Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(5); 2014 > Article
-
Case Study
Nodular Fasciitis of the Parotid Gland, Masquerading as Pleomorphic Adenoma - Chung Su Hwang, Chang Hun Lee, Ahrong Kim, Nari Shin, Won Young Park, Min Gyoung Park1, Do Youn Park
-
Korean Journal of Pathology 2014;48(5):366-370.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.366
Published online: October 27, 2014
Department of Pathology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
1Department of Pathology, Dong-A University College of Medicine, Busan, Korea
- Corresponding Author: Chang Hun Lee, M.D. Department of Pathology and Medical Research Institute, Pusan National University Hospital, Pusan National University School of Medicine, 179 Gudeok-ro, Seo-gu, Busan 602-739, Korea Tel: +82-51-240-7422 Fax: +82-51-240-7579 E-mail: cnlee@pusan.ac.kr
• Received: May 19, 2014 • Revised: August 7, 2014 • Accepted: August 19, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- It is difficult to distinguish nodular fasciitis (NF) from other neoplasm of the parotid gland, especially pleomorphic adenoma (PA) by fine needle aspiration cytology. A 39-year-old female noticed a mass in the parotid region. The aspirate material showed cohesive parts composed of the cells that had oval or spindle-shaped nuclei and relatively abundant cytoplasm and some cells with plasmacytoid features. The background substance was fibromyxoid. PA was diagnosed based on the cytologic findings. Subsequently, parotidectomy was performed and NF was diagnosed based on histologic and immunohistochemical findings. NF in the parotid region is rare and may be misdiagnosed as other benign or malignant tumors of the parotid gland. The clinical history of rapid growth and the presence of mitoses and inflammatory cells help to distinguish NF from PA. In addition, immunohistochemical stains for smooth muscle actin and CD68 are useful to confirm the diagnosis of NF.
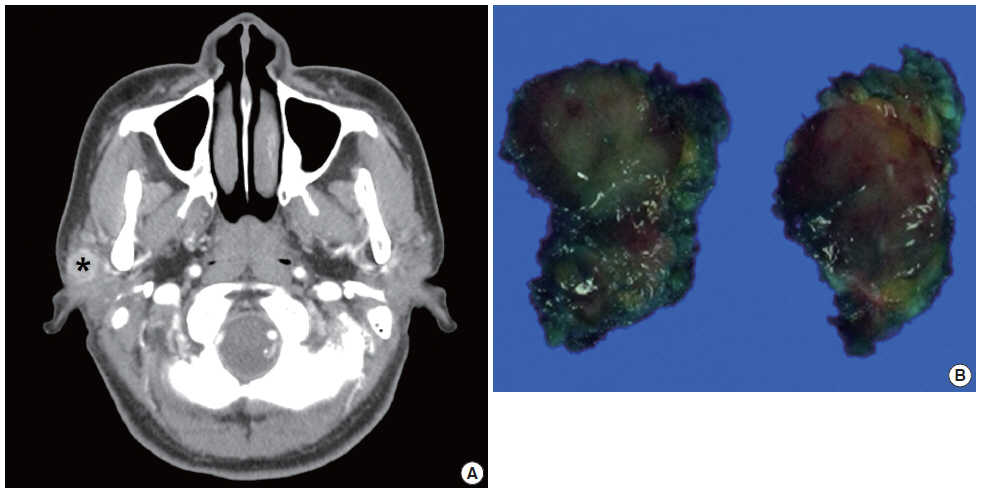
- The patient was a 39-year-old female who had a mass in the right parotid region. The mass was found 2 months previously and had subsequently showed a gradual increase in size. No history of trauma or injury was identified. On physical examination, a firm, mobile, well-circumscribed mass was palpable in the subcutaneous tissue of the right cheek. No nerve paralysis or paresthesia was identified. At the same time, the patient was diagnosed with papillary carcinoma in the right thyroid gland by fine needle aspiration cytology (FNAC). Before the patient underwent a right hemithyroidectomy, ultrasonography and FNAC were performed. Ultrasonography showed a 2.3-cm-sized irregular shaped mass in the right parotid gland with ill-defined margins. Subsequently, computed tomography scan showed a 1.9×1.3×1.2-cm-sized, heterogeneously enhancing lobulated mass (Fig. 1A). Laboratory examination data were within normal limits. FNAC was performed for the mass of the right parotid region.
- Cytology
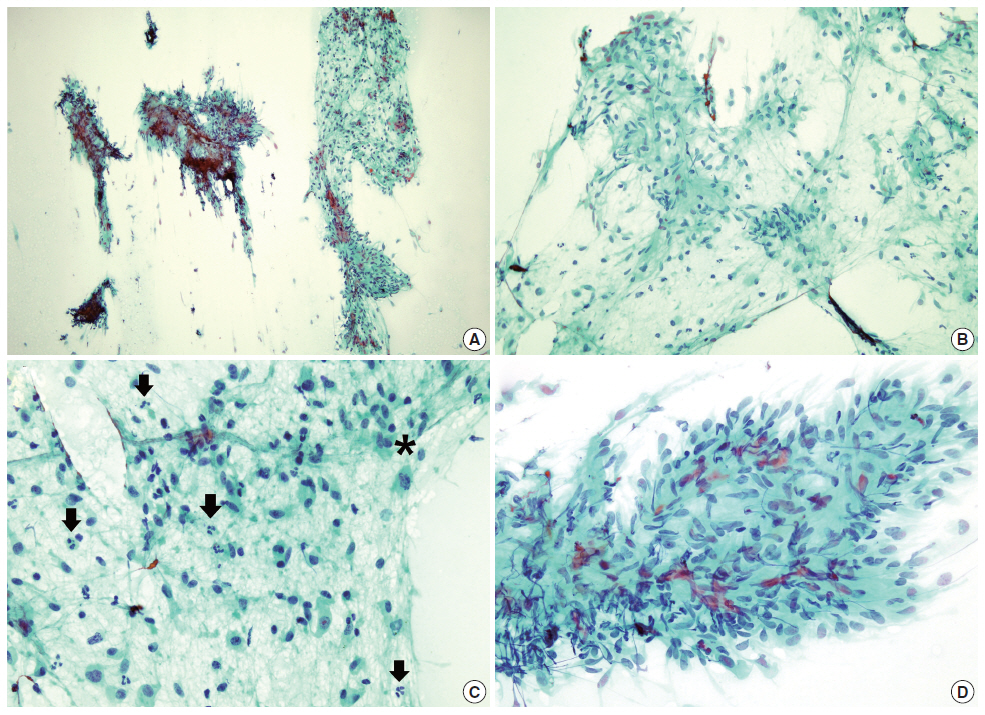
- Papanicolaou staining from the aspirate showed moderately increased cellularity and was composed of isolated and cohesive cells with loose fibrous stroma. In cohesive parts, the cells consisted of short bundles or fascicles that did not form a characteristic pattern of arrangement. Most of the cells had oval or spindle-shaped nuclei and relatively abundant cytoplasm with rather indistinct cytoplasmic outline. Some cells had plasmacytoid features. Cellular pleomorphism was minimal. Inflammatory cells were also diffusely scattered (Fig. 2). Neither necrosis nor brisk mitotic activity was seen. Giemsa staining revealed that the mucoid-like stromal materials were metachromatic, which was reminiscent of the fibromyxoid matrix that is commonly seen in PA. Therefore, PA was initially diagnosed based on the cytologic findings.
- Histology
- After FNAC diagnosis of the mass, superficial parotidectomy was performed for treatment and histological confirmation. The removed specimen was 2.3 g in weight and 2.4×1.8×1.2 cm in dimensions. On section, the tumor was well demarcated, but not encapsulated. The cut surface of the mass was solid and gray white (Fig. 1B).
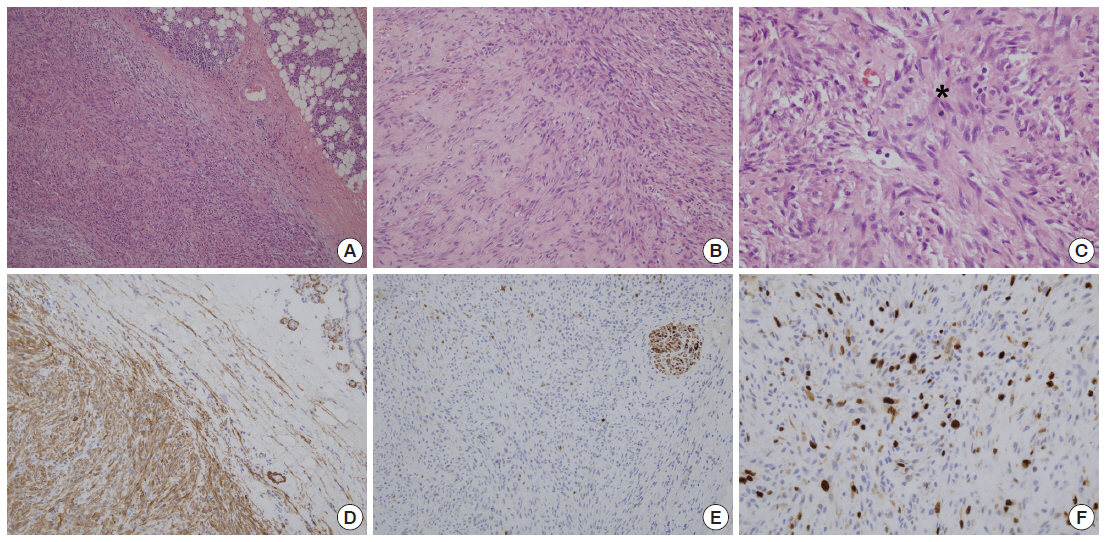
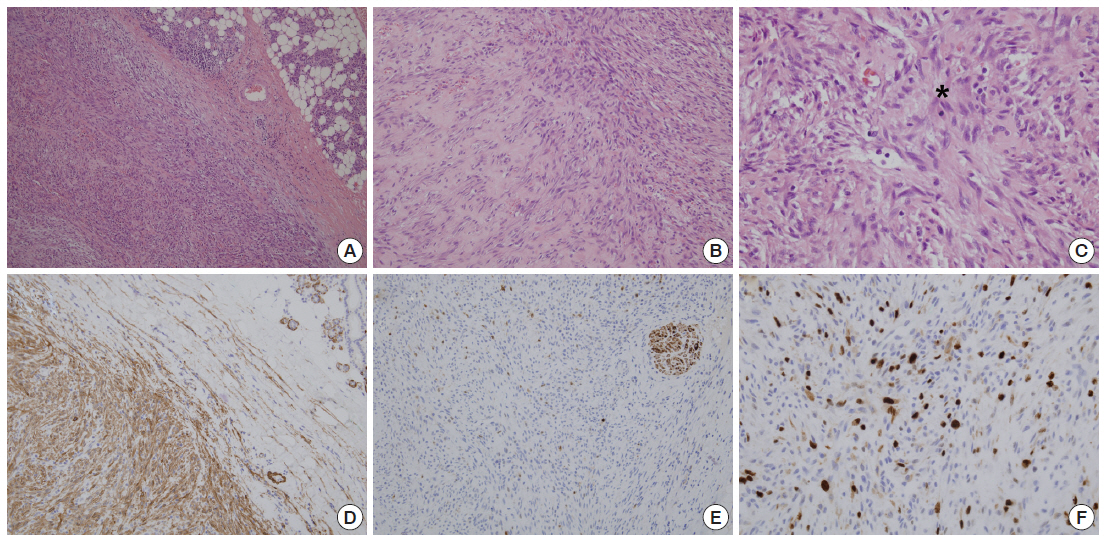
- Histologically, the mass was predominantly composed of relatively uniform spindle cells which showed moderate cellularity. These spindle-shaped cells were arranged in short fascicles arranged in haphazard pattern. Focally, a storiform growth pattern was also noted. The tumor cells had centrally located round to oval nuclei with finely granular chromatin and one or two inconspicuous nucleoli. The stromal part was predominantly collagenous with focal myxoid areas. Glands or squamous structures were not noted. Some inflammatory cells including lymphocytes, neutrophils, and histiocytes were found. Many small thin-walled vessels and extravasated erythrocytes were scattered diffusely. Regarding mitotic count, about 8 per 10 high power fields were found, but no atypical mitotic figure was identified (Fig. 3A–C). There was no necrosis. Combined with these histologic findings, NF was diagnosed. Additionally, immunohistochemical staining was performed. The spindle cells showed positivity to vimentin (1:1,000, V9, Zymed, South San Francisco, CA, USA), smooth muscle actin (1A4, 1:400, Dako, Glostrup, Denmark), but negativity to S-100 (1:1,000, polyclonal antibody, Novocastra, Newcastle Upon Tyne, UK), desmin (1:200, D33, Dako), CD34 (1:400, QBEnd-10, Dako), and pan-cytokeratin (1:500, AE1&AE3, Labvision, Fremont, CA, USA) (Fig. 3D–F). The results of immunohistochemical staining supported the diagnosis of NF
CASE REPORT
- Soft-tissue tumors or lesions of the head and neck are very rare compared with the far more common epithelial tumors that arise in these anatomic locations. Among those lesions, NF arising in the parotid gland is especially exceptional, so it is essentially impossible to give any estimate of their relative frequency.
- Most cases of NF spontaneously resolve in 1–16 weeks after diagnosis based on FNAC findings[4]. It is thought that NF is triggered by a local injury or local inflammatory episode. However, only a small number of patients are confirmed to have histories of trauma[5]. In our patient, no trauma history was present.
- Until now, there have been few reports about the cytologic features of NF arising in a salivary gland such as the parotid, and there are no definite criteria that aid pathologists in diagnosing NF of the gland based on cytology alone. According to the reports which have been published so far, the general cytology of NF shows aspirate material characterized by smears of low to moderate cellularity with a varying degree of myxoid background. The cells, which are composed of mainly fibrillary spindle cells, some plasmacytoid cells and ganglion-like cells, lie singly and in small clusters. Cytoplasm is present in moderate amounts and has a wispy flowing appearance. Nuclei are bland with an open chromatin pattern and small but distinct nucleoli. The cells do not show bona fide cytologic atypia or atypical mitoses.
- The cytologic features of NF are often similar to many benign or malignant neoplasms arising in the parotid area, especially PA. PA is the most common salivary neoplasm and is 10 times more common in the parotid gland than in the submandibular gland. Therefore, if FNAC is performed from the tumor of the parotid area and the aspirated material shows many spindle shaped cells or plasmacytoid cells with a myxoid background, the cytologists who examine the sample may suspect at first glance that the tumor is PA, instead of NF. In comparison with NF, the typical cytology of PA consists of small round or cuboidal epithelial cells and occasional spindled or plasmacytoid mesenchymal cells with fibrillary, myxochondroid stromal substance. The tumor can also show a varied cytomorphologic appearance and a varied proportion between epithelial and mesenchymal components. However, because FNAC itself represents only a small portion of the tumor, the aspirated material of the mass can reveal a different proportion of cell components and limited cytologic features each time, compared with those of typical PA.
- Some literature and case reports suggest criteria to distinguish NF from PA in cytology. If the aspirate material is composed of plasmacytoid cells on the whole, this more strongly PA. The presence of chondromyxoid matrix also helps to diagnose PA rather than NF. On the contrary, NF may be suspected when inflammatory cells or mitoses are seen in the aspirate materials[6]. In the present case, some plasmacytoid cells were seen, but their presence was not enough to consider PA. Although no ganglion-like cells or multinucleated cells were present, we found that inflammatory cells were frequently scattered in all slides and one mitotic cell was also present when reviewing our cytology slides (Fig. 2C). Otherwise, ductal or sheet-like epithelial structures which may be present in FNAC of PA were not noted in this case. The clinical course may also be important. In particular, rapid growth of the lesion is helpful for suggesting NF. Typically, it presents as a rapid-forming mass that is recognized in 1–2 weeks and it is out of ordinary for the lesion to be present for more than 3 months before surgery[7]. Immunohistochemistry can provide evidence to support the diagnosis of NF. Kong and Cha[8] reported that an immunohistochemical stain for smooth muscle actin showed cytoplasmic reactivity in the spindle cells, but immunohistochemical stain for CD68 can be also helpful when smooth muscle actin is negative, because of both the fibrohistiocytic and myofibroblastic characters of the cells. Therefore, if there is a clinical history of rapid tumor growth, NF can be suggested in the differential diagnosis, and pathologists subsequently can make an effort to find mitotic cells, multinucleated cells, ganglion-like cells or inflammatory cells such as neutrophils or lymphocytes. It may be helpful to perform immunohistochemical staining for alpha-smooth muscle actin and CD68 in case of the presence of these cytologic features suggesting NF.
- In some cases, spindle cell-dominant NF may be difficult to cytologically distinguish from fibromatosis. However, the latter lacks a myxoid background, actual mitotic activity, and inflammation. Immunohistochemically, β-catenin may be helpful in the differential diagnosis, because nuclear positive staining for β-catenin is the specific finding in fibromatosis, whereas it is absent in NF. A benign peripheral nerve sheath tumor such as neurofibroma may also be included in the list of differential diagnoses. Neurofibroma commonly consists of a slender wavy spindle cell population of low to moderate cellularity which may be accompanied by a loose mucoid background. However, NF shows short fibrillary spindle cells often arranged in a whorllike pattern. In confusing cases, immunohistochemical stain for S-100 protein, using cell block or liquid-based preparation samples, is very helpful[9]. Among seriously misinterpreted cases of NF, a majority were misdiagnosed as low-grade sarcoma from frequent mitoses, cellular spindle cell population, and low-grade nuclear atypia[9], leading to unnecessary extensive surgical resection. Taking a careful look at the FNAC smear, however, NF can be distinguished by its more loosely arranged short bundles of fibroblasts, abundant isolated cells, prominent mucoid matrix, mild cytologic atypia, absence of atypical mitoses, and no necrotic background.
- In conclusion, the cytologic diagnosis of NF in the parotid region may be difficult to distinguish from other neoplasms, especially PA, because of its extreme rarity and cytologic similarity. However, it is crucial to precisely diagnose NF since it is a self-limiting reactive process treated by conservative surgery, or even follow-up, whereas PA should be treated with superficial parotidectomy[10]. Therefore, careful evaluation for smears with spindle cell components is required to avoid a misdiagnosis of spindle cell-dominant lesion arising in the parotid region.
DISCUSSION
Acknowledgments
Fig. 1.Computed tomography image and gross finding of the parotid mass. (A) Computed tomography scan shows a 1.9×1.3×1.2-cm-sized, heterogeneously enhancing lobulated mass (*). (B) The excised tumor is relatively well demarcated, but not encapsulated. The cut surface of the mass is solid and gray white.
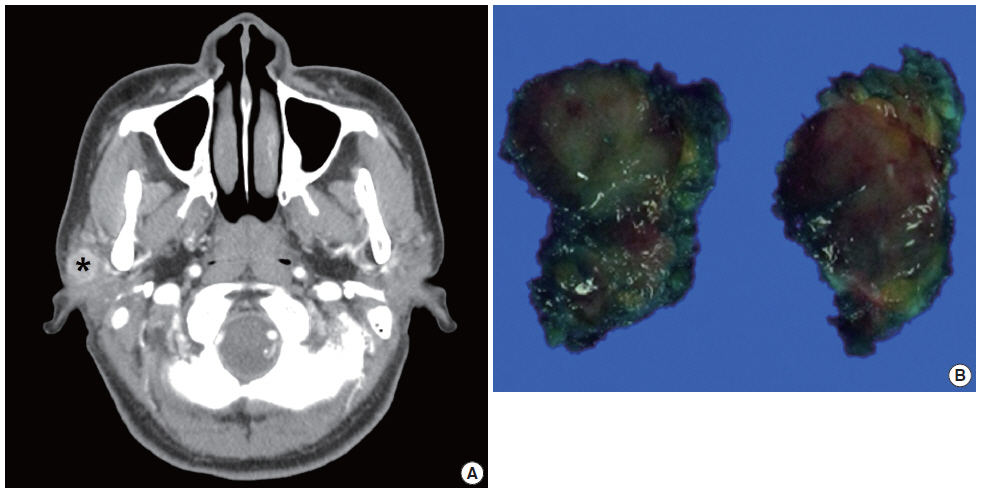

Fig. 2.The cytologic findings of the parotid mass. (A) On low power, the aspirate shows moderately increased cellular areas and rather fibrous stroma. (B) The cells in the loose fibrocellular part have oval or spindle-shaped nuclei with abundant cytoplasm and are arranged in short loose bundles with myxoid background. (C) Some cells seem to have plasmacytoid features and many inflammatory cells are present (arrows). One mitotic cell is also found (*). (D) The cells in some areas consist of tight fascicles (A–D, Papanicolaou stain).
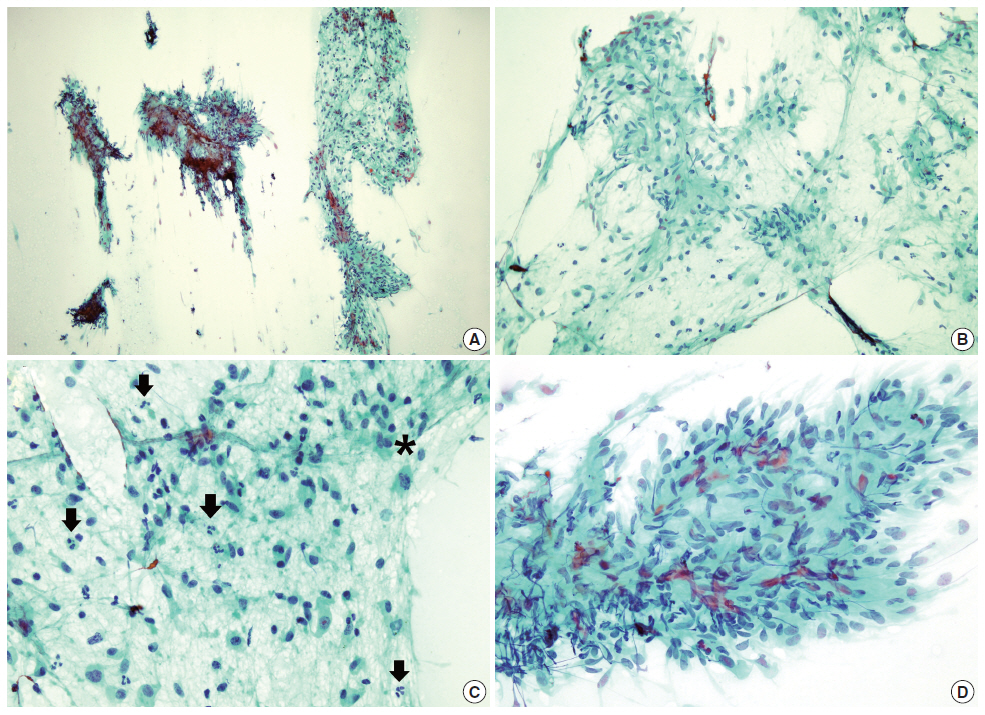

Fig. 3.The histologic and immunohistochemical findings of the parotid mass. (A) The tumor is composed of relatively uniform spindle-shaped cells arranged in short fascicles. (B) The stroma focally shows loose myxoid features and diffusely scattered extravasated erythrocytes are also noted. (C) A mitotic cell (*) is present. The spindle cells are positive for alpha-smooth muscle actin (D), and negative for S-100 (E). (F) Ki-67 nuclear labeling cells are mildly increased.

- 1. Peng WX, Kudo M, Yamamoto T, et al. Nodular fasciitis in the parotid gland: a case report and review of the literature. Diagn Cytopathol 2013; 41: 829-33. ArticlePubMed
- 2. Borumandi F, Cascarini L, Mallawaarachchi R, Sandison A. The chameleon in the neck: nodular fasciitis mimicking malignant neck mass of unknown primary. Int J Surg Case Rep 2012; 3: 501-3. ArticlePubMedPMC
- 3. Saad RS, Takei H, Lipscomb J, Ruiz B. Nodular fasciitis of parotid region: a pitfall in the diagnosis of pleomorphic adenomas on fine-needle aspiration cytology. Diagn Cytopathol 2005; 33: 191-4. ArticlePubMed
- 4. Hidir Y, Arslan HH, Gunhan O, Satar B. Case report: nodular fasciitis of the parotid region. J Laryngol Otol 2011; 125: 1312-4. ArticlePubMed
- 5. Bernstein KE, Lattes R. Nodular (pseudosarcomatous) fasciitis, a nonrecurrent lesion: clinicopathologic study of 134 cases. Cancer 1982; 49: 1668-78. ArticlePubMed
- 6. Abendroth CS, Frauenhoffer EE. Nodular fasciitis of the parotid gland. Report of a case with presentation in an unusual location and cytologic differential diagnosis. Acta Cytol 1995; 39: 530-4. PubMed
- 7. Maly B, Maly A. Nodular fasciitis of the breast: report of a case initially diagnosed by fine needle aspiration cytology. Acta Cytol 2001; 45: 794-6. PubMed
- 8. Kong CS, Cha I. Nodular fasciitis: diagnosis by fine needle aspiration biopsy. Acta Cytol 2004; 48: 473-7. PubMed
- 9. Enzinger FM, Weiss SW. Soft tissue tumors. 4th ed. St. Louis: Mosby, 2001; 247-307.
- 10. Foschini MP, Scarpellini F, Gown AM, Eusebi V. Differential expression of myoepithelial markers in salivary, sweat and mammary glands. Int J Surg Pathol 2000; 8: 29-37. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- A case report of nodular fasciitis of the parotid gland: An entity of concern
Andrea Varazzani, Laura Tognin, Silvia Eleonora Gazzani, Luigi Corcione, Tito Poli
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2024; 36(3): 422. CrossRef - Cytologic findings of nodular fasciitis in the parotid region
Yoshihiro KATO, Keiko TSUCHIDO, Makoto YAMADA, Yasuhiro AKAZAWA, Shogo MIZUNO, Yoshiro OTSUKI, Shin-ichi SHIMIZU, Hiroshi KOBAYASHI
The Journal of the Japanese Society of Clinical Cytology.2024; 63(3): 129. CrossRef - A Case of Parotid Nodular Fasciitis in Children and Literature Review
豆豆 屈
Advances in Clinical Medicine.2024; 14(10): 854. CrossRef - A Rare Case of Parotid Nodular Fasciitis in a Six-Month-Old Female
Mazin Merdad, Linah Qasim, Mohammed Nujoom, Hani Z Marzouki, Abdulaziz Neazy
Cureus.2023;[Epub] CrossRef - A rare case of nodular fasciitis presenting as a parotid tumor: Clues to cytodiagnosis
Seetu Palo, Chitrawati Bal Gargade
Journal of Laboratory Physicians.2023; 16: 124. CrossRef - Condylar Reshape in Orthognathic Surgery: Morphovolumetric and Densitometric Analysis Based on 3D Imaging and Digital Workflow
Vincenzo Abbate, Giovanni Audino, Giovanni Dell’Aversana Orabona, Marco Friscia, Paola Bonavolontà, Carmelo Lo Faro, Umberto Committeri, Carlos Navarro Cuéllar, Giorgio Iaconetta, Luigi Califano
Journal of Maxillofacial and Oral Surgery.2022; 21(2): 501. CrossRef - Nodular fasciitis of the submandibular gland
Ting Suen Wong, Richard Wei Chern Gan, Laszlo Karsai, Bun Yin Winson Wong
BMJ Case Reports.2022; 15(4): e245584. CrossRef - Nodular fasciitis in cervicofacial region: a rare case description and literature review
Vincenzo Abbate, Giovanni Dell’Aversana Orabona, Giovanni Audino, Antonio Romano, Paola Bonavolontà, Daniela Russo, Silvia Varricchio, Roberto Ferrigno, Giorgio Iaconetta, Luigi Califano
Oral Surgery.2022; 15(4): 550. CrossRef - Nodular fasciitis of the parotid gland engulfing the facial nerve: a conservative approach
Stephen Bennett, Kristian Hutson, Olakunle Ajayi, Andreas Hilger
BMJ Case Reports.2019; 12(10): e231203. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Nodular Fasciitis of the Parotid Gland, Masquerading as Pleomorphic Adenoma



Fig. 1. Computed tomography image and gross finding of the parotid mass. (A) Computed tomography scan shows a 1.9×1.3×1.2-cm-sized, heterogeneously enhancing lobulated mass (*). (B) The excised tumor is relatively well demarcated, but not encapsulated. The cut surface of the mass is solid and gray white.
Fig. 2. The cytologic findings of the parotid mass. (A) On low power, the aspirate shows moderately increased cellular areas and rather fibrous stroma. (B) The cells in the loose fibrocellular part have oval or spindle-shaped nuclei with abundant cytoplasm and are arranged in short loose bundles with myxoid background. (C) Some cells seem to have plasmacytoid features and many inflammatory cells are present (arrows). One mitotic cell is also found (*). (D) The cells in some areas consist of tight fascicles (A–D, Papanicolaou stain).
Fig. 3. The histologic and immunohistochemical findings of the parotid mass. (A) The tumor is composed of relatively uniform spindle-shaped cells arranged in short fascicles. (B) The stroma focally shows loose myxoid features and diffusely scattered extravasated erythrocytes are also noted. (C) A mitotic cell (*) is present. The spindle cells are positive for alpha-smooth muscle actin (D), and negative for S-100 (E). (F) Ki-67 nuclear labeling cells are mildly increased.
Fig. 1.
Fig. 2.
Fig. 3.
Nodular Fasciitis of the Parotid Gland, Masquerading as Pleomorphic Adenoma

 E-submission
E-submission