Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(6); 2025 > Article
-
Original Article
Modified plasma-thrombin method using patient-derived plasma for cell block preparation in endobronchial ultrasound–guided transbronchial fine-needle aspiration -
Xizhe Zhang1,*
 , Chunli Tang1,*
, Chunli Tang1,* , Yingying Gu1
, Yingying Gu1 , Zeyun Lin1,2
, Zeyun Lin1,2 , Shiqi Tang1
, Shiqi Tang1 , Anzi Tan1
, Anzi Tan1 , Mengshi Li1
, Mengshi Li1 , Zhucheng Chen1
, Zhucheng Chen1 , Yuying Chen1
, Yuying Chen1 , Shi-yue Li1
, Shi-yue Li1 , Juhong Jiang1
, Juhong Jiang1
-
Journal of Pathology and Translational Medicine 2025;59(6):434-443.
DOI: https://doi.org/10.4132/jptm.2025.08.20
Published online: November 11, 2025
1State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
2Department of Clinical Laboratory, Guangzhou Development District Hospital, Guangzhou, China
-
Corresponding Author Juhong Jiang, PhD State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong 510120, China Tel: +86-20-81567845, Fax: +86-20-83395961, E-mail: juhongjiang2006@163.com
Shi-yue Li, PhD State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong 510120, China Tel: +86-20-81566640, Fax: +86-20-83395961, E-mail: lishiyue@188.com - *Xizhe Zhang and Chunli Tang contributed equally to this work.
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,351 Views
- 80 Download
Abstract
-
Background
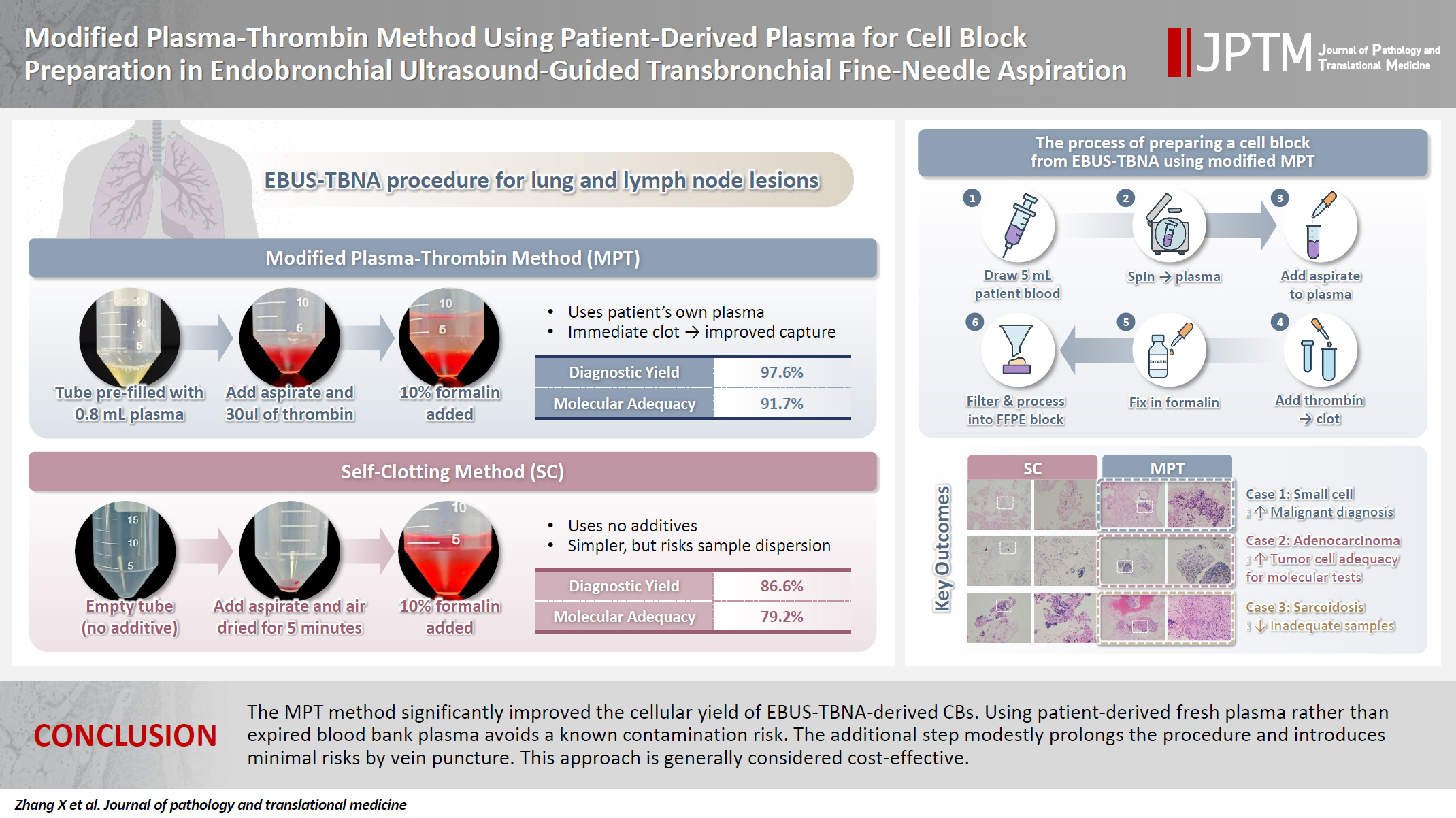
- The plasma-thrombin method, which uses expired blood bank plasma as an ancillary component, has been widely used in cell block (CB) preparation. However, the application of expired blood bank plasma raises concerns about nucleic acid contamination. This study investigated the feasibility of using patient-derived plasma as a substitute for blood bank plasma in the modified plasma-thrombin (MPT) method for CB preparation in endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) samples.
-
Methods
- A prospective study was conducted to compare the adequacy of CB preparation between a previously used self-clotting (SC) method and the MPT method. The EBUS-TBNA specimens from each targeted lesion were divided into paired samples: one processed using the SC method and the other using the MPT method, substituting the blood bank plasma with patient-derived plasma.
-
Results
- A total of 82 paired EBUS-TBNA samples from 59 patients were analyzed. The diagnostic yield of the SC method and the MPT method was 86.6% and 97.6%, respectively. Among patients diagnosed with non–small cell lung cancer, the adequacy rate for molecular testing was 79.2% with the SC method and 91.7% with the MPT method.
-
Conclusions
- The MPT method significantly improved the cellular yield of EBUS-TBNA–derived CBs. Using patient-derived fresh plasma rather than expired blood bank plasma avoids a known contamination risk. The additional step modestly prolongs the procedure and introduces minimal risks by vein puncture. This approach is generally considered cost-effective.
- Cell blocks (CBs) are preparations in which cytologic material is collected and processed into a paraffin-embedded block, similar to formalin-fixed paraffin-embedded (FFPE) tissue in surgical pathology. CBs are primarily obtained from the pellet formed by cells and small solid particles in a cell suspension derived from needle rinses of aspirates, effusions, washings, or brushings [1-3]. A key advantage of CBs is that hematoxylin and eosin (H&E)–stained slides provide well-preserved cytomorphology while maintaining tissue architecture in cell clusters and tissue fragments. Moreover, the concentration of diagnostic material within a limited area facilitates rapid examination [1]. CBs also enable the preparation of multiple serial sections for ancillary studies, including special stains, immunohistochemistry, and molecular testing [4]. Furthermore, they can be archived long-term for potential future diagnostic studies or research applications.
- Unlike histological tissue preparation in surgical pathology, there is no standardized method for CB preparation, with various in-house and commercially available techniques in use. A 2014 survey found that while more than 10 CB preparation methods are used, including HistoGel (27%), plasma-thrombin (PT) (33%), and the Cellient automated system (8%), up to 44% of cytopathologists are dissatisfied with CB quality, highlighting the challenges they present in routine practice [5].
- Endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) is an effective technique for obtaining tissue and cytologic samples from mediastinal or hilar lymph nodes and lung parenchyma for diagnostic purposes [6,7]. Although cytomorphologic examination of cytologic slides, such as direct smears or liquid-based preparations, is an essential diagnostic tool for evaluating aspirates obtained by EBUS-TBNA, CBs allow for reproducible morphological and immunohistochemical characterization of lesions. Traditionally, CBs are prepared by rinsing the aspiration needle with normal saline or RPMI medium into a centrifuge tube. The solution is then centrifuged to form a cell pellet, which is processed into a CB. To enhance cell adhesion and clot formation, other CB preparation methods using PT, HistoGel, or sodium alginate have been employed [8-10]. However, even when all aspirated material is retrieved completely by needle rinsing and concentration, the cellular yield of CBs remains inconsistent. A primary limitation of these methods is that they focus on concentrating the cellular material after it has already been diluted during the needle rinsing, rather than preventing cell loss before processing.
- The “tissue coagulum clot,” also known as “self-clotting” (SC) technique, an alternative approach designed to improve cellular yield compared to the traditional needle rinse method. In the SC method described by Shi et al. (2018) [11], aspirated material for CB preparation is directly expelled onto the bottom or sidewall of a 50 mL centrifuge tube, where it subsequently forms a clot and air-dries. If the air-dried sample lacks sufficient blood or is too small in quantity, plasma and thrombin are added to form an artificial clot. The final needle rinse and collection medium, composed of RPMI, is then combined with the clot and air-dried sample. CBs are then prepared in the histology laboratory using the conventional PT method. While CB preparation—including PT-enhanced SC and the traditional PT method—can be complex, the SC technique prevents cellular material that does not form a tissue coagulum from dispersing into the collection medium. This significantly minimizes cellular loss during subsequent processing.
- In the modified plasma-thrombin (MPT) method, as described by Aisagbonhi et al. (2018) [9], the fine-needle aspiration (FNA) sample is directly rinsed into previously aliquoted pooled fresh plasma. Two drops of thrombin are then added and gently mixed, leading to immediate clot formation. The clot is immediately transferred to a tissue bag and cassette, fixed in formalin, and processed using standard histological methods. The MPT method eliminates the need for centrifugation to concentrate the sample, thereby reducing processing time and avoiding tissue deformation caused by centrifugation. Moreover, immediate formalin fixation improves the preservation of both single-cell cytomorphology and the architecture of tissue fragments.
- The traditional PT method, including needle rinsing and sample centrifugation, has been widely adopted as an inexpensive and simple approach despite the lack of rigorous comparisons with alternative CB techniques or validation against the gold standard of FFPE histology tissue [12]. However, using expired blood bank plasma or pooled fresh plasma as an ancillary component in the traditional PT method and MPT method raises several concerns. First, expired plasma exhibits variability in clotting ability, leading to inconsistencies in clot formation time and clot size, which can result in an uneven cell distribution through the cutting levels of the CB. Furthermore, the use of discarded human plasma introduces a theoretical risk of nucleic acid contamination or the presence of biological factors that may interfere with sensitive downstream molecular testing. This concern is increasingly relevant as cytology specimens are frequently used for molecular analysis. A study by Sung et al. [13] demonstrated that all tested expired fresh frozen donor plasma contained high-quality amplifiable DNA. Their findings, along with those of other researchers, caution against using pooled blood bank plasma for CB preparation due to potential genomic DNA interference, particularly in specimens with low cellularity [14].
- Beginning in February 2022, a modified SC method based on the technique described by Shi et al. [11] was implemented for the CB processing of EBUS-TBNA samples in the authors’ institute. Unlike the original method, this approach eliminates the ancillary PT step to mitigate concerns regarding nucleic acid contamination. In this method, EBUS-TBNA samples designated for CB preparation are expelled directly into a centrifuge tube, allowing clot formation. A 10% formalin solution is then added. In the histology laboratory, a disposable paint filter with 100-µm fine nylon mesh is placed over a beaker, and a piece of embedding paper is positioned inside the filter. The sample from the centrifuge tube is poured into this device, where tissue clots and dispersed cellular material are captured by the embedding paper. The paper is then wrapped and processed as a routine histology sample [15].
- The current study aimed to investigate the feasibility of using the MPT method described by Aisagbonhi et al. [9] while substituting patient-derived plasma for pooled blood bank plasma in CB preparation. By obtaining 5 mL of whole blood from the patient before the procedure, an adapted MPT method was developed and compared with the previous SC method. A prospective study was conducted to evaluate the adequacy of CB preparation between these two methods.
INTRODUCTION
- Study design and participants
- This single-center, prospective study was conducted to compare the SC and MPT methods for CB preparation in the pulmonary clinic of the First Affiliated Hospital of Guangzhou Medical University from January to December 2024. Adults aged 18 years or older with undiagnosed mediastinal lymphadenopathy or mediastinal masses, as well as patients with lung cancer undergoing staging, were included.
- Thrombin and plasma preparation
- Thrombin from bovine plasma (Absin, Shanghai, China) was used. The product was supplied as a lyophilized powder with an activity of 2,000 units/mg protein. It was reconstituted in normal saline containing 0.1% bovine serum albumin to a final concentration of 100 units/mL. The solution was aliquoted into polymerase chain reaction (PCR) tubes (100 µL per tube) and stored at −20°C. Only the required number of aliquots was thawed before use.
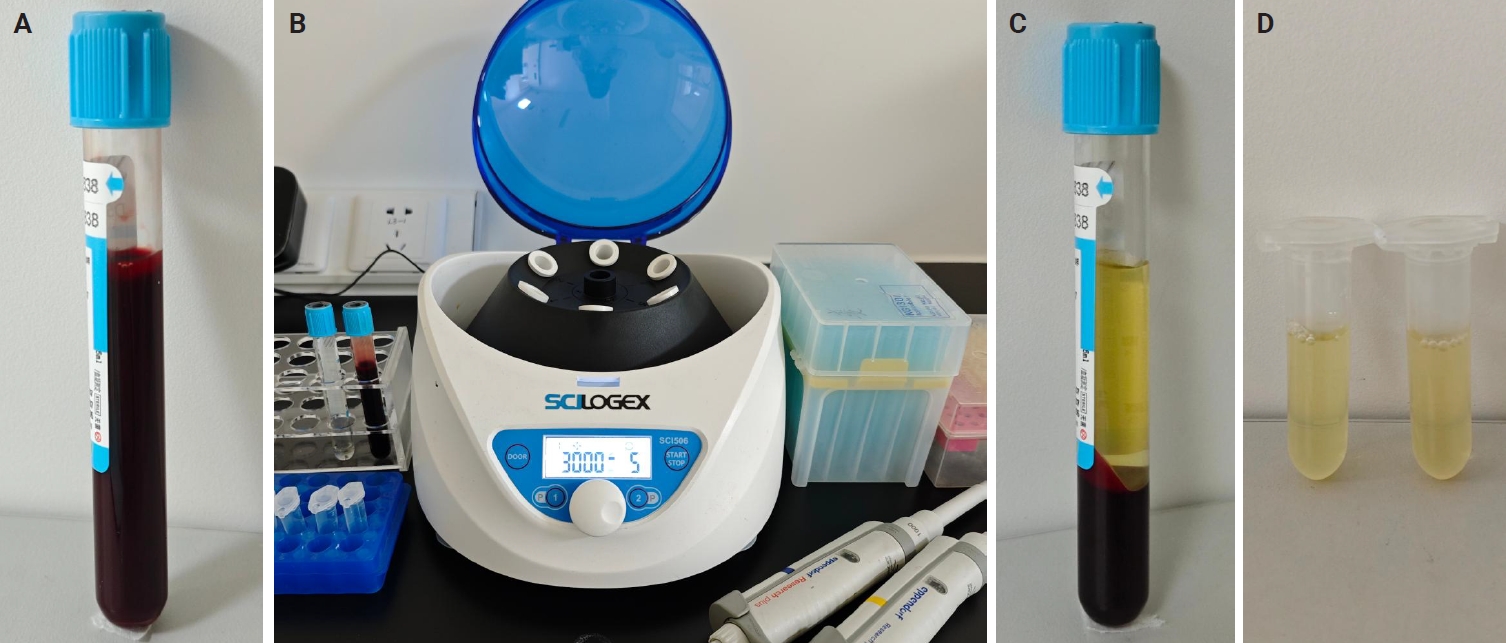
- A mini centrifuge and other necessary materials were set up in the bronchoscopy unit (Fig. 1). Before the EBUS-TBNA procedure, 5 mL of venous blood was drawn from the patient by a nurse in the bronchoscopy unit using sodium citrate as an anticoagulant. The blood sample was centrifuged at 3,000 rpm for 5 minutes, and the supernatant (plasma) was transferred to a 2-mL Eppendorf tube for further use. The details of these processes are illustrated in Fig. 1.
- Sample acquisition
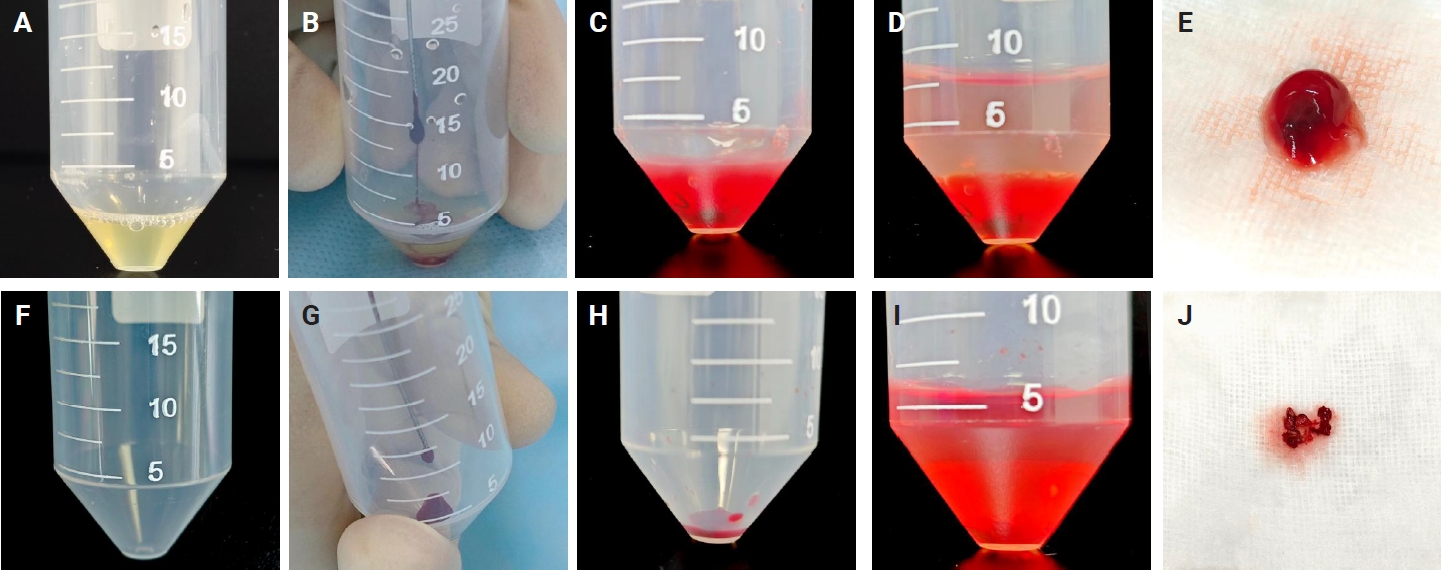
- Two 50-mL centrifuge tubes labeled tube A and tube B were prepared for each targeted lesion. Tube A was pre-filled with 0.8 mL of plasma for the MPT method, while tube B contained no additives for the SC method. Each EBUS-TBNA procedure was performed by a physician in the bronchoscopy unit of the pulmonary diseases clinic. Aspiration material was obtained from a targeted lymph node (LN) or lesion in the lung, trachea, or mediastinum using a 21-gauge or 22-gauge cytology needle. The material inside the cytology needle was gently expelled using the wire stylet provided with the needle kit, depositing the aspirate into the bottom of the respective centrifuge tube. Four passes were obtained from each targeted LN or lesion. For cases 1–10, 21–30, and 41–50, the first and third passes were expelled into tube A (pre-filled with plasma), while the second and fourth passes were expelled into tube B (without any additives). For cases 11–20, 31–40, and 51–59, the first and third passes were expelled into tube B, while the second and fourth passes were expelled into tube A. Targeted LNs or lesions with fewer than four passes were excluded from the study.
- Following sample acquisition, 30 µL of thrombin was added to tube A. The tube was gently flicked to mix the thrombin, resulting in immediate clot formation. The sample in tube B, which lacked clotting material, was air-dried for 5 minutes. A 10% formalin solution was then added to both tube A and tube B. Samples were then submitted to the pathology laboratory for processing. The details of these processes are illustrated in Fig. 2.
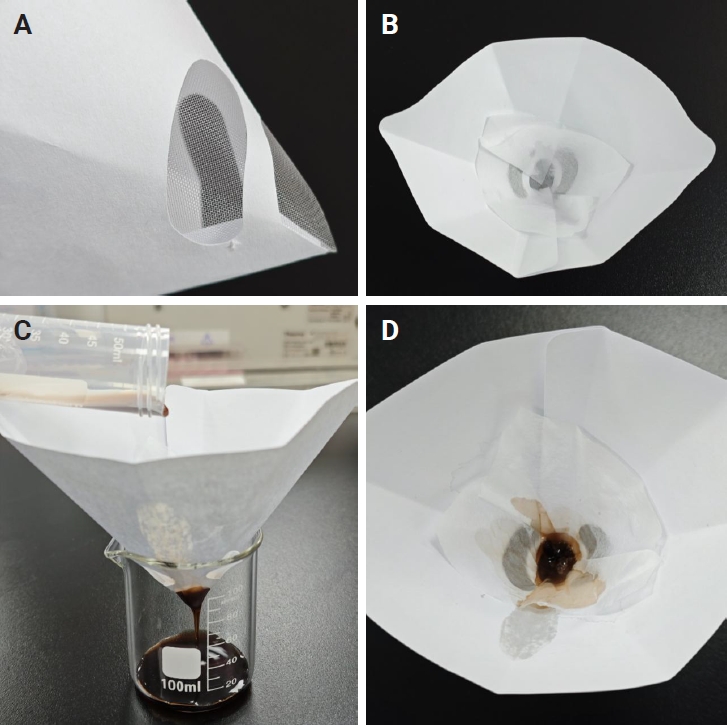
- In the pathology laboratory, a disposable paint filter with 100-µm fine nylon mesh was positioned over a beaker, with a piece of embedding paper placed inside the filter. The sample contents from each centrifuge tube were poured into this filter, allowing both tissue clots and dispersed cellular components to be captured by the embedding paper. The embedding paper was wrapped and processed as a standard histology specimen. The setup and filtration workflow are illustrated in Fig. 3.
- H&E-stained FFPE sections and immunohistochemical analysis
- The FFPE samples were cut at a standard thickness of 4 µm and stained with H&E following routine protocols. Immunohistochemistry was performed on cases in which the pathologist deemed additional analysis necessary for diagnostic clarification. Immunohistochemical staining was carried out using the Ventana-Roche automated immunostainer following standard staining protocols.
- Slide review
- H&E-stained FFPE sections were independently reviewed by two pathologists (J.J. and Y.G.), blinded and without knowledge of the CB preparation method. Sections from each case were systematically examined and compared. According to the World Health Organization (WHO) Reporting System for Lung Cytopathology and established criteria for evaluating inadequate EBUS-derived specimens [16-18], samples from both preparation methods were categorized into five diagnostic groups: “insufficient/inadequate/nondiagnostic,” “benign,” “atypical,” “suspicious for malignancy,” and “malignant.” The “insufficient/inadequate/nondiagnostic” category was applied to cases with limited material resulting from low cellularity, suboptimal preparation, poor fixation or staining, or obscuring artifacts such as blood, inflammation, or necrosis that preclude reliable interpretation. Samples displaying cells or structures consistent with non-malignant conditions were classified as “benign,” whereas those displaying clear malignant cytologic features were categorized as “malignant.” Specimens categorized as “atypical” demonstrated findings predominantly characteristic of benign lesions and minor atypical features that raised the possibility of malignancy, though insufficient in quantity or quality for a definitive diagnosis. The “suspicious for malignancy” category was used when cytologic features strongly suggested malignancy but the cellular material was limited in quantity or quality, rendering a definitive diagnosis impossible despite overall specimen adequacy. For malignant cases, tumor cell quantity and fraction were systematically assessed. Tumor cellularity was estimated at three levels: low, moderate, and high. Low cellularity was defined as having 0–200 tumor cells or a tumor cell fraction <10%, which was considered insufficient for molecular testing. Moderate cellularity was defined as having 200–1,000 tumor cells and a tumor cell fraction of ≥10%, which was deemed suitable for molecular testing. High cellularity was defined as having more than 1,000 tumor cells and a tumor cell fraction of ≥10%, which was considered optimal for molecular testing.
- Statistical analysis
- McNemar's test was used to compare the diagnostic yield and molecular test adequacy rate between the SC and MPT methods in paired samples. A p-value of <.05 was considered statistically significant. All statistical analyses were performed using SPSS software ver. 25.0 (IBM Corp., Armonk, NY, USA).
MATERIALS AND METHODS
- Patient characteristics
- A total of 82 paired EBUS-TBNA samples were collected from 59 patients. Each case had four successful passes from the target LN or lesion, which were divided into paired samples. One sample was processed using the SC method, while the other was processed using the MPT method. Patient demographics, pathological data, and the distribution of sampling stations are summarized in Table 1.
- Diagnostic performance
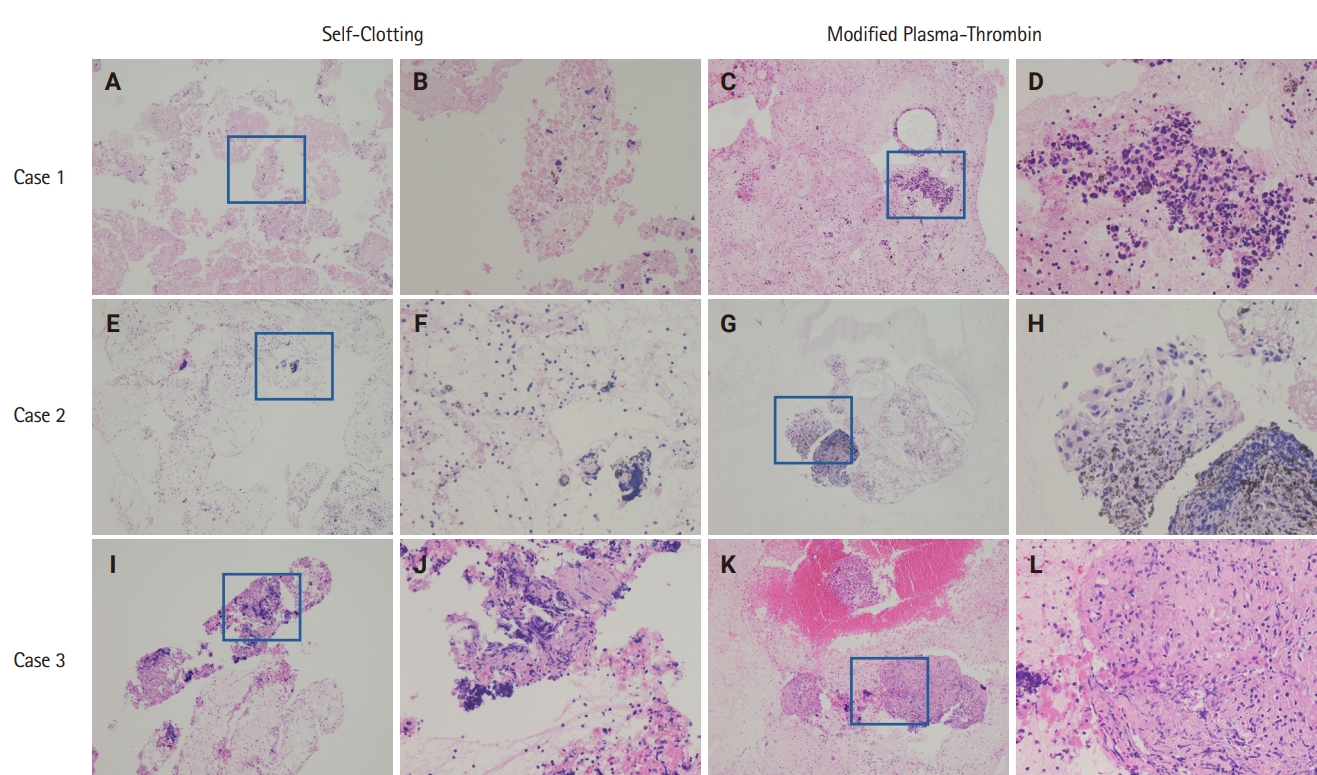
- Cellular morphology, including architectural and nuclear preservation, was comparable between the two CB preparation methods. In the SC method samples, tissue fragments were observed either wrapped in blood clots or dispersed on CB sections. On the other hand, samples processed with the MPT method showed tissue fragments and cellular material enmeshed in blood clots or pale pink eosinophilic artifacts.
- The SC method demonstrated an overall diagnostic yield of 86.6% (71/82), with 49 malignant and 22 benign cases, while 13.4% (11/82) of the samples were deemed inadequate. On the other hand, the MPT method achieved a diagnostic yield of 97.6% (80/82), with 55 malignant and 25 benign cases, and only 2.4% (2/82) of samples classified as inadequate. McNemar's test revealed a statistically significant difference in diagnostic yield between the SC and MPT methods (p = .016). The diagnostic performance of both methods is summarized in Table 2. Fig. 4 presents representative microscopic images of EBUS-TBNA samples deemed inadequate by the SC method but adequate by the MPT method.
- Tumor cellularity assessment in non–small cell lung cancer cases
- Tumor cellularity was evaluated in CB sections of 48 paired samples diagnosed with non–small cell lung cancer, as this subgroup is most commonly considered for targeted therapy. Adequacy for molecular testing was defined as having more than 200 tumor cells and a tumor cell fraction ≥10%. The SC method demonstrated an adequacy rate of 79.2% (38/48), whereas the MPT method achieved an adequacy rate of 91.7% (44/48). A statistically significant difference in adequacy rates was observed between the two methods (p = .041). The tumor cellularity distribution for both methods is presented in Table 3.
- Immunocytochemistry
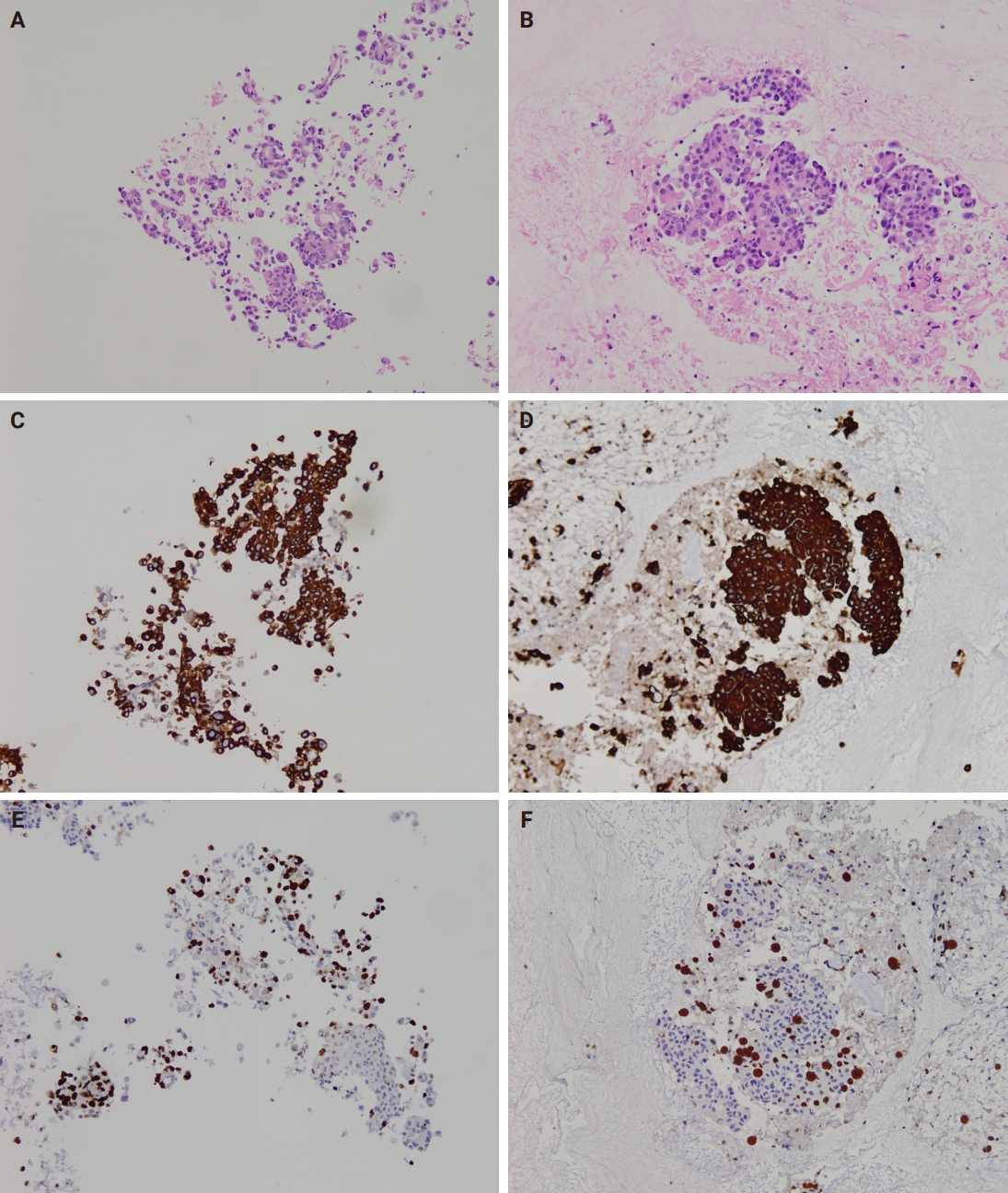
- Immunocytochemistry results for both CB preparation methods showed no difference in staining quality regarding staining intensity. However, occasional faint brown background staining was observed in MPT CBs due to trapped plasma, but this did not interfere with immunocytochemical evaluation. Representative images of cytoplasmic (cytokeratin 7) and nuclear (Ki-67) antigen staining on CB sections prepared by both methods are shown in Fig. 5.
- Time consumption
- The additional processing time required for the MPT method in the bronchoscopy unit, including blood sampling, plasma separation, and clot formation, was approximately 10 minutes.
RESULTS
- The PT method is most commonly used for CB preparation due to its simplicity, low cost, and high satisfaction rate relative to other methods [12]. However, the use of expired blood bank plasma in this process is not recommended for the preparation of CBs due to nucleic acid contamination concerns. To address this issue, this study reports the development of the MPT method, which uses patient-derived plasma as a substitute for expired blood bank plasma. The findings of this study demonstrate that the MPT method using patient-derived plasma improves the cellularity of CB preparations from EBUS-TBNA samples. Patient-derived plasma was easily obtained and convenient for use in the procedure unit. The use of fresh patient-derived plasma in the MPT method offers several advantages over expired blood bank plasma. First, patient-derived plasma avoids a known contamination risk. Second, the stable clotting properties of fresh patient plasma ensure immediate clot formation and predictable clot size, contributing to increased cellular yield by promoting even cell concentration.
- Several factors influence the cellular yield of the MPT method, including plasma volume and the plasma-to-thrombin ratio. A lower plasma volume produces CBs with more concentrated cellular material. In this study, the plasma volume used was approximately equal to that of the cellular sediment. Moreover, a small volume of thrombin (30 µL) was added, in contrast to previous reports where 3–5 drops were used. To ensure consistent clotting efficiency, the thrombin working solution was aliquoted into 100 µL portions in 0.2-mL PCR tubes and stored at −20°C. Only the required number of aliquots was thawed for use, preventing variability in clotting activity.
- The MPT method developed in this study offers several advantages. Compared with the previously reported normal saline needle rinsing and PT method, which involves forced spraying and dispersion of the material using a syringe filled with saline, the SC and MPT methods minimize tissue disruption by expelling the coagulum with minimal mechanical force. Moreover, immediate fixation of the clot in formalin prevents potential antigenicity loss for immunohistochemistry that may occur upon exposure to saline or water. Furthermore, the use of the filtration method in both techniques eliminates the need for centrifugation, reducing processing time and avoiding tissue deformation caused by centrifugation. This approach preserves cellular morphology and prevents the loss of cell pellets during supernatant decanting.
- In previously reported tissue coagulum clot methods or the SC method without the ancillary PT method, aspirated material that was not sufficiently bloody and failed to form a tissue coagulum clot became dispersed upon the addition of formalin, leading to potential sample loss during processing. The MPT method addresses this issue by allowing the aspirated material to stream directly into the tube containing plasma, followed by the addition of thrombin, which enmeshes the cellular material within a clot. This process ensures that dispersed cells or necrotic tissue are retained during subsequent processing. It not only reduces the loss of tumor cellularity but also maintains the tissue structure, which is vital for the diagnosis of cases such as lymphoma. It also helps to keep the necrotic tissue in granuloma nodules, which is important in distinguishing sarcoidosis or tuberculosis.
- Incorporating routine blood sampling and plasma separation into bronchoscopy workflows at high-volume centers may lead to a considerable increase in workload. Therefore, this technique may be more efficiently applied in selected patient groups, such as those with suspected or confirmed thoracic malignancy or those with lesion sizes and locations that may be challenging to visualize or sample via EBUS-TBNA. Alternatively, an additional 5-mL blood sample could be collected during routine laboratory testing before EBUS-TBNA. In such cases, plasma separation could be performed in the laboratory, and the prepared plasma then delivered to the bronchoscopy unit.
- Several limitations should be acknowledged. First, patient-derived plasma was not directly compared with expired blood bank plasma for CB preparation. Second, the MPT method was not evaluated against the traditional normal saline needle rinsing and PT method. Third, statistical validation comparing the MPT method with other CB techniques, including the collodion bag and sodium alginate methods, was not conducted to determine the optimal approach. Finally, although this study did not include image-guided FNAs from the liver, lung, pancreas, or other organs, the findings are likely applicable to these sample types as well.
- In conclusion, patient-derived plasma serves as an effective alternative to expired blood bank plasma for PT-based CB preparation in routine practice. The MPT method developed in this study is a safe, cost-effective approach that poses no additional risk to patients. We encourage the adoption of this method in other image-guided FNAs.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Guangzhou Medical University (reference number: ES-2023-134-03, January 05, 2024). Procedures involving human subjects were conducted following the Declaration of Helsinki, 1975, as revised in 1983. The study was conducted following institutional ethical guidelines, and written informed consent was obtained for blood collection.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: JJ. Data curation: XZ. Investigation: XZ, AT. Funding acquisition: JJ. Methodology: ML, ZC, YC. Project administration: SL. Resources: CT. Supervision: YG. Validation: ZL, ST. Writing—original draft: JJ. Writing—review & editing: SL. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant number 81772814] and the Open Project of the State Key Laboratory of Respiratory Disease [grant number SKLRD-OP-202205]. The funding sources had no role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
| Method | Tumor cellularity | Molecular test adequacy rate, n (%) | p-value | ||
|---|---|---|---|---|---|
| Low | Moderate | High | |||
| Self-clotting | 10 | 18 | 22 | 38/48 (79.2) | .041 |
| Modified plasma thrombin | 4 | 24 | 20 | 44/48 (91.7) | |
Low, with <200 tumor cells/section and/or a tumor cell fraction <10%, insufficient for molecular testing; Moderate, with 200–1,000 tumor cells/section and a tumor cell fraction ≥10 %, suitable for molecular testing; High, with >1,000 tumor cells/section and a tumor cell fraction ≥10%, advantageous for molecular testing.
- 1. Karnauchow PN, Bonin RE. "Cell-block" technique for fine needle aspiration biopsy. J Clin Pathol 1982; 35: 688.ArticlePubMedPMC
- 2. Burt AD, Smillie D, Cowan MD, Adams FG. Fine needle aspiration cytology: experience with a cell block technique. J Clin Pathol 1986; 39: 114-5. Article
- 3. Olson NJ, Gogel HK, Williams WL, Mettler FA. Processing of aspiration cytology samples: an alternative method. Acta Cytol 1986; 30: 409-12. PubMed
- 4. da Cunha Santos G, Saieg MA, Troncone G, Zeppa P. Cytological preparations for molecular analysis: a review of technical procedures, advantages and limitations for referring samples for testing. Cytopathology 2018; 29: 125-32. ArticlePubMedPDF
- 5. Crapanzano JP, Heymann JJ, Monaco S, Nassar A, Saqi A. The state of cell block variation and satisfaction in the era of molecular diagnostics and personalized medicine. Cytojournal 2014; 11: 7.ArticlePubMedPMC
- 6. Tyan CC, Machuca T, Czarnecka K, et al. Performance of endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of isolated mediastinal and hilar lymphadenopathy. Respiration 2017; 94: 457-64. ArticlePubMedPDF
- 7. Rahman KK, Mohapatra PR, Panigrahi MK, Purkait S, Bhuniya S. Comparison of endobronchial ultrasound-guided transbronchial needle aspiration cytology versus cell blocks in adults with undiagnosed mediastinal lymphadenopathy. Lung India 2021; 38: 425-30. ArticlePubMedPMC
- 8. Torous VF, Chen Y, VanderLaan PA. Comparison of plasma-thrombin, HistoGel, and CellGel cell block preparation methods with paired ThinPrep slides in the setting of mediastinal granulomatous disease. J Am Soc Cytopathol 2019; 8: 52-60. ArticlePubMed
- 9. Aisagbonhi O, Birungi A, Atwine R, et al. Modified plasma-thrombin method of cell block preparation for fine-needle aspiration biopsies in resource-limited settings. Am J Clin Pathol 2018; 150: 137-45. ArticlePubMed
- 10. Gupta S, Gautam U, Susheilia S, Bansal B, Uppal R, Srinivasan R. Sodium alginate versus plasma thrombin cell blocks in diagnostic cytopathology: a comparative analysis. Acta Cytol 2022; 66: 72-8. ArticlePubMedPDF
- 11. Shi Y, Chiaffarano J, Yee-Chang M, et al. Self-clotting method improves cell block preparation. Cancer Cytopathol 2018; 126: 190-9. ArticlePubMedPDF
- 12. Jing X, Li QK, Bedrossian U, Michael CW. Morphologic and immunocytochemical performances of effusion cell blocks prepared using 3 different methods. Am J Clin Pathol 2013; 139: 177-82. ArticlePubMedPDF
- 13. Sung S, Sireci AN, Remotti HE, et al. Plasma-thrombin cell blocks: Potential source of DNA contamination. Cancer Cytopathol 2019; 127: 771-7. ArticlePubMedPDF
- 14. Balassanian R, Ng DL, van Zante A. Stop using expired plasma for cell blocks. Cancer Cytopathol 2019; 127: 737-8. ArticlePubMedPDF
- 15. Lin Z, Huang L, Tang S, et al. Comparison of three specimen collection techniques in tissue coagulum clot-based cell block preparation of endobronchial ultrasound-guided transbronchial needle aspiration. Pathol Res Pract 2025; 265: 155730.ArticlePubMed
- 16. Schmitt FC, Bubendorf L, Canberk S, et al. The World Health Organization reporting system for lung cytopathology. Acta Cytol 2023; 67: 80-91. ArticlePubMedPDF
- 17. Jeffus SK, Joiner AK, Siegel ER, et al. Rapid on-site evaluation of EBUS-TBNA specimens of lymph nodes: comparative analysis and recommendations for standardization. Cancer Cytopathol 2015; 123: 362-72. ArticlePubMed
- 18. Vuorisalo A, Huhtala H, Paavonen T, Kholova I. Insufficient endobronchial ultrasound-guided transbronchial needle aspiration specimens. When and why? The analysis of criteria and reasons behind the insufficient specimens. Diagn Cytopathol 2024; 52: 271-87. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
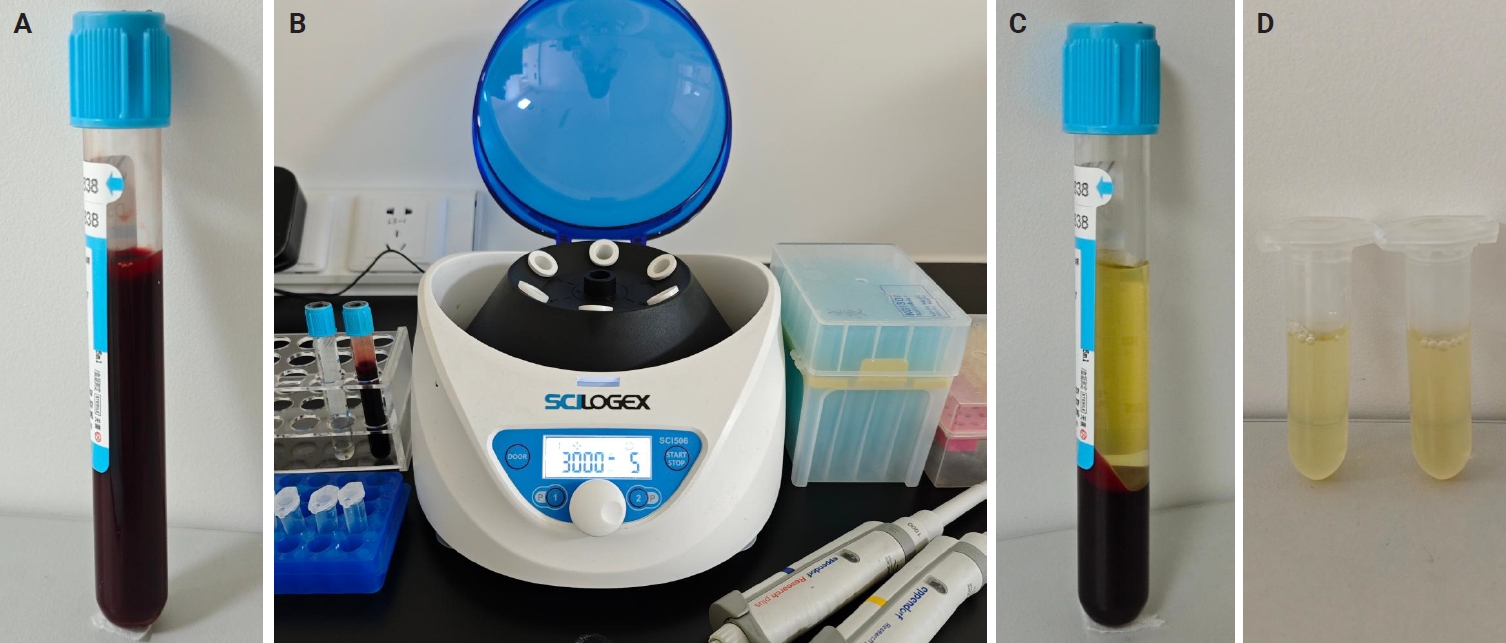
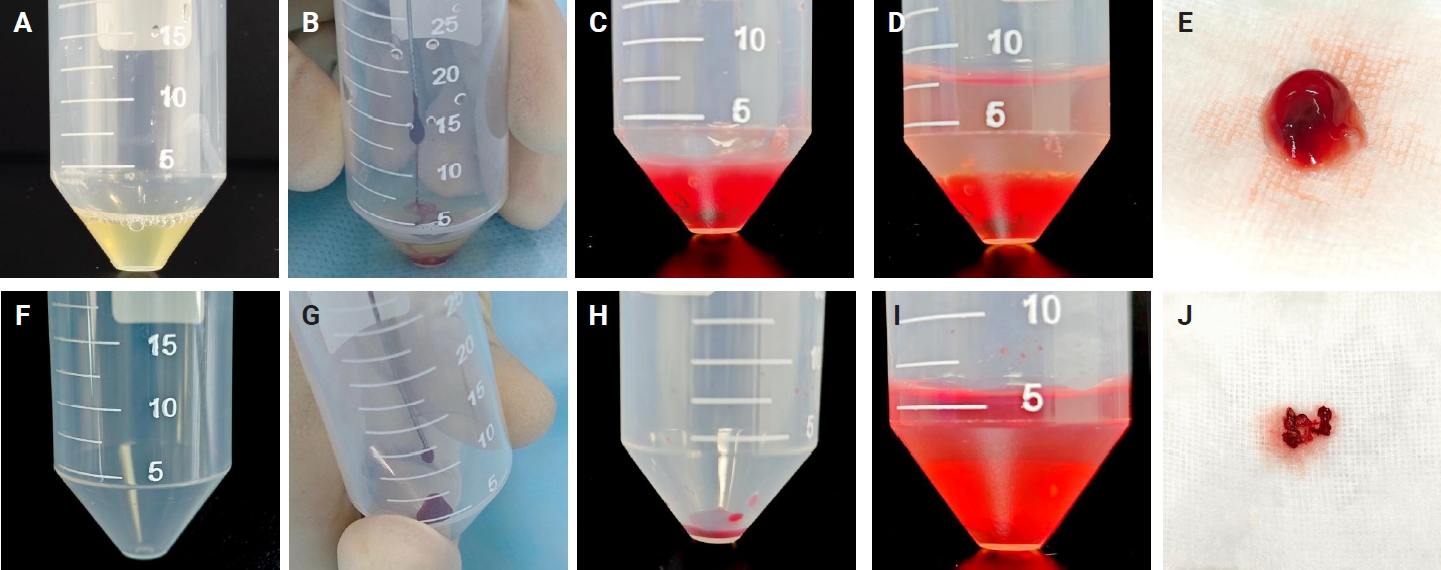
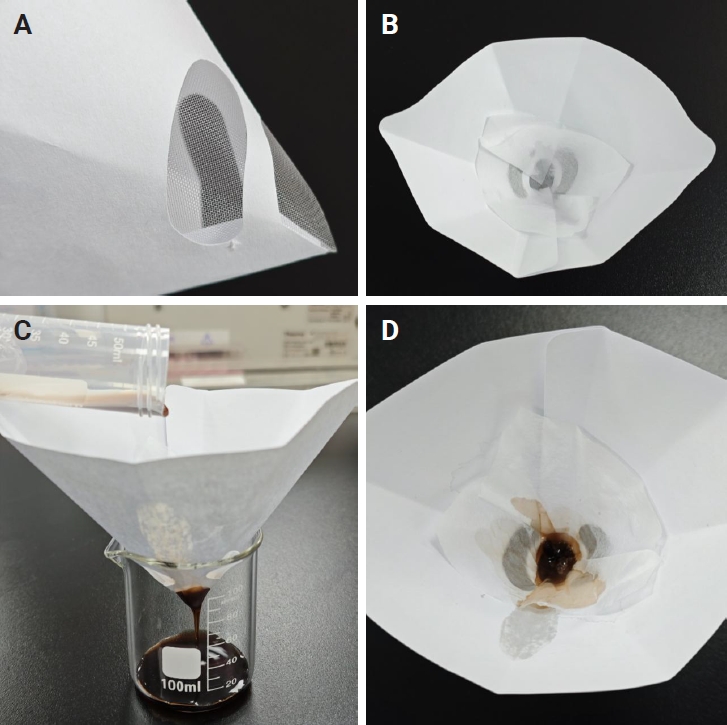
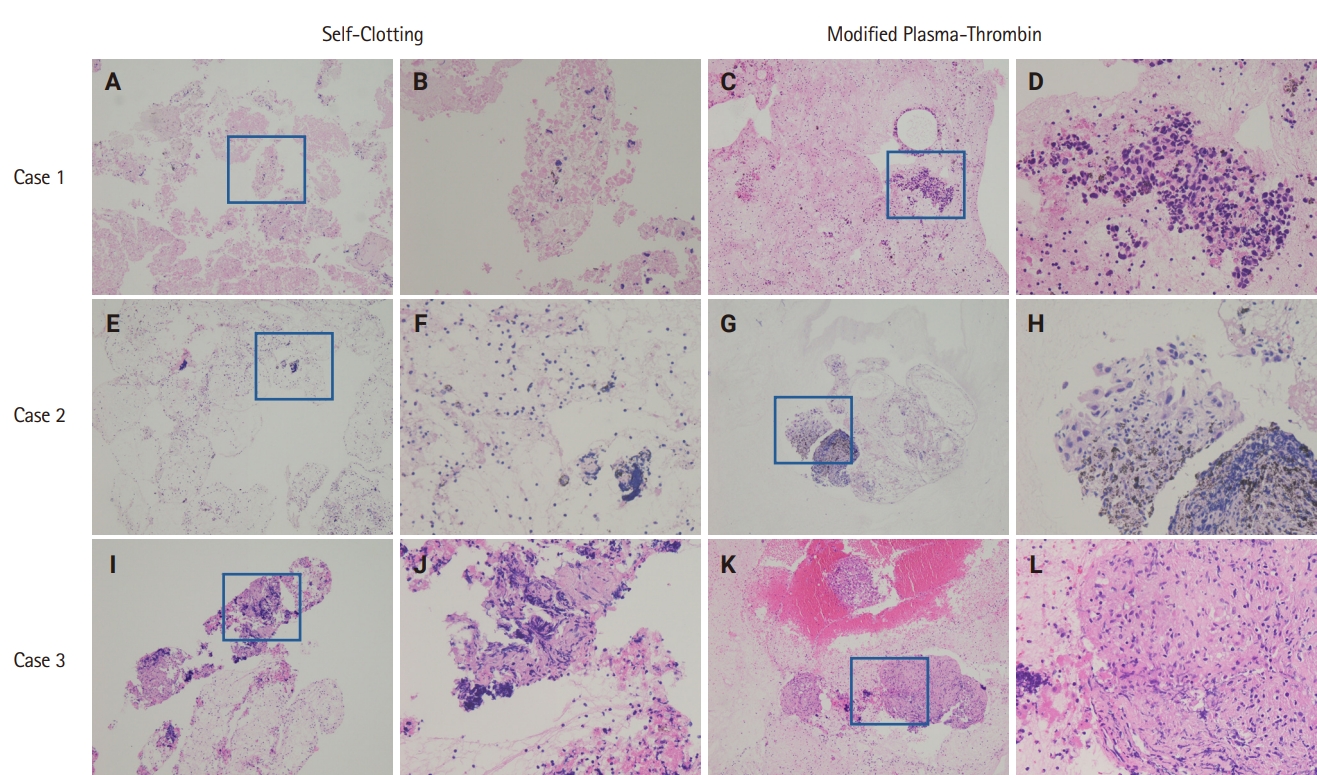
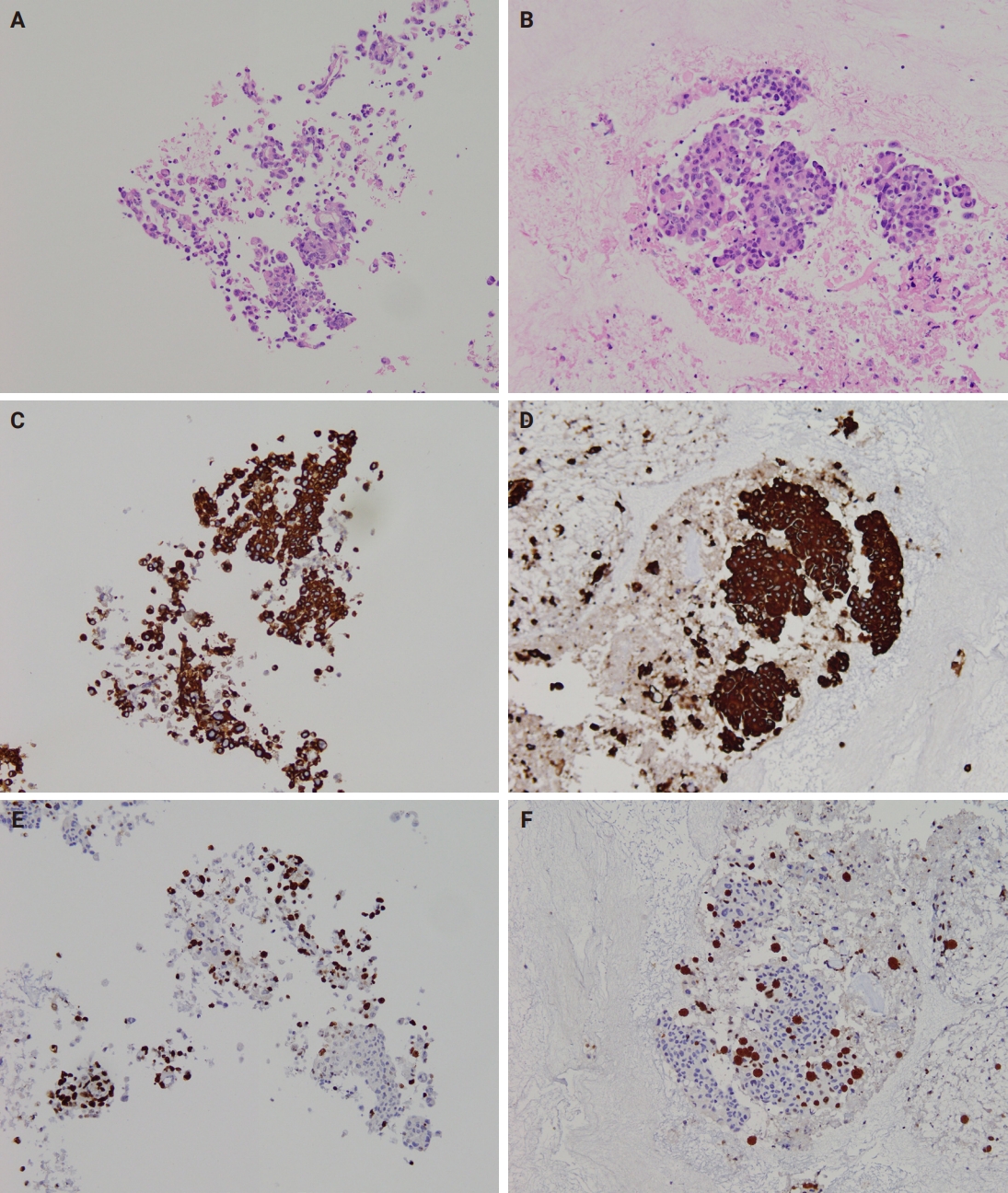

Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Graphical abstract
| Characteristic | Value |
|---|---|
| Age (yr), median (range) | 65 (21-79) |
| Sex, n (%) | |
| Male | 41 (69.5) |
| Female | 18 (30.5) |
| Lung lesion | 16 lesions |
| Hilum of right lung | 1 |
| Right upper lobe | 2 |
| Right lower lobe | 6 |
| Right middle lobe | 2 |
| Left upper lobe | 2 |
| Left lower lobe | 3 |
| Lymph node | 66 lesions |
| 4L | 2 |
| 4R | 10 |
| 7# | 28 |
| 10L | 2 |
| 10R | 2 |
| 11L | 5 |
| 11R | 7 |
| 12R | 10 |
| Malignancy | 39 cases |
| Adenocarcinoma | 24.0 |
| Squamous cell carcinoma | 4.0 |
| Lymphoepithelial carcinoma | 2.0 |
| Small cell lung carcinoma | 3.0 |
| Large cell neuroendocrine carcinoma | 1.0 |
| NSCLC NOS | 5.0 |
| Nonmalignant diagnosis | 18 cases |
| Benign reactive lymph node | 12.0 |
| Tuberculosis | 4.0 |
| Sarcoidosis | 2.0 |
| Inadequate specimen | 2 cases |
| Method | Insufficient/inadequate/non-diagnostic | Benign | Atypical | Suspicious for malignancy | Malignant | Diagnostic yield, n (%) | p-value |
|---|---|---|---|---|---|---|---|
| Self-clotting | 10 | 22 | 0 | 0 | 49 | 71/82 (86.6) | 0.016 |
| Modified plasma-thrombin | 2 | 25 | 0 | 0 | 55 | 80/82 (97.6) |
| Method | Tumor cellularity | Molecular test adequacy rate, n (%) | p-value | ||
|---|---|---|---|---|---|
| Low | Moderate | High | |||
| Self-clotting | 10 | 18 | 22 | 38/48 (79.2) | .041 |
| Modified plasma thrombin | 4 | 24 | 20 | 44/48 (91.7) | |
NSCLC, non–small cell lung cancer; NOS, not otherwise specified.
Low, with <200 tumor cells/section and/or a tumor cell fraction <10%, insufficient for molecular testing; Moderate, with 200–1,000 tumor cells/section and a tumor cell fraction ≥10 %, suitable for molecular testing; High, with >1,000 tumor cells/section and a tumor cell fraction ≥10%, advantageous for molecular testing.

 E-submission
E-submission