no difference between FAs and FTCs in our study. Han et al. reported a distinct architectural pattern called “microfollicles in trabecular arrangement” and demonstrated that this pattern was more frequently observed in neoplastic lesion than in non-neoplastic condition [42]. Although this pattern was more common in FTCs, the differences were not statistically significant in the present study. Further validation in large-scale cohort is required.
- We further categorized FTCs into three histologic subtypes (MIFTC, AIFTC, and WIFTC) in order to find out which features are associated with more aggressive histologic subtypes. Of note, AIFTCs and WIFTCs presented with larger tumor size than MIFTCs. Nuclear enlargement and hyperchromatism were also more common in AIFTCs and WIFTCs. Additionally, WIFTCs more frequently showed nuclear hyperchromatism than MIFTCs, suggesting certain morphologic features are associated with more aggressive histologic subtypes. These results are in line with the concept proposed by Cervino et al. that certain cytologic features such as nuclear size and the amount of colloid gradually change from benign nodular hyperplasia to follicular neoplasm [43].
- Our study has some limitations. First, this was a retrospective study. Second, data were collected from 10 individual institutes, mostly tertiary hospitals, leading to a selection bias. Lastly, the slide review was performed by 10 cytopathologists, which might have caused interobserver variability. However, this is so far the first study to evaluate the differences in FNAC category distribution and preoperative FNAC category of follicular neoplasm among Asian countries.
- Countries that have higher incidence of PTC generally showed higher frequency of FNAC diagnostic category V (SM) and VI (malignant), than those with lower incidence of PTC, suggesting the incidence of thyroid tumors in an institute is related to the distribution of FNAC diagnostic category. FTCs were more often diagnosed as diagnostic category IV (SFN) in Japan and India than in other countries, which is probably associated with different FNAC classification system. Finally, certain nuclear features were more frequently observed in malignancy. And some were more commonly noted in AIFTCs or WIFTCs than in MIFTCs, suggesting that identifying these cytologic features is important to keep those cases under clinical follow-up, which is a traditional clinical approach in Asia to identify higher-risk tumors for surgery.
- In conclusion, our study demonstrated the difference in preoperative FNAC diagnostic categories of FAs and FTCs in five Asian countries, which might be related to different reporting systems and thyroid cancer incidence in each country. Recognizing certain cytologic features associated with FTC can improve diagnostic accuracy in preoperative FNAC.
DISCUSSION
Supplementary Information
Ethics Statement
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2012-652-309), and the requirement for informed consent was waived.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: KK (Kennichi Kakudo), SYP. Data curation: HYN. Formal analysis: HYN. Investigation: HYN, MH, SS, KK (Kaori Kameyama), CKJ, SJS, SA, JFH, YZ, ZL. Methodology: AB, HYN. Project administration: HYN, AB. Resources: MH, SS, KK (Kaori Kameyama), CKJ, SJS, SA, JFH, YZ, ZL. Supervision: AB, KK (Kennichi Kakudo), SYP. Visualization: HYN. Writing—original draft: HYN. Writing—review & editing: AB, KK (Kennichi Kakudo), SYP. Approval of final manuscript: all authors.
Conflicts of Interest
C.K.J., Y.Z., Z.L., A.B., K.K. and S.Y.P., contributing editors of the Journal of Pathology and Translational Medicine, were not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.


| FNAC diagnostic category | FA (n = 112) |
FTC |
p-valuea | p-valueb | |||
|---|---|---|---|---|---|---|---|
| FTC all (n = 110) | MIFTC (n = 67) | AIFTC (n = 20) | WIFTC (n = 23) | ||||
| Original diagnoses | |||||||
| I | 3 (2.7) | 4 (3.6) | 3 (4.5) | 1 (5.0) | 0 | .720 | > .999 |
| II | 31 (27.7) | 12 (10.9) | 8 (11.9) | 3 (15.0) | 1 (4.3) | .002c | .762 |
| III | 31 (27.7) | 30 (27.3) | 21 (31.3) | 5 (25.0) | 4 (17.4) | .946 | .231 |
| IV | 42 (37.5) | 57 (51.8) | 30 (44.8) | 10 (50.0) | 17 (73.9) | .032c | .065 |
| V | 5 (4.5) | 3 (2.7) | 2 (3.0) | 1 (5.0) | 0 | .722 | > .999 |
| VI | 0 | 4 (3.6) | 3 (4.5) | 0 | 1 (4.3) | .059 | .647 |
| Revised diagnoses | |||||||
| I | 5 (4.5) | 5 (4.5) | 4 (6.0) | 1 (5.0) | 0 | .977 | .647 |
| II | 30 (26.8) | 12 (10.9) | 8 (11.9) | 0 | 4 (17.4) | .003c | .762 |
| III | 27 (24.1) | 25 (22.7) | 18 (26.9) | 4 (20.0) | 3 (13.0) | .685 | .196 |
| IV | 50 (44.6) | 62 (56.4) | 32 (47.8) | 14 (70.0) | 16 (69.6) | .107 | .023c |
| V | 0 | 4 (3.6) | 3 (4.5) | 1 (5.0) | 0 | .059 | > .999 |
| VI | 0 | 2 (1.8) | 2 (3.0) | 0 | 0 | .244 | .519 |
Values are presented as number (%).
FNAC, fine needle aspiration cytology; FA, follicular adenoma; FTC, follicular thyroid carcinoma; MIFTC, minimally invasive follicular thyroid carcinoma; AIFTC, encapsulated angioinvasive follicular thyroid carcinoma; WIFTC, widely invasive follicular thyroid carcinoma.
aFA versus FTC;
bMIFTC versus AIFTC and WIFTC;
cStatistically significant difference; for comparison between surgical diagnosis, chi-square test was used.
| Clinical and cytologic parameters | FA (n = 107) |
FTC |
p-valuea | p-valueb | |||
|---|---|---|---|---|---|---|---|
| FTC all (n = 105) | MIFTC (n = 63) | AIFTC (n = 19) | WIFTC (n = 23) | ||||
| USG | .001c | .644 | |||||
| Low suspicious | 37 (34.6) | 17 (16.2) | 11 (17.5) | 3 (15.8) | 3 (13.0) | ||
| Intermediate to high suspicious | 49 (45.8) | 70 (66.7) | 41 (65.1) | 11 (57.9) | 18 (78.3) | ||
| NA | 21 (19.6) | 18 (17.1) | 11 (17.5) | 5 (26.3) | 2 (8.7) | ||
| Tumor size (cm) | 3.2 (0.6–9.0) | 3.5 (0.7–8.5) | 3.3 (0.7–8.5) | 3.8 (0.8–6.5) | 4.0 (1.4–8.2) | .128 | .022c |
| Cellularity ≥ 60 cells | 97 (90.7) | 95 (90.5) | 56 (88.9) | 19 (100) | 20 (87.0) | .965 | .736 |
| Pattern/arrangement | |||||||
| Microfollicles ≥ 50% | 49 (45.8) | 65 (61.9) | 37 (58.7) | 13 (68.4) | 15 (65.2) | .019c | .412 |
| Microfollicles in trabecular arrangement | 37 (34.6) | 46 (43.8) | 27 (42.9) | 8 (42.1) | 11 (47.8) | .169 | .810 |
| Solid or trabecular pattern | 57 (53.3) | 54 (51.4) | 32 (50.8) | 10 (52.6) | 12 (52.2) | .788 | .873 |
| Dyshesive cells, extensive | 9 (8.4) | 22 (21.0) | 11 (17.5) | 6 (31.6) | 5 (21.7) | .010c | .282 |
| Macrofollicles ≥ 50% | 13 (12.1) | 8 (7.6) | 5 (7.9) | 0 | 3 (13.0) | .270 | >.999 |
| Nuclear features | |||||||
| Nuclear crowding, marked | 14 (13.1) | 29 (27.6) | 17 (27.0) | 6 (31.6) | 6 (26.1) | .009c | .859 |
| Nuclear enlargement | 74 (69.2) | 80 (76.2) | 43 (68.3) | 17 (89.5) | 20 (87) | .251 | .017c |
| Nuclear pleomorphism | 12 (11.2) | 23 (21.9) | 15 (23.8) | 5 (26.3) | 3 (13) | .036c | .563 |
| Nuclear hyperchromatism | 35 (32.7) | 44 (41.9) | 19 (30.2) | 12 (63.2) | 13 (56.5) | .166 | .003c |
| Coarse chromatin | 23 (21.5) | 29 (27.6) | 17 (27.0) | 9 (47.4) | 3 (13.0) | .300 | .859 |
| Prominent nucleoli | 2 (1.9) | 8 (7.6) | 5 (7.9) | 3 (15.8) | 0 | .059 | > .999 |
| Colloid quantity | .572 | .503 | |||||
| Absent | 61 (57.0) | 68 (64.8) | 41 (65.1) | 9 (47.4) | 18 (78.3) | ||
| Thin | 34 (31.8) | 28 (26.7) | 16 (25.4) | 9 (47.4) | 3 (13.0) | ||
| Thick | 9 (8.4) | 8 (7.6) | 6 (9.5) | 1 (5.3) | 1 (4.3) | ||
| Thin and thick | 3 (2.8) | 1 (1.0) | 0 | 0 | 1 (4.3) | ||
| Colloid quantity | .556 | > .999 | |||||
| Absent to focal | 93 (86.9) | 94 (89.5) | 56 (88.9) | 16 (84.2) | 22 (95.7) | ||
| Extensive | 14 (13.1) | 11 (10.5) | 7 (11.1) | 3 (15.8) | 1 (4.3) | ||
| Cystic change | 6 (5.6) | 8 (7.6) | 7 (11.1) | 1 (5.3) | 0 | .555 | .140 |
Values are presented as number (%) or mean (range).
FA, follicular adenoma; FTC, follicular thyroid carcinoma; MIFTC, minimally invasive follicular thyroid carcinoma; AIFTC, encapsulated angioinvasive follicular thyroid carcinoma; WIFTC, widely invasive follicular thyroid carcinoma; USG, ultrasonography; NA, not available.
aFA vs. FTC;
bMIFTC vs. AIFTC and WIFTC;
cStatistically significant difference; for comparison between surgical diagnosis, chi-square test was used.
- 1. Baloch ZW, Livolsi VA. Follicular-patterned lesions of the thyroid: the bane of the pathologist. Am J Clin Pathol 2002; 117: 143-50. ArticlePubMed
- 2. Zacks JF, de las Morenas A, Beazley RM, O’Brien MJ. Fine-needle aspiration cytology diagnosis of colloid nodule versus follicular variant of papillary carcinoma of the thyroid. Diagn Cytopathol 1998; 18: 87-90. ArticlePubMed
- 3. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017; 27: 1341-6. ArticlePubMed
- 4. Deveci MS, Deveci G, LiVolsi VA, Baloch ZW. Fine-needle aspiration of follicular lesions of the thyroid: diagnosis and follow-up. Cytojournal 2006; 3: 9.PubMedPMC
- 5. Wu HH, Rose C, Elsheikh TM. The Bethesda system for reporting thyroid cytopathology: an experience of 1,382 cases in a community practice setting with the implication for risk of neoplasm and risk of malignancy. Diagn Cytopathol 2012; 40: 399-403. ArticlePubMed
- 6. Yang J, Schnadig V, Logrono R, Wasserman PG. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer 2007; 111: 306-15. ArticlePubMed
- 7. Bommanahalli B, Bhat R, Rupanarayan R. A cell pattern approach to interpretation of fine needle aspiration cytology of thyroid lesions: a cyto-histomorphological study. J Cytol 2010; 27: 127-32. ArticlePubMedPMC
- 8. Bongiovanni M, Sykiotis GP, La Rosa S, et al. Macrofollicular variant of follicular thyroid carcinoma: a rare underappreciated pitfall in the diagnosis of thyroid carcinoma. Thyroid 2020; 30: 72-80. ArticlePubMed
- 9. Na HY, Moon JH, Choi JY, et al. Preoperative diagnostic categories of fine needle aspiration cytology for histologically proven thyroid follicular adenoma and carcinoma, and Hurthle cell adenoma and carcinoma: analysis of cause of under- or misdiagnoses. PLoS One 2020; 15: e0241597. ArticlePubMedPMC
- 10. Vuong HG, Ngo HT, Bychkov A, et al. Differences in surgical resection rate and risk of malignancy in thyroid cytopathology practice between Western and Asian countries: a systematic review and meta-analysis. Cancer Cytopathol 2019; 128: 238-49. ArticlePubMedPDF
- 11. Kim M, Park HJ, Min HS, et al. The use of the Bethesda System for Reporting Thyroid Cytopathology in Korea: a nationwide multicenter survey by the Korean Society of Endocrine Pathologists. J Pathol Transl Med 2017; 51: 410-7. ArticlePubMedPMCPDF
- 12. Bychkov A, Kakudo K, Hong S. Current practices of thyroid fine-needle aspiration in Asia: a missing voice. J Pathol Transl Med 2017; 51: 517-20. ArticlePubMedPMCPDF
- 13. Kakudo K, Higuchi M, Hirokawa M, Satoh S, Jung CK, Bychkov A. Thyroid FNA cytology in Asian practice-Active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017; 28: 455-66. ArticlePubMedPDF
- 14. Kakudo K, Jung CK, Liu Z, et al. The Asian Thyroid Working Group, from 2017 to 2023. J Pathol Transl Med 2023; 57: 289-304. ArticlePubMedPMCPDF
- 15. Jung CK, Hong S, Bychkov A, Kakudo K. The use of fine-needle aspiration (FNA) cytology in patients with thyroid nodules in Asia: a brief overview of studies from the Working Group of Asian Thyroid FNA Cytology. J Pathol Transl Med 2017; 51: 571-8. ArticlePubMedPMCPDF
- 16. Japan Thyroid Association. Guidelines for clinical practice for the management of thyroid nodules in Japan 2013. Tokyo: Nankodo Co., Ltd, 2013.
- 17. Kini SR. Thyroid cytopathology: an atlas and text. Philadelphia: Lippincott Williams and Wilkins, 2008.
- 18. Lloyd R, Osamura R, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. Lyon: IARC Press, 2017; 4th.
- 19. Ha EJ, Na DG, Baek JH. Korean Thyroid Imaging Reporting and Data System: current status, challenges, and future perspectives. Korean J Radiol 2021; 22: 1569-78. ArticlePubMedPMCPDF
- 20. Miranda-Filho A, Lortet-Tieulent J, Bray F, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol 2021; 9: 225-34. ArticlePubMed
- 21. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid cancer incidence trends in the United States: association with changes in professional guideline recommendations. Thyroid 2020; 30: 1132-40. ArticlePubMed
- 22. Rana C, Vuong HG, Nguyen TQ, et al. The incidence of noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a meta-analysis assessing worldwide impact of the reclassification. Thyroid 2021; 31: 1502-13. ArticlePubMed
- 23. Bychkov A, Jung CK, Liu Z, Kakudo K. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice: perspectives for surgical pathology and cytopathology. Endocr Pathol 2018; 29: 276-88. ArticlePubMedPMCPDF
- 24. Bychkov A, Hirokawa M, Jung CK, et al. Low rate of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian Practice. Thyroid 2017; 27: 983-4. ArticlePubMed
- 25. Zhu Y, Li Y, Jung CK, et al. Histopathologic assessment of capsular invasion in follicular thyroid neoplasms: an observer variation study. Endocr Pathol 2020; 31: 132-40. ArticlePubMedPDF
- 26. Bhattacharyya N. Survival and prognosis in Hurthle cell carcinoma of the thyroid gland. Arch Otolaryngol Head Neck Surg 2003; 129: 207-10. ArticlePubMed
- 27. Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the united states during 1996. U.S. and German Thyroid Cancer Study Group. An American College of Surgeons Commission on Cancer Patient Care Evaluation study. Cancer 2000; 89: 202-17. ArticlePubMed
- 28. Agarwal S, Bychkov A, Jung CK, et al. The prevalence and surgical outcomes of Hurthle cell lesions in FNAs of the thyroid: a multi-institutional study in 6 Asian countries. Cancer Cytopathol 2019; 127: 181-91. PubMed
- 29. Thompson LD, Poller DN, Kakudo K, Burchette R, Nikiforov YE, Seethala RR. An international interobserver variability reporting of the nuclear scoring criteria to diagnose noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a validation study. Endocr Pathol 2018; 29: 242-9. ArticlePubMedPDF
- 30. Liu Z, Bychkov A, Jung CK, et al. Interobserver and intraobserver variation in the morphological evaluation of noninvasive follicular thyroid neoplasm with papillary-like nuclear features in Asian practice. Pathol Int 2019; 69: 202-10. ArticlePubMedPDF
- 31. Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid 2016; 26: 1535-40. ArticlePubMed
- 32. Lee JH, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet 2014; 384: 1848.Article
- 33. Kakudo K, Kameyama K, Miyauchi A, Nakamura H. Introducing the reporting system for thyroid fine-needle aspiration cytology according to the new guidelines of the Japan Thyroid Association. Endocr J 2014; 61: 539-52. ArticlePubMed
- 34. Kameyama K, Sasaki E, Sugino K, Ito K. The Japanese Thyroid Association Reporting System of thyroid aspiration cytology and experience from a high-volume center, especially in indeterminate category. J Basic Clin Med 2015; 4: 70-4.
- 35. Satoh S, Yamashita H, Kakudo K. Thyroid Cytology: the Japanese system and experience at Yamashita Thyroid Hospital. J Pathol Transl Med 2017; 51: 548-54. ArticlePubMedPMCPDF
- 36. Boon ME, Lowhagen T, Willems JS. Planimetric studies on fine needle aspirates from follicular adenoma and follicular carcinoma of the thyroid. Acta Cytol 1980; 24: 145-8. PubMed
- 37. Crissman JD, Drozdowicz S, Johnson C, Kini SR. Fine needle aspiration diagnosis of hyperplastic and neoplastic follicular nodules of the thyroid: a morphometric study. Anal Quant Cytol Histol 1991; 13: 321-8. PubMed
- 38. Luck JB, Mumaw VR, Frable WJ. Fine needle aspiration biopsy of the thyroid. Differential diagnosis by videoplan image analysis. Acta Cytol 1982; 26: 793-6. PubMed
- 39. Wright RG, Castles H, Mortimer RH. Morphometric analysis of thyroid cell aspirates. J Clin Pathol 1987; 40: 443-5. ArticlePubMedPMC
- 40. Gupta S, Savala R, Gupta N, Dey P. Fractal dimension and chromatin textural analysis to differentiate follicular carcinoma and adenoma on fine needle aspiration cytology. Cytopathology 2020; 31: 491-3. ArticlePubMedPDF
- 41. Savala R, Dey P, Gupta N. Artificial neural network model to distinguish follicular adenoma from follicular carcinoma on fine needle aspiration of thyroid. Diagn Cytopathol 2018; 46: 244-9. ArticlePubMedPDF
- 42. Han K, Ha HJ, Kong JS, et al. Cytological features that differentiate follicular neoplasm from mimicking lesions. J Pathol Transl Med 2018; 52: 110-20. ArticlePubMedPMCPDF
- 43. Cervino JM, Paseyro P, Grosso OF, Maggiolo J. The cytological examination of the thyroid gland and its anatomo-clinical correlations. An Fac Med Univ Repub Montev Urug 1962; 47: 128-43. PubMed
REFERENCES
Figure & Data
References
Citations

- Misdiagnosed follicular adenoma with 11 year postoperative liver and lung metastases a case report and literature review
Kai-Li Yang, Heng-Tong Han, Shou-Hua Li, Xiao-Xiao Li, Ze Yang, Li-Bin Ma, Yong-Xun Zhao
Discover Oncology.2025;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

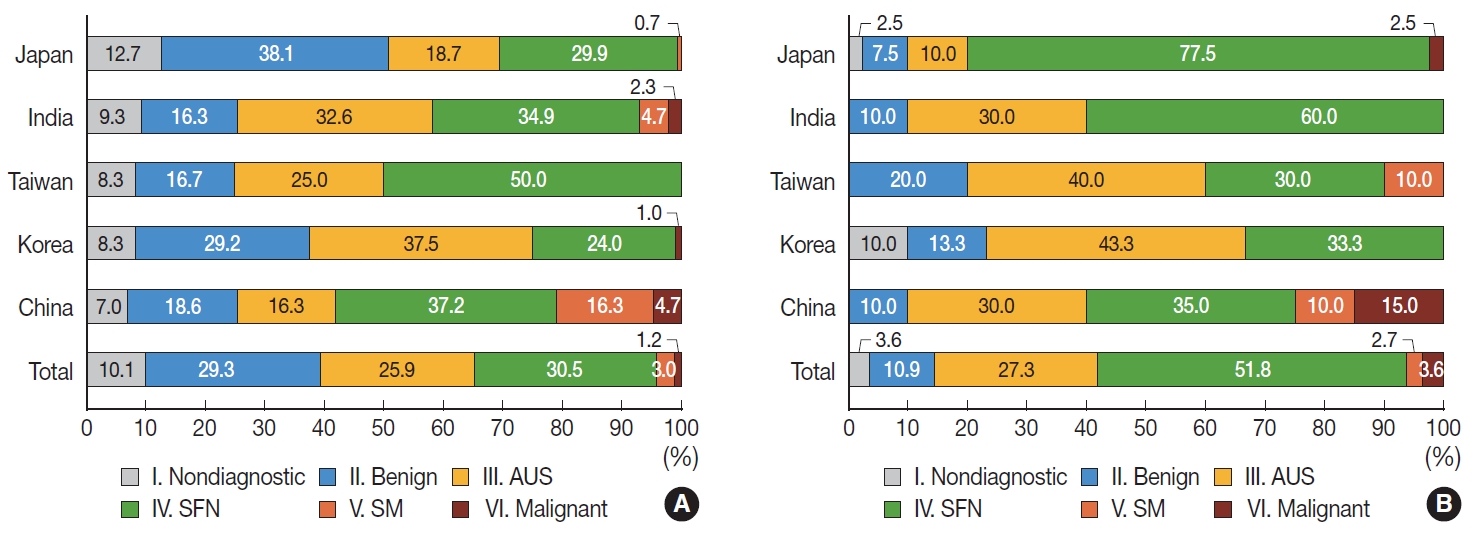
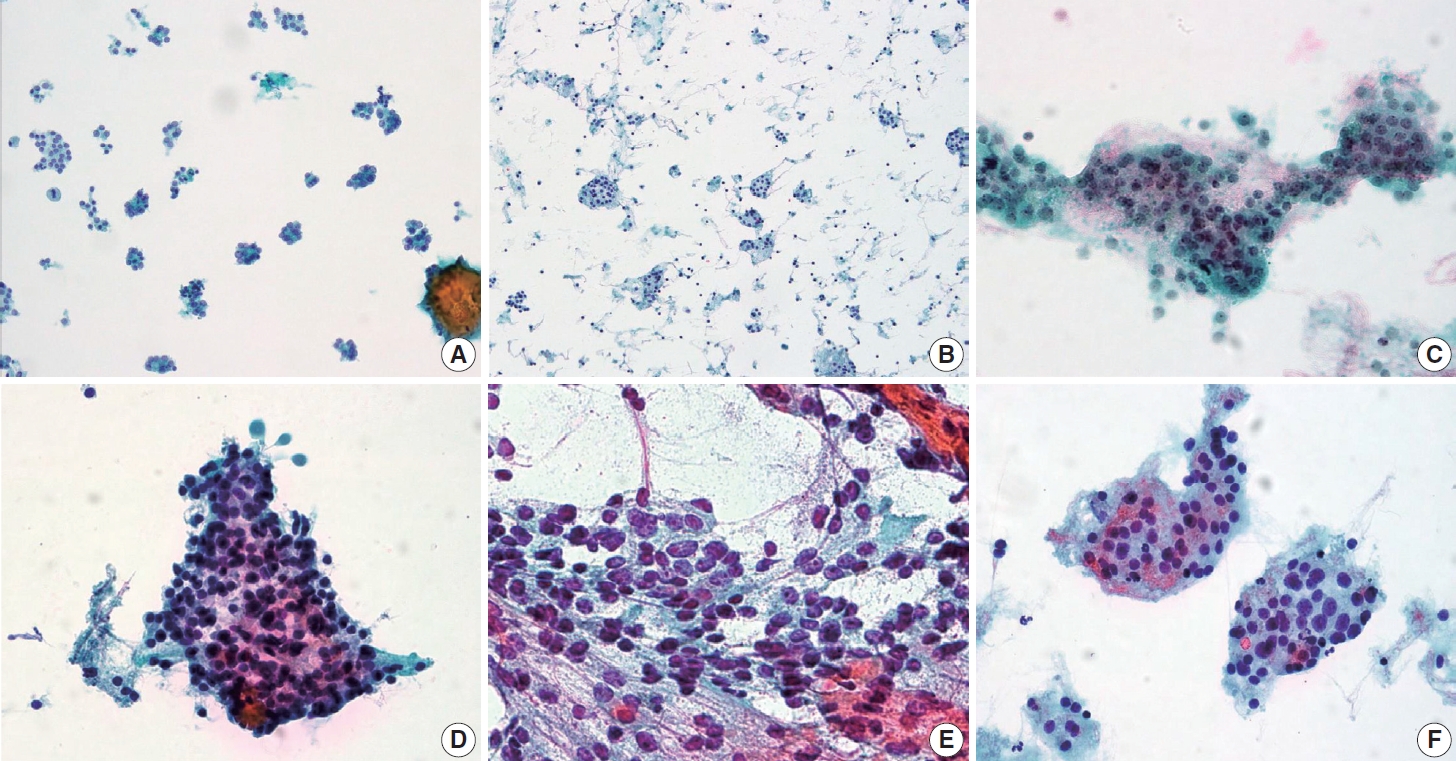

Fig. 1.
Fig. 2.
Fig. 3.
Graphical abstract
| FNAC diagnostic category | FA (n = 112) | FTC |
p-value |
p-value |
|||
|---|---|---|---|---|---|---|---|
| FTC all (n = 110) | MIFTC (n = 67) | AIFTC (n = 20) | WIFTC (n = 23) | ||||
| Original diagnoses | |||||||
| I | 3 (2.7) | 4 (3.6) | 3 (4.5) | 1 (5.0) | 0 | .720 | > .999 |
| II | 31 (27.7) | 12 (10.9) | 8 (11.9) | 3 (15.0) | 1 (4.3) | .002 |
.762 |
| III | 31 (27.7) | 30 (27.3) | 21 (31.3) | 5 (25.0) | 4 (17.4) | .946 | .231 |
| IV | 42 (37.5) | 57 (51.8) | 30 (44.8) | 10 (50.0) | 17 (73.9) | .032 |
.065 |
| V | 5 (4.5) | 3 (2.7) | 2 (3.0) | 1 (5.0) | 0 | .722 | > .999 |
| VI | 0 | 4 (3.6) | 3 (4.5) | 0 | 1 (4.3) | .059 | .647 |
| Revised diagnoses | |||||||
| I | 5 (4.5) | 5 (4.5) | 4 (6.0) | 1 (5.0) | 0 | .977 | .647 |
| II | 30 (26.8) | 12 (10.9) | 8 (11.9) | 0 | 4 (17.4) | .003 |
.762 |
| III | 27 (24.1) | 25 (22.7) | 18 (26.9) | 4 (20.0) | 3 (13.0) | .685 | .196 |
| IV | 50 (44.6) | 62 (56.4) | 32 (47.8) | 14 (70.0) | 16 (69.6) | .107 | .023 |
| V | 0 | 4 (3.6) | 3 (4.5) | 1 (5.0) | 0 | .059 | > .999 |
| VI | 0 | 2 (1.8) | 2 (3.0) | 0 | 0 | .244 | .519 |
| Clinical and cytologic parameters | FA (n = 107) | FTC |
p-value |
p-value |
|||
|---|---|---|---|---|---|---|---|
| FTC all (n = 105) | MIFTC (n = 63) | AIFTC (n = 19) | WIFTC (n = 23) | ||||
| USG | .001 |
.644 | |||||
| Low suspicious | 37 (34.6) | 17 (16.2) | 11 (17.5) | 3 (15.8) | 3 (13.0) | ||
| Intermediate to high suspicious | 49 (45.8) | 70 (66.7) | 41 (65.1) | 11 (57.9) | 18 (78.3) | ||
| NA | 21 (19.6) | 18 (17.1) | 11 (17.5) | 5 (26.3) | 2 (8.7) | ||
| Tumor size (cm) | 3.2 (0.6–9.0) | 3.5 (0.7–8.5) | 3.3 (0.7–8.5) | 3.8 (0.8–6.5) | 4.0 (1.4–8.2) | .128 | .022 |
| Cellularity ≥ 60 cells | 97 (90.7) | 95 (90.5) | 56 (88.9) | 19 (100) | 20 (87.0) | .965 | .736 |
| Pattern/arrangement | |||||||
| Microfollicles ≥ 50% | 49 (45.8) | 65 (61.9) | 37 (58.7) | 13 (68.4) | 15 (65.2) | .019 |
.412 |
| Microfollicles in trabecular arrangement | 37 (34.6) | 46 (43.8) | 27 (42.9) | 8 (42.1) | 11 (47.8) | .169 | .810 |
| Solid or trabecular pattern | 57 (53.3) | 54 (51.4) | 32 (50.8) | 10 (52.6) | 12 (52.2) | .788 | .873 |
| Dyshesive cells, extensive | 9 (8.4) | 22 (21.0) | 11 (17.5) | 6 (31.6) | 5 (21.7) | .010 |
.282 |
| Macrofollicles ≥ 50% | 13 (12.1) | 8 (7.6) | 5 (7.9) | 0 | 3 (13.0) | .270 | >.999 |
| Nuclear features | |||||||
| Nuclear crowding, marked | 14 (13.1) | 29 (27.6) | 17 (27.0) | 6 (31.6) | 6 (26.1) | .009 |
.859 |
| Nuclear enlargement | 74 (69.2) | 80 (76.2) | 43 (68.3) | 17 (89.5) | 20 (87) | .251 | .017 |
| Nuclear pleomorphism | 12 (11.2) | 23 (21.9) | 15 (23.8) | 5 (26.3) | 3 (13) | .036 |
.563 |
| Nuclear hyperchromatism | 35 (32.7) | 44 (41.9) | 19 (30.2) | 12 (63.2) | 13 (56.5) | .166 | .003 |
| Coarse chromatin | 23 (21.5) | 29 (27.6) | 17 (27.0) | 9 (47.4) | 3 (13.0) | .300 | .859 |
| Prominent nucleoli | 2 (1.9) | 8 (7.6) | 5 (7.9) | 3 (15.8) | 0 | .059 | > .999 |
| Colloid quantity | .572 | .503 | |||||
| Absent | 61 (57.0) | 68 (64.8) | 41 (65.1) | 9 (47.4) | 18 (78.3) | ||
| Thin | 34 (31.8) | 28 (26.7) | 16 (25.4) | 9 (47.4) | 3 (13.0) | ||
| Thick | 9 (8.4) | 8 (7.6) | 6 (9.5) | 1 (5.3) | 1 (4.3) | ||
| Thin and thick | 3 (2.8) | 1 (1.0) | 0 | 0 | 1 (4.3) | ||
| Colloid quantity | .556 | > .999 | |||||
| Absent to focal | 93 (86.9) | 94 (89.5) | 56 (88.9) | 16 (84.2) | 22 (95.7) | ||
| Extensive | 14 (13.1) | 11 (10.5) | 7 (11.1) | 3 (15.8) | 1 (4.3) | ||
| Cystic change | 6 (5.6) | 8 (7.6) | 7 (11.1) | 1 (5.3) | 0 | .555 | .140 |
Values are presented as number (%). FNAC, fine needle aspiration cytology; FA, follicular adenoma; FTC, follicular thyroid carcinoma; MIFTC, minimally invasive follicular thyroid carcinoma; AIFTC, encapsulated angioinvasive follicular thyroid carcinoma; WIFTC, widely invasive follicular thyroid carcinoma. FA versus FTC; MIFTC versus AIFTC and WIFTC; Statistically significant difference; for comparison between surgical diagnosis, chi-square test was used.
Values are presented as number (%) or mean (range). FA, follicular adenoma; FTC, follicular thyroid carcinoma; MIFTC, minimally invasive follicular thyroid carcinoma; AIFTC, encapsulated angioinvasive follicular thyroid carcinoma; WIFTC, widely invasive follicular thyroid carcinoma; USG, ultrasonography; NA, not available. FA vs. FTC; MIFTC vs. AIFTC and WIFTC; Statistically significant difference; for comparison between surgical diagnosis, chi-square test was used.

 E-submission
E-submission