Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(4); 2012 > Article
-
Original Article
Clinicopathologic Implications of PIWIL2 Expression in Colorectal Cancer - Sun-Ju Oh, Su-Mi Kim, Young-Ok Kim, Hee-Kyung Chang
-
Korean Journal of Pathology 2012;46(4):318-323.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.4.318
Published online: August 23, 2012
Department of Pathology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
- Corresponding Author: Young-Ok Kim, M.D. Department of Pathology, Kosin University Gospel Hospital, Kosin University College of Medicine, 262 Gamcheon-ro, Seo-gu, Busan 602-702, Korea. Tel: +82-51-990-6744, Fax: +82-51-241-7420, 10highpowerfield@gmail.com
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- There are no established reports about the expression of the Piwil gene, a subfamily of the Piwi gene involved in RNA silencing and self-renewal, in colorectal carcinomas. It is known that the degree of PIWIL2 expression is higher in colorectal carcinomas. But its clinicopathologic significance remains undetermined. This study reassessed the relationship between PIWIL2 expression and the clinicopathologic parameters in colorectal carcinomas.
-
Methods
- An immunohistochemistry of PIWIL2 expression was done in 60 cases of colorectal carcinoma. This was followed by an analysis of the correlation between PIWIL2 expression and clinicopathologic features and a survival analysis.
-
Results
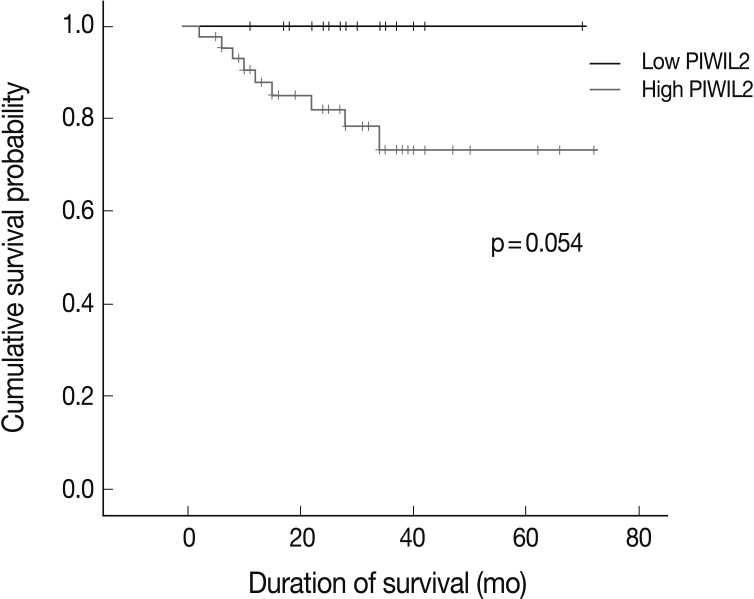
- There were 44 cases (73.3%) where the degree of PIWIL2 expression was relatively higher. The high degree of PIWIL2 expression was significantly correlated with the lower degree of differentiation (p=0.039), deep invasion (p=0.019) and perineural invasion (p=0.027). The overall survival was longer in patients with the lower degree of PIWIL2 expression than in those with the higher degree of PIWIL2 expression.
-
Conclusions
- Our results showed that the degree of PIWIL2 expression was relatively higher in colorectal carcinomas and it was significantly correlated with variable clinicopathologic indicators for a poor prognosis. This indicates that PIWIL2-positive cells contribute to the progression of colorectal cancer.
- Patient selection and sample collection
- Primary tumor samples were collected from 60 patients (n=60) who had been diagnosed with colorectal adenocarcinoma at Kosin University Gospel Hospital in Busan, Korea. The patients had undergone a colectomy between January 2006 and December 2010, where cases were identified retrospectively from clinicopathologic data. Inclusion criteria were the histopathologic diagnosis of colorectal adenocarcinoma, the availability of clinical follow-up data and the availability of paraffin-embedded tissue specimens. The following clinicopathologic characteristics were evaluated for their relevance to protein expression or long-term survival: age (>65 years vs ≤65 years), sex, tumor location (proximal, distal, and rectal), invasion depth (T1, T2 vs T3, T4), pathologic stage (p-stage; I, II vs III, IV), lymph node status, distant metastasis, lymphatic invasion, venous invasion, and perineural invasion. The tumor differentiation grade was classified into two groups: the low-grade (well-to-moderately differentiated tumors) group and the high-grade (poorly-differentiated tumors) one. No patients received chemoradiation treatment preoperatively. Invasion depth and staging were determined using tumor, node and metastasis system of the American Joint Committee on Cancer.9 In our series, the median follow-up period was 28 months (range, 2 to 72 months). The overall survival (OS) was calculated from the date of surgery to the date of last follow-up or death (whichever occurred first).
- Immunohistochemistry
- For the immunohistochemical analysis, 4-µm thick sections were cut consecutively from the blocks. This was followed by deparaffinization in xylene and rehydration in a graded alcohol series. The following steps were performed by BOND-MAX autostaining system (Leica Microsystems, Wetzlar, Germany). Antigen retrieval was performed in pH 6.0 citratephosphate buffer using a microwave. The primary antibody against PIWIL2 was a rabbit polyclonal antibody against PIWIL2 (1:100, Abcam, Cambridge, UK). Slides were counter-stained with Meyer's hematoxylin. Negative controls were treated similarly without primary antibodies.
- Evaluation of immunohistochemical staining
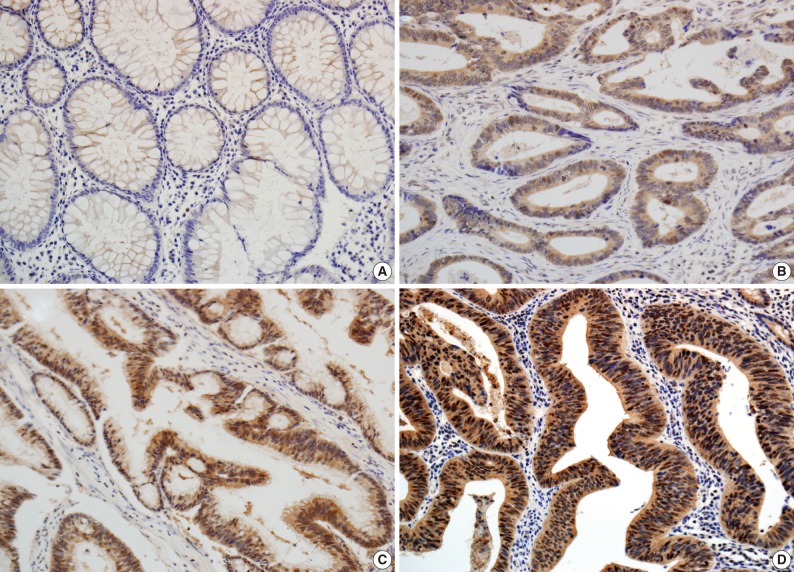
- The scoring method of He et al.6 was used for the immunohistochemical staining evaluation of both the intensity and the proportion of reactive tumor cells. Slides were examined under low power (×40 to ×100) microscopy to identify the regions containing the highest percentage of PIWIL2-positive cells (hot spot) in the cancer nest. Ten hot spots inside the tumor tissue were selected, which was followed by a semi-quantitative evaluation of the expression of PIWIL2 with high power (×400) microscopy. The cell-positive score was graded: <5%, 0 (-); 5-25%, 1 (+); 25-50%, 2 (++); and >50%, 3 (+++). In order to evaluate the intensity of PIWIL2, tumor cells were compared with normal epithelium. Cytoplasmic and nuclear staining in tumor cells, whose staining property was stronger than that in normal epithelium, was considered positive. The staining intensity score was graded: 0, negative (-); 1, weak (+); 2, moderate (++); and 3, strong staining (+++) (Fig. 1). Immunoreactive scores were calculated by adding the cell-positive score and staining intensity score and then dividing by 2. Those with low (immunoreactive scores <2) and high (immunoreactive scores ≥2) were grouped separately for statistical analysis. The evaluation was performed twice, with the evaluator being blind to the clinical details.
- Statistical analysis
- Statistical analyses were performed using SPSS ver. 20.0 (SPSS Inc., Chicago, IL, USA). The correlation between protein expression and categorical variables was compared, if appropriate, using the Pearson χ2 test or Fisher exact probability test. Survival curves were estimated using the Kaplan-Meier method. The distribution of survival was comparatively studied using the log-rank test. Independent prognostic factors were determined using Cox's proportional hazard model. Statistical significance was set at p<0.05.
MATERIALS AND METHODS
- Clinicopathologic findings
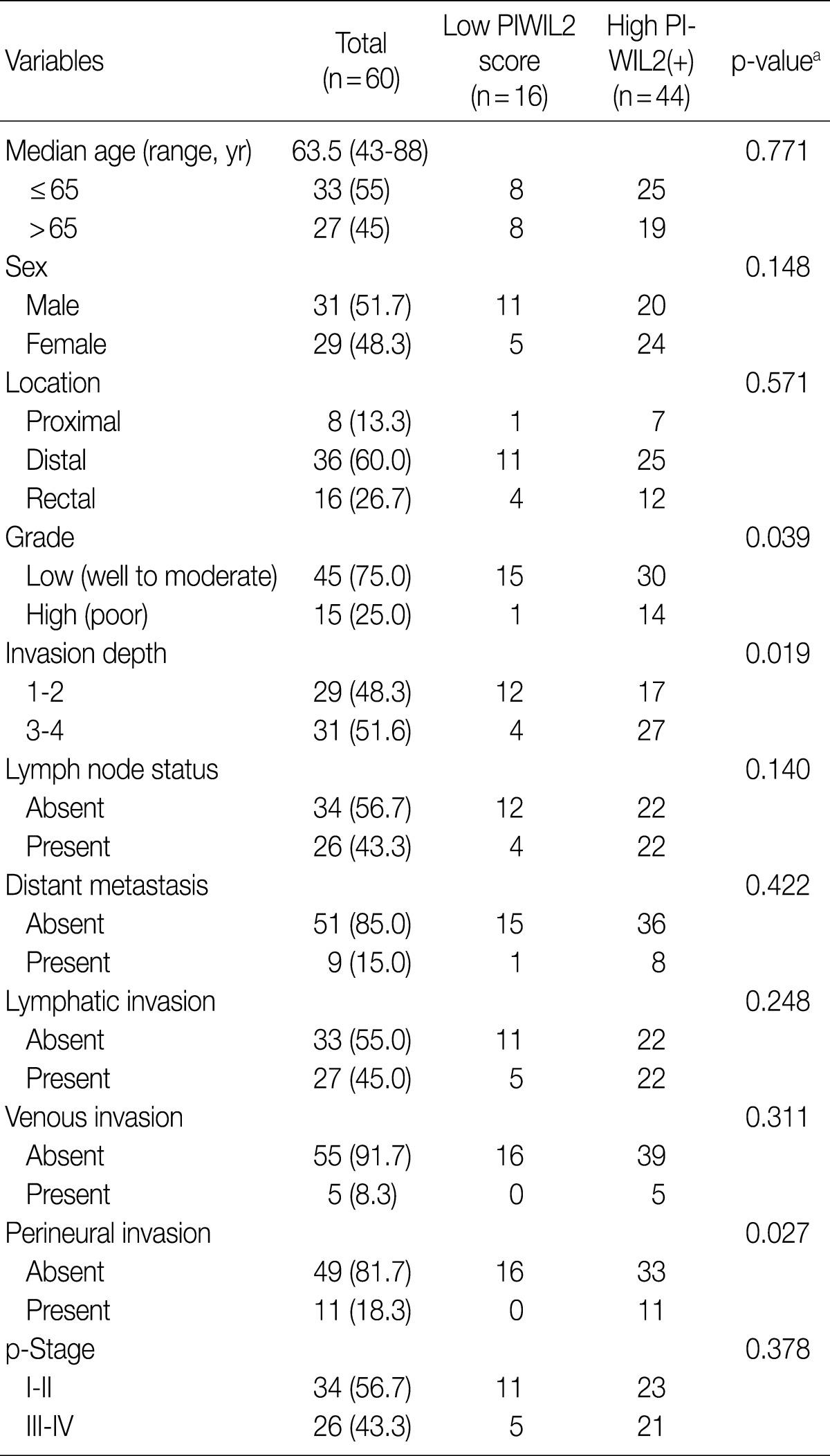
- In this retrospective study, we reviewed clinicopathologic findings from the 60 patients (31 men and 29 women). The mean age was 63.5 years (range, 43 to 88 years) and the mean follow-up period was 28 months (range, 2 to 72 months). Of the 60 resected cases of colorectal cancer, the locations of the cancers were the proximal colon (from the cecum to the ascending colon) in 8 patients, the distal colon (from the transverse colon to the rectosigmoid junction) in 36 patients and the rectum in 16 patients. In addition, there were 15 cases of colorectal cancer with a high-grade differentiation. The invasion depth was limited (T1 or T2) in 29 patients and advanced (T3 or T4) in 31 patients. There were 26 cases of colorectal cancer with a regional lymph metastasis and 9 cases with a distant metastasis. In addition, there were 27 cases of lymphatic invasion, 5 cases of vascular invasion and 11 cases of perineural invasion. Furthermore, there were 26 cases of colorectal cancer with a high p-stage (III and IV) (Table 1).
- Expression of PIWIL2
- In the non-cancerous surface and glandular epithelium of the colon, immunohistochemistry was positive for PIWIL2; it was stained in the nuclear and cytonuclear pattern, whose intensity ranged from entirely negative to weakly positive. In the neoplastic glands, PIWIL2 showed the same immunohistochemistry expression as the adjacent non-cancerous glands but it had a much stronger intensity. In contrast, PIWIL2 expression was mainly seen in the cytoplasmic regions on the surface of tumor and in the cytonuclear pattern of the invasive front of the colorectal cancer. There were 44 cases (73.3%) of colorectal cancer with high degree of PIWIL2 expression of an immunoreactive score ≥2.
- An independent comparison between PIWIL2 immunoreactivity and clinicopathologic characteristics
- Table 1 shows the correlation between PIWIL2 expression and clinicopathologic features in our clinical series of patients with colorectal cancer. The degree of PIWIL2 expression was higher in patients with a higher T stage (p=0.019). The higher degree of PIWIL2 expression was significantly more correlated with the lower degree of differentiation as compared with the lower degree of PIWIL2 expression (p=0.039). In addition, there was a significant correlation between the high degree of PIWIL2 expression and perineural invasion (p=0.027). There were no significant differences in such clinicopathologic features as age, sex, location, tumor grade, lymph node status, distant metastasis, lymphatic or venous invasion, and p-stage between the group of patients with lower PIWIL2 scores and that of their counterparts with higher PIWIL2 scores. There was a tendency, however, that the higher expression of PIWIL2 protein was correlated with older age, distal location, lymph node metastasis, distant metastasis, lymphatic invasion, venous invasion, and adverse pathological stages.
- Survival analysis
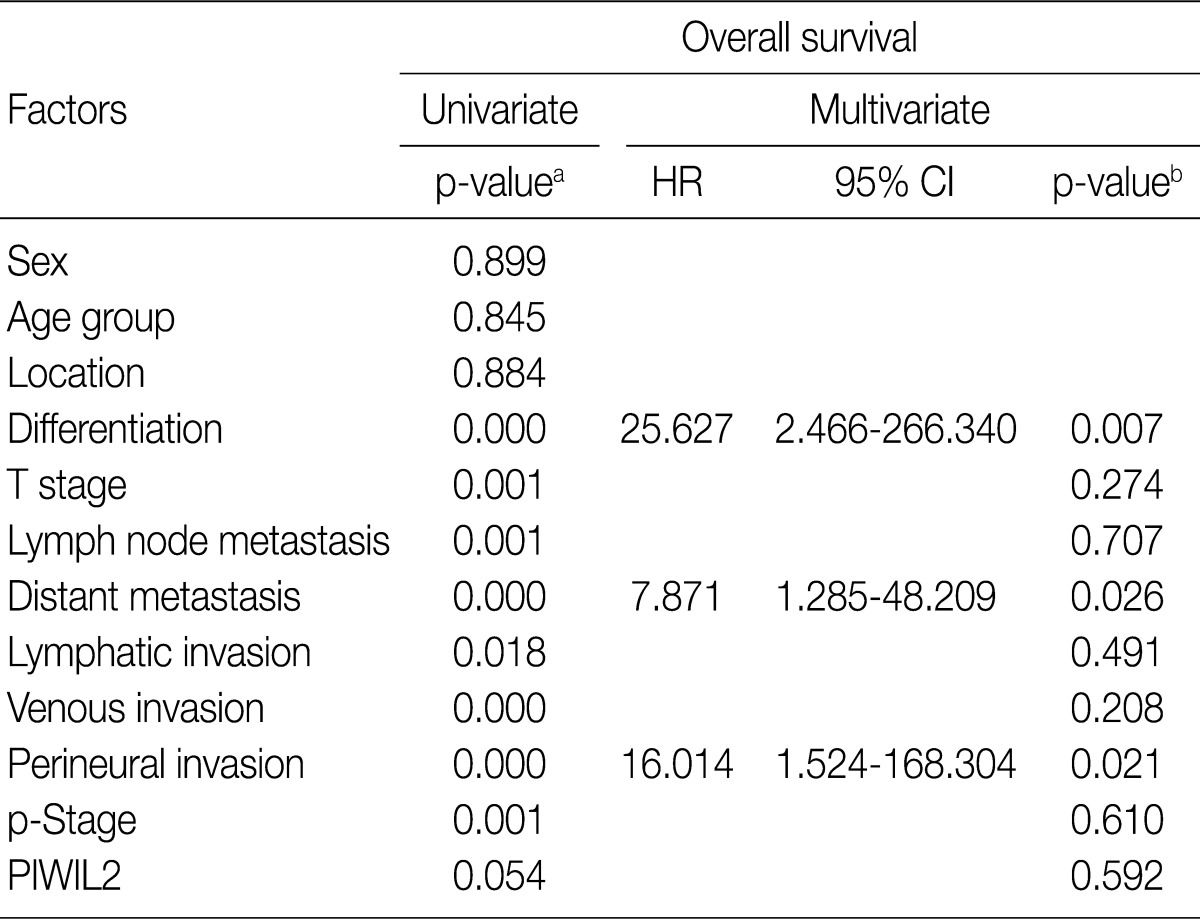
- For the survival analysis, Kaplan-Meier curves were plotted for univariate analysis. At the time of analysis, there were 9 cases (15%) of cancer-specific deaths. Cancer-specific survival was highly correlated with tumor differentiation, depth of invasion, lymph node metastasis, distant metastasis, lymphatic invasion, venous invasion, perineural invasion, and p-stage. Patients with low PIWIL2 scores had a longer OS compared to their counterparts high PIWIL2 scores (median survival period, 28 months vs 12 months, respectively), although survival benefits showed a statistically borderline significance (p=0.054) (Fig. 2). Multivariate analysis using the Cox proportional hazard model was performed to evaluate an independent predictor in patients with colorectal cancer. As shown in Table 2, there was a correlation between the OS and various clinicopathologic parameters. The OS was poorly correlated with sex, age and tumor location. But it was highly correlated with tumor differentiation, T stage, lymph node metastasis, distant metastasis, lymphatic invasion, venous invasion, perineural invasion and p-stage. On multivariate analysis, tumor differentiation (p=0.007), distant metastasis (p=0.026), and perineural invasion (p=0.021) were significantly associated with low OS. On multivariate analysis, however, PIWIL2 was not an independent prognostic factor (p=0.592).
RESULTS
- PIWIL2 belongs to the Piwi gene family, whose members are characterized by conserved PAZ and PIWI domains and play crucial roles in the self-renewal mechanism of stem and germ cells, RNA silencing and translational regulation in different organisms during evolution.4,10,11 These genes are highly conserved during evolution and play essential roles in stem-cell self-renewal, gametogenesis and RNA interference (RNAi) in diverse organisms ranging from Arabidopsis to humans.10,12
- Recent studies have shown that PIWIL2 is widely expressed in various types of human cancer and these include prostate, breast, gastrointestinal, ovarian, and endometrial cancers. It has also been reported that it is also prevalently expressed in breast tumor, rhabdomyosarcoma, and medulloblastoma in an animal experimental model using mice.5-7 Several previous studies have reported that Piwi genes play an important role in the tumorigenesis of various cancer cells. Liu et al.13 showed that the proportion of PIWIL1-positive cells was increased from 10% in normal gastric tissues to 76% in gastric cancer. Some investigators have also studied Piwi gene expression in breast and cervical cancers, thus describing it as a highly promising factor for its application to the cancer diagnosis and treatment.6,7,14
- Although identification of biomarkers for the detection of colon cancer is a critical issue for the treatment and cure of colon cancer, there are only a limited number of reports on PIWIL2 expression in colorectal cancers. Li et al.8 first conducted a systematic immunohistochemical study of human ARGONAUTE proteins in a cohort of 75 colon cancer specimens. According to these authors, ARGONAUTE proteins were over-expressed in colon cancer relative to its adjacent non-cancer tissue. These authors indicate that the expression of EIF2C2-4 and PIWIL4 might be increased in advanced tumors with a distant metastasis. This suggests that both genes might be involved in the promotion of tumor invasion. Furthermore, the above authors also suggested that the higher degree of PIWIL2 expression was significantly correlated with the occurrence of colon cancer and PIWIL2 might be a novel marker with early diagnostic significance in patients with colorectal cancer. But they failed to discover a significant correlation between Piwi expression and clinicopathologic parameters (age, sex, histological grade, lymph node status or p-stage). In our series, however, a positive immunohistochemistry of PIWIL2 was significantly correlated with a poorer differentiation of the tumor, direct tumor invasion and perineural invasion. The significance of PIWIL2 expression as a prognostic factor remains undetermined in patients with colorectal cancer despite its clear correlation with several clinicopathologic parameters of a prognostic value.
- He et al.6 suggested that PIWIL2 be used as a biomarker for early detection of cervical cancers because it appeared in various stages of cervical neoplasia. In this study, the degree of PIWIL2 expression was relatively higher in adenocarcinoma tissues as compared with their adjacent non-neoplastic ones (Fig. 1). These findings indicate that PIWIL2 might play an important role in tumor development. But we failed to determine whether PIWIL2 expression could solely be used as a biomarker in making a diagnosis of early colon cancer. This is because we did not examine it in the precancerous lesions. Further extensive studies are therefore warranted to establish the diagnostic value of PIWIL2 including precancerous lesions such as adenoma in patients with early colon cancer.
- How do ARGONAUTE proteins play an important role in colonic carcinogenesis? One possible mechanism is the regulation of PIWIL2 over the expression of genes related to proliferation and apoptosis. PIWIL2 mediates gene regulation through small RNA and methylation.15 A subset of small RNA species, termed Piwi-interacting RNAs, has been characterized in mammalian testis; these small RNAs interact with PIWI proteins (PIWIL1 and PIWIL2) to silence transpositional function in the germ cell genome.5 This implies that Piwi-interacting RNAs determines the specificity for DNA methylation, which is known to be a key component of gene regulation in cancer cells.
- For the specific mechanism of PIWIL2-mediating gene regulation in colorectal tumorigenesis, at the molecular level, several studies on the Piwi family5,11,16 demonstrated that PIWIL2 acts as an oncogene by inhibition of apoptosis and promotion of proliferation via STAT3/Bcl-XL signaling. PIWIL2 activates the expression of Bcl-XL, a member of the bcl-2 gene family, which serves as regulators of cell death. PIWIL2 was also able to induce STAT3 expression, which functions as downstream effectors of cytokine and growth factor receptor signaling. Persistent signaling of specific STATs has been demonstrated to directly contribute to the oncogenesis by stimulating cell proliferation and preventing apoptosis. Lee et al.14 demonstrated that PIWIL2 is expressed predominantly in breast cancer stem cells modulating their proliferation and anti-apoptotic state through the STAT3/Bcl-XL signaling pathway. Our results support that PIWIL2 expression is associated with the progression of colorectal cancer. But further studies are warranted to identify correlation between PIWIL2 and other factors related to cell proliferation and apoptosis.
- In this study, an immunohistochemistry was the only measure that we took to disclose the role of PIWIL2 in colorectal cancer. But the immunohistochemical findings are not necessarily consistent with the results of chromosomal analysis. Further complementary molecular studies are therefore warranted to advocate our results about PIWIL2 expression. This should particularly apply to the function of PIWIL2 as a cancer stem cell and the mechanisms by which PIWIL2 regulates the pathways of proliferation and anti-apoptosis, specifically through the STAT3/Bcl-XL signaling pathway.
- In conclusion, our results showed that the degree of PIWIL2 expression was relatively higher in colorectal cancer tissues and it was significantly correlated with a variety of clinicopathologic indicators for a poor prognosis. This indicates that PIWIL2-positive cells contribute to the progression of colorectal cancer. Our study demonstrated that the PIWIL2 protein may be used as a prognostic factor in the context of colorectal cancer. But further studies are warranted to examine the clinical significance of PIWIL2 and its usefulness as a biomarker.
DISCUSSION
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277-300. ArticlePubMed
- 2. Gao JX. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med 2008; 12: 67-96. ArticlePubMed
- 3. Carmell MA, Xuan Z, Zhang MQ, Hannon GJ. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev 2002; 16: 2733-2742. ArticlePubMed
- 4. Sasaki T, Shiohama A, Minoshima S, Shimizu N. Identification of eight members of the Argonaute family in the human genome small star, filled. Genomics 2003; 82: 323-330. ArticlePubMed
- 5. Lee JH, Schütte D, Wulf G, et al. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet 2006; 15: 201-211. ArticlePubMed
- 6. He G, Chen L, Ye Y, et al. Piwil2 expressed in various stages of cervical neoplasia is a potential complementary marker for p16. Am J Transl Res 2010; 2: 156-169. PubMedPMC
- 7. Liu JJ, Shen R, Chen L, et al. Piwil2 is expressed in various stages of breast cancers and has the potential to be used as a novel biomarker. Int J Clin Exp Pathol 2010; 3: 328-337. PubMedPMC
- 8. Li L, Yu C, Gao H, Li Y. Argonaute proteins: potential biomarkers for human colon cancer. BMC Cancer 2010; 10: 38.ArticlePubMedPMCPDF
- 9. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 2010; 7th ed. New York: Springer, 143-164.
- 10. Unhavaithaya Y, Hao Y, Beyret E, et al. MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J Biol Chem 2009; 284: 6507-6519. ArticlePubMedPMC
- 11. Lee JH, Engel W, Nayernia K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev 2006; 73: 173-179. ArticlePubMed
- 12. Kuramochi-Miyagawa S, Kimura T, Ijiri TW, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 2004; 131: 839-849. ArticlePubMedPDF
- 13. Liu X, Sun Y, Guo J, et al. Expression of hiwi gene in human gastric cancer was associated with proliferation of cancer cells. Int J Cancer 2006; 118: 1922-1929. ArticlePubMed
- 14. Lee JH, Jung C, Javadian-Elyaderani P, et al. Pathways of proliferation and antiapoptosis driven in breast cancer stem cells by stem cell protein piwil2. Cancer Res 2010; 70: 4569-4579. ArticlePubMedPDF
- 15. Kuramochi-Miyagawa S, Watanabe T, Gotoh K, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 2008; 22: 908-917. ArticlePubMedPMC
- 16. Ye Y, Yin DT, Chen L, et al. Identification of Piwil2-like (PL2L) proteins that promote tumorigenesis. PLoS One 2010; 5: e13406.ArticlePubMedPMC
REFERENCES
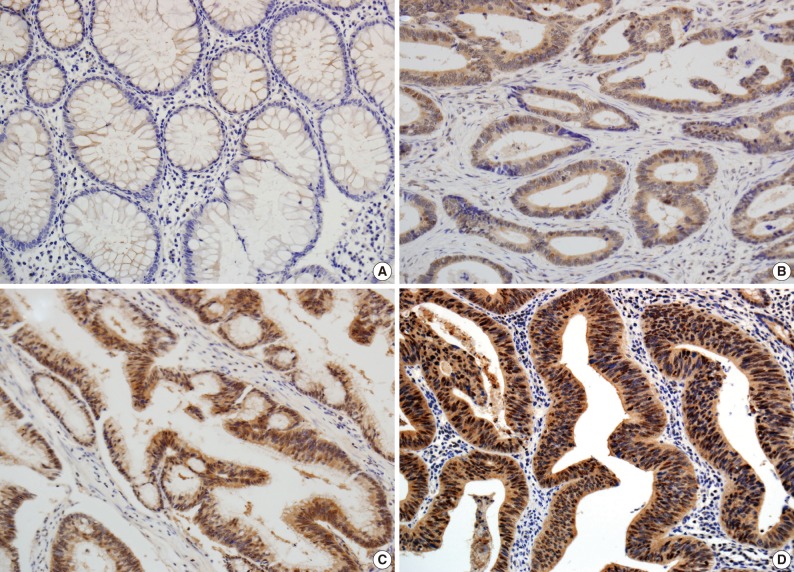
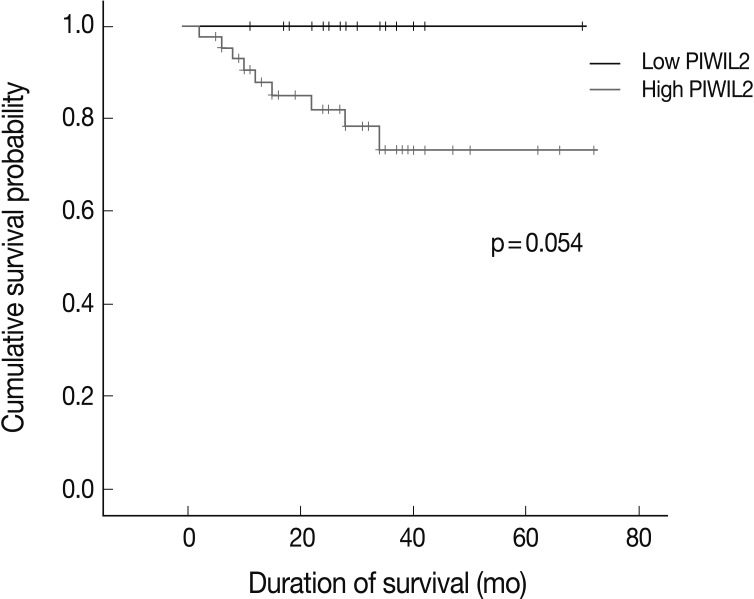
Figure & Data
References
Citations

- Piwil-2 represents a poor prognosticator in Merkel cell carcinomas that regulates oncoproteins, cell cycle arrest and SOX-2 expression
Stefan Janik, Johannes Pammer, Elisabeth Simader, Ulana Kotowski, Stefan Grasl, Roland Houben, Dragan Copic, Michael Mildner, Goran Mitulovic, Martin Bilban, Sophia Derdak, Markus Unterwurzacher, Klaus Kratochwill, Boban M. Erovic
Scientific Reports.2025;[Epub] CrossRef - Critical appraisal of the piRNA-PIWI axis in cancer and cancer stem cells
Elena Garcia-Borja, Frantisek Siegl, Rosana Mateu, Ondrej Slaby, Aleksi Sedo, Petr Busek, Jiri Sana
Biomarker Research.2024;[Epub] CrossRef - PIWI-interacting RNAs (PiRNAs) as emerging biomarkers and therapeutic targets in biliary tract cancers: A comprehensive review
Sahar Ahmadi Asouri, Esmat Aghadavood, Hamed Mirzaei, Alireza Abaspour, Mohammad Esmaeil Shahaboddin
Heliyon.2024; 10(13): e33767. CrossRef - Piwi-interacting RNAs (piRNAs) and Colorectal Carcinoma: Emerging Non-invasive diagnostic Biomarkers with Potential
Therapeutic Target Based Clinical Implications
Suman Kumar Ray, Sukhes Mukherjee
Current Molecular Medicine.2023; 23(4): 300. CrossRef - piRNA: A promising biomarker in early detection of gastrointestinal cancer
Melika Ameli Mojarad, Mandana Ameli Mojarad, Bahador Shojaee, Ehsan Nazemalhosseini-Mojarad
Pathology - Research and Practice.2022; 230: 153757. CrossRef - The function of novel small non‐coding RNAs (piRNAs, tRFs) and PIWI protein in colorectal cancer
Mandana AmeliMojarad, Melika AmeliMojarad, Jian Wang
Cancer Treatment and Research Communications.2022; 31: 100542. CrossRef - Morbid Obesity in Women Is Associated with an Altered Intestinal Expression of Genes Related to Cancer Risk and Immune, Defensive, and Antimicrobial Response
Ailec Ho-Plágaro, Cristina Rodríguez-Díaz, Concepción Santiago-Fernández, Carlos López-Gómez, Sara García-Serrano, Flores Martín-Reyes, Francisca Rodríguez-Pacheco, Alberto Rodríguez-Cañete, Guillermo Alcaín-Martínez, Luis Vázquez-Pedreño, Sergio Valdés,
Biomedicines.2022; 10(5): 1024. CrossRef - Emerging roles of PIWI-interacting RNAs (piRNAs) and PIWI proteins in head and neck cancer and their potential clinical implications
Trisha Chattopadhyay, Priyajit Biswal, Anthony Lalruatfela, Bibekanand Mallick
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2022; 1877(5): 188772. CrossRef - Cytokine-mediated crosstalk between cancer stem cells and their inflammatory niche from the colorectal precancerous adenoma stage to the cancerous stage: Mechanisms and clinical implications
Guanglin Cui, Ziqi Wang, Hanzhe Liu, Zhigang Pang
Frontiers in Immunology.2022;[Epub] CrossRef - piRNAs and PIWI proteins: From biogenesis to their role in cancer
Negar Balmeh, Samira Mahmoudi, Anasik Karabedianhajiabadi
Gene Reports.2021; 22: 101013. CrossRef - Epigenetic roles of PIWI proteins and piRNAs in colorectal cancer
Fatemeh Sadoughi, Seyyed Mehdi Mirhashemi, Zatollah Asemi
Cancer Cell International.2021;[Epub] CrossRef - PIWI interacting RNAs perspectives: a new avenues in future cancer investigations
Pooneh Mokarram, Maryam Niknam, Mohammadamin Sadeghdoust, Farnaz Aligolighasemabadi, Morvarid Siri, Sanaz Dastghaib, Hassan Brim, Hassan Ashktorab
Bioengineered.2021; 12(2): 10401. CrossRef - PIWI family proteins as prognostic markers in cancer: a systematic review and meta-analysis
Alexios-Fotios A. Mentis, Efthimios Dardiotis, Nicholas A. Romas, Athanasios G. Papavassiliou
Cellular and Molecular Life Sciences.2020; 77(12): 2289. CrossRef Identification of Genomic Alterations of Perineural Invasion in Patients with Stage II Colorectal Cancer
Hao Su, Chen Chang, Jiajie Hao, Xin Xu, Mandula Bao, Shou Luo, Chuanduo Zhao, Qian Liu, Xishan Wang, Zhixiang Zhou, Haitao Zhou
OncoTargets and Therapy.2020; Volume 13: 11571. CrossRef- Biological function and molecular mechanism of piRNA in cancer
Ghanbar Mahmoodi Chalbatani, Hassan Dana, Feridon Memari, Elahe Gharagozlou, Shirin Ashjaei, Peyman Kheirandish, Vahid Marmari, Habibollah Mahmoudzadeh, Farnaz Mozayani, Ali Reza Maleki, Ehsan Sadeghian, Elham Zainali Nia, Seyed Rohollah Miri, Neda zainal
Practical Laboratory Medicine.2019; 13: e00113. CrossRef - Molecular and Functional Characterization of the Somatic PIWIL1/piRNA Pathway in Colorectal Cancer Cells
Assunta Sellitto, Konstantinos Geles, Ylenia D’Agostino, Marisa Conte, Elena Alexandrova, Domenico Rocco, Giovanni Nassa, Giorgio Giurato, Roberta Tarallo, Alessandro Weisz, Francesca Rizzo
Cells.2019; 8(11): 1390. CrossRef - Emerging roles of piRNAs in cancer: challenges and prospects
Ye Cheng, Qian Wang, Wei Jiang, Yonghua Bian, Yang zhou, Anxing Gou, Wenling Zhang, Kai Fu, Weihong Shi
Aging.2019; 11(21): 9932. CrossRef - Differential expression of PIWIL2 in papillary thyroid cancers
Ibrahim Halil Erdogdu, Onder Yumrutas, M. Ozgur Cevik, Ibrahim Bozgeyik, Miyase Erdogdu, Hacı Mehmet Inan, Haydar Bagis
Gene.2018; 649: 8. CrossRef - High expression of PIWIL2 promotes tumor cell proliferation, migration and predicts a poor prognosis in glioma
Jinquan Li, Li Xu, Zhen Bao, Peng Xu, Hao Chang, Jingjing Wu, Yuanqi Bei, Liuwan Xia, Peizhang Wu, Gang Cui
Oncology Reports.2017; 38(1): 183. CrossRef - The meaning of PIWI proteins in cancer development
Monika Litwin, Anna Szczepańska-Buda, Aleksandra Piotrowska, Piotr Dzięgiel, Wojciech Witkiewicz
Oncology Letters.2017; 13(5): 3354. CrossRef - An unusual intragenic promoter ofPIWIL2contributes to aberrant activation of oncogenicPL2L60
Shan-Shan Liu, Ning Liu, Meng-Yao Liu, Lei Sun, Wu-Yan Xia, Hong-Min Lu, Yu-Jie Fu, Guo-Liang Yang, Juan-Jie Bo, Xiao-Xing Liu, Haizhong Feng, Hailong Wu, Lin-Feng Li, Jian-Xin Gao
Oncotarget.2017; 8(28): 46104. CrossRef - Manipulations in HIWI level exerts influence on the proliferation of human non-small cell lung cancer cells
YUGUANG WANG, JIA LIU, GUANGYAO WU, FANG YANG
Experimental and Therapeutic Medicine.2016; 11(5): 1971. CrossRef - High expression of piwi-like RNA-mediated gene silencing 1 is associated with poor prognosis via regulating transforming growth factor-β receptors and cyclin-dependent kinases in breast cancer
JIWEI CAO, GANG XU, JING LAN, QINGQING HUANG, ZUXIONG TANG, LIPING TIAN
Molecular Medicine Reports.2016; 13(3): 2829. CrossRef - PIWI-Interacting RNAs in Gliomagenesis: Evidence from Post-GWAS and Functional Analyses
Daniel I. Jacobs, Qin Qin, Michael C. Lerro, Alan Fu, Robert Dubrow, Elizabeth B. Claus, Andrew T. DeWan, Guilin Wang, Haifan Lin, Yong Zhu
Cancer Epidemiology, Biomarkers & Prevention.2016; 25(7): 1073. CrossRef - Hiwi downregulation, mediated by shRNA, reduces the proliferation and migration of human hepatocellular carcinoma cells
YINGJUN XIE, YONGSHENG YANG, DEGANG JI, DAN ZHANG, XIAOXIAO YAO, XUEWEN ZHANG
Molecular Medicine Reports.2015; 11(2): 1455. CrossRef - Emerging roles for PIWI proteins in cancer
Y. Tan, L. Liu, M. Liao, C. Zhang, S. Hu, M. Zou, M. Gu, X. Li
Acta Biochimica et Biophysica Sinica.2015; 47(5): 315. CrossRef - Genetic variants in noncoding PIWI‐interacting RNA and colorectal cancer risk
Haiyan Chu, Liping Xia, Xiaonan Qiu, Dongying Gu, Linjun Zhu, Jing Jin, Gaoyun Hui, Qiuhan Hua, Mulong Du, Na Tong, Jinfei Chen, Zhengdong Zhang, Meilin Wang
Cancer.2015; 121(12): 2044. CrossRef - Piwil2 modulates the invasion and metastasis of prostate cancer by regulating the expression of matrix metalloproteinase-9 and epithelial-mesenchymal transitions
YANFENG YANG, XUEPEI ZHANG, DONGKUI SONG, JINXING WEI
Oncology Letters.2015; 10(3): 1735. CrossRef - Piwil 2 Expression Is Correlated with Disease-Specific and Progression-Free Survival of Chemotherapy-Treated Bladder Cancer Patients
Helge Taubert, Sven Wach, Rudolf Jung, Michael Pugia, Bastian Keck, Simone Bertz, Elke Nolte, Robert Stoehr, Jan Lehmann, Carsten-H. Ohlmann, Michael Stöckle, Bernd Wullich, Arndt Hartmann
Molecular Medicine.2015; 21(1): 371. CrossRef - Overexpression of Hiwi Promotes Growth of Human Breast Cancer Cells
Da-Wei Wang, Zhao-Hui Wang, Ling-Ling Wang, Yang Song, Gui-Zhen Zhang
Asian Pacific Journal of Cancer Prevention.2014; 15(18): 7553. CrossRef - Lactobacillus acidophilus and Lactobacillus crispatus Culture Supernatants Downregulate Expression of Cancer-testis Genes in the MDA-MB-231 Cell Line
Rosa Azam, Soudeh Ghafouri-Fard, Mina Tabrizi, Mohammad-Hossein Modarressi, Reza Ebrahimzadeh-Vesal, Maryam Daneshvar, Maryam Beigom Mobasheri, Elahe Motevaseli
Asian Pacific Journal of Cancer Prevention.2014; 15(10): 4255. CrossRef - Expression Profiles of PIWIL2 Short Isoforms Differ in Testicular Germ Cell Tumors of Various Differentiation Subtypes
Ildar V. Gainetdinov, Yulia V. Skvortsova, Elena A. Stukacheva, Oksana S. Bychenko, Sofia A. Kondratieva, Marina V. Zinovieva, Tatyana L. Azhikina, Jian-Xin Gao
PLoS ONE.2014; 9(11): e112528. CrossRef - PIWIL2 induces c-Myc expression by interacting with NME2 and regulates c-Myc-mediated tumor cell proliferation
Youlin Yao, Chao Li, Xiaoyan Zhou, Yu Zhang, Yilu Lu, Jianhui Chen, Xulei Zheng, Dachang Tao, Yunqiang Liu, Yongxin Ma
Oncotarget.2014; 5(18): 8466. CrossRef - Novel dimensions of piRNAs in cancer
Yuping Mei, David Clark, Li Mao
Cancer Letters.2013; 336(1): 46. CrossRef


Fig. 1
Fig. 2


p-Stage, pathologic stage. aFisher exact test, p<0.05.
HR, harzard ratio; CI, confidence interval; p-Stage, pathologic stage. aKaplan-Meier method using log-rank test, p<0.05; bCox proportional hazard model, p<0.05.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article