Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(5); 2014 > Article
-
Brief Case Report
Pulmonary Hodgkin Lymphoma in a Patient with Crohn’s Disease - Jae-Young Park, Juhie Lee
-
Korean Journal of Pathology 2014;48(5):387-389.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.387
Published online: October 27, 2014
Department of Pathology, Kyung Hee University School of Medicine, Seoul, Korea
- Corresponding Author: Juhie Lee, M.D. Department of Pathology, Kyung Hee University Hospital, 23 Kyungheedae-ro, Dongdaemun-gu, Seoul 130-872, Korea Tel: +82-2-958-8741, Fax: +82-2-958-8730, E-mail: leejuhie@khmc.or.kr
• Received: July 30, 2013 • Revised: October 1, 2013 • Accepted: October 10, 2013
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
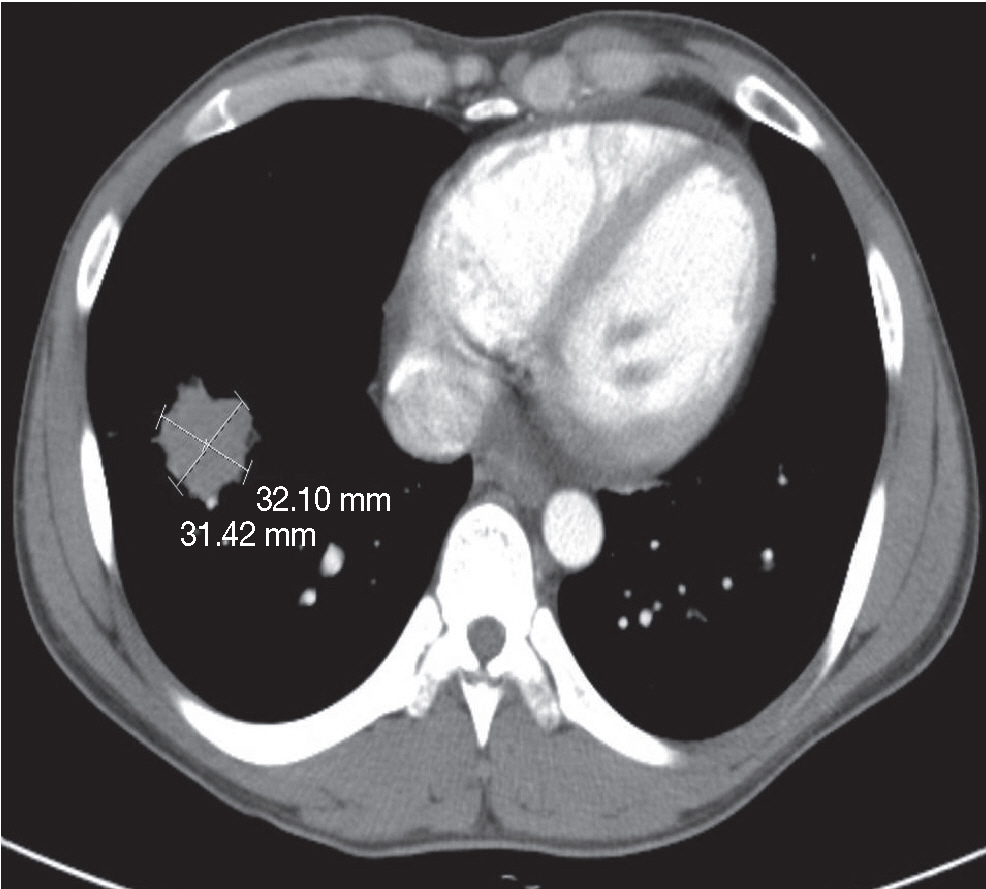
- A 27-year-old male was admitted to the emergency room with nausea, vomiting and diffuse abdominal pain. He had a longstanding history of CD (since 2009) and was being treated with prednisone (50 mg/day), mesalazine (1.5 g/day), and azathioprine (50 mg/day). He received adalimumab (tumor necrosis factor-α [TNF-α] antagonist) twice (160 mg at week 0 and 80mg at week 2) in order to control recurrent abdominal discomfort and diarrhea. Abdominal computed tomography (CT) and X-ray revealed a small bowel obstruction and a suspicious masslike lesion in the right lower lobe of lung. Subsequent chest CT showed a mass approximately 3 cm in diameter in the right lower lobe (Fig. 1) and another two nodules in the right upper lobe with multiple lymphadenopathy in the mediastinum, neck, and supraclavicular areas. We performed segmental resection of the small bowel, wedge resection of the right lower lobe of lung, and mediastinal lymph node biopsy.
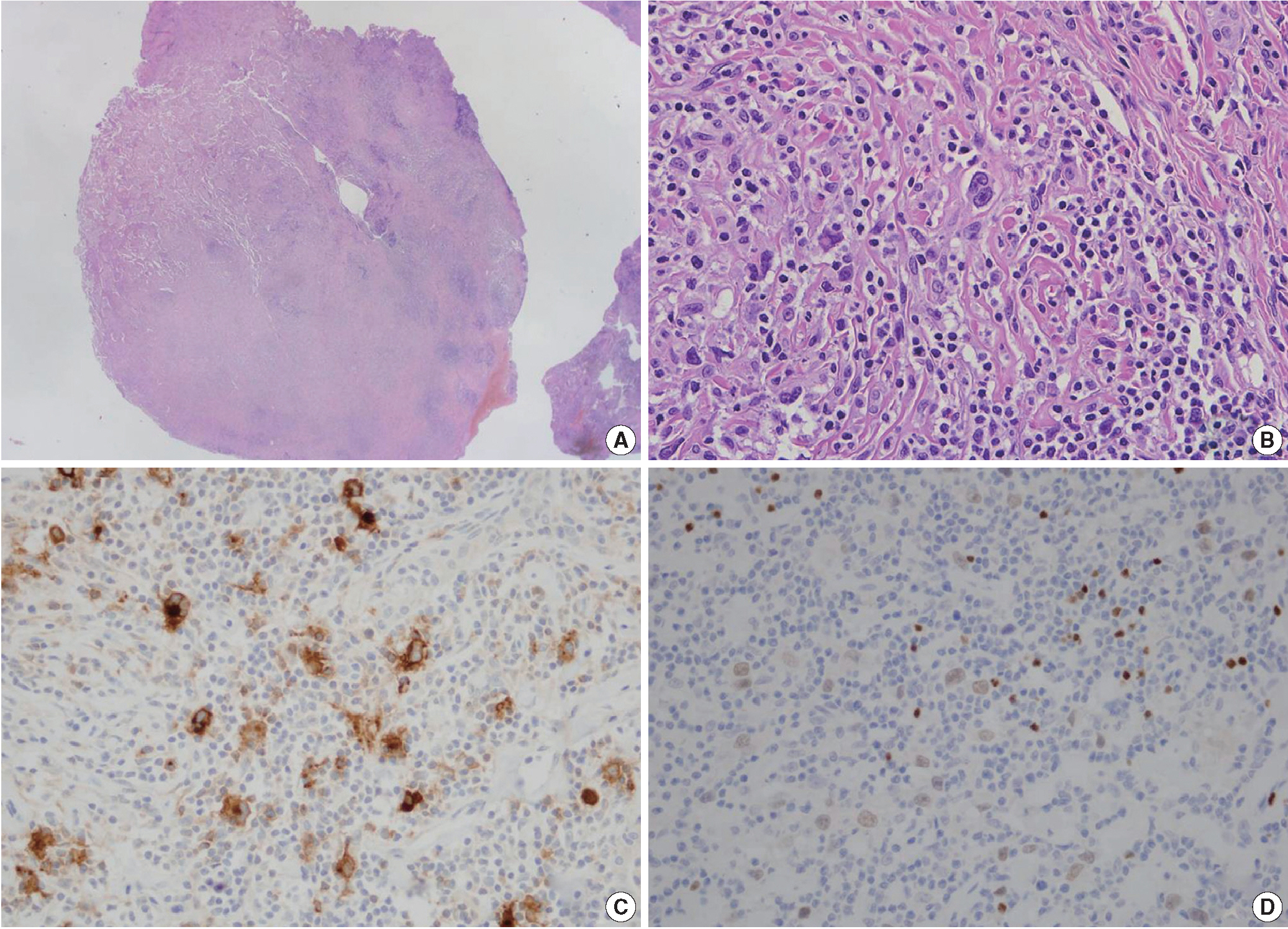
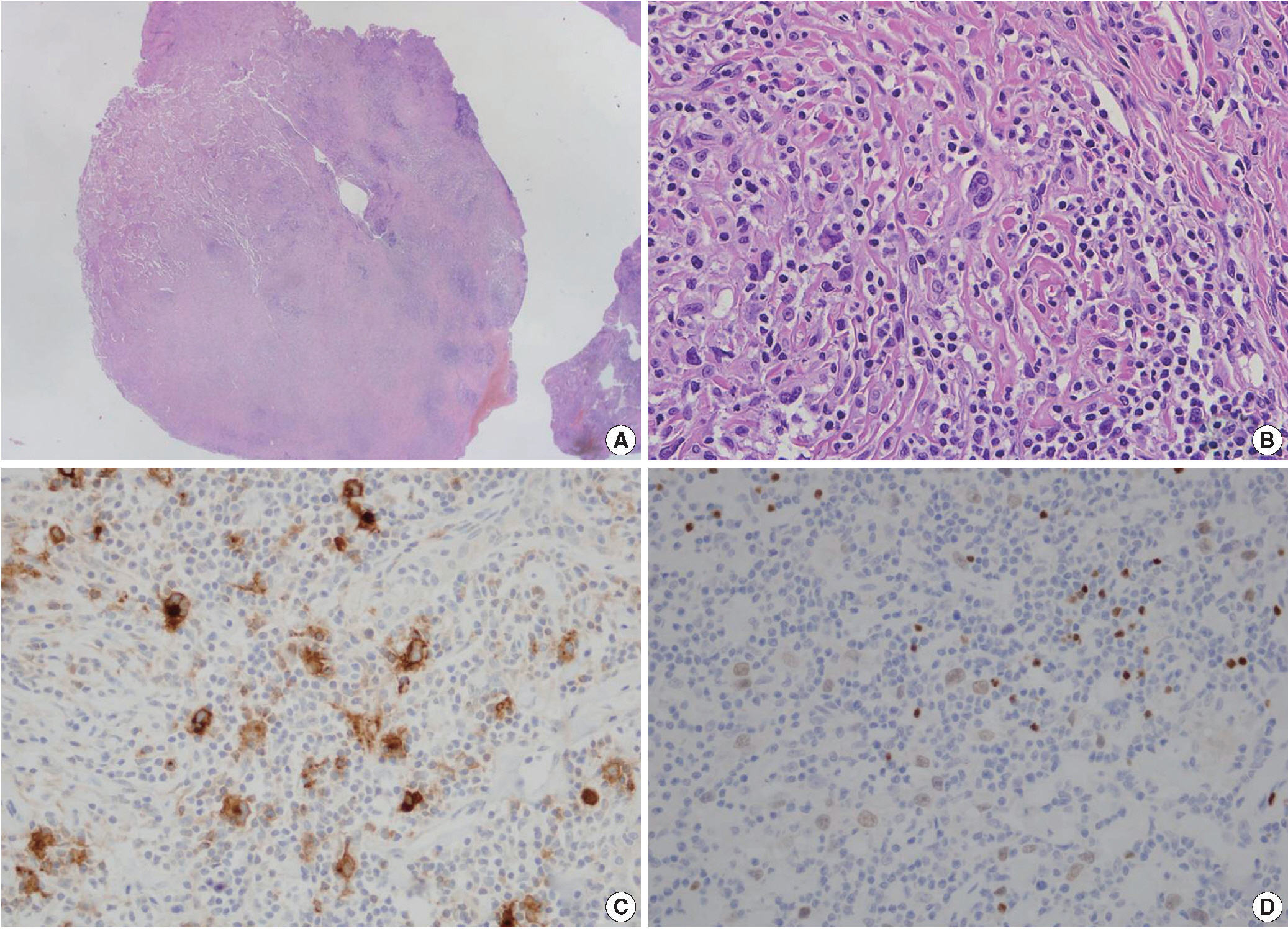
- The histopathologic findings of the lung showed a nodular growth pattern, separated by fibrous bands. The lymphoid nodule included scattered large atypical cells, resembling Hodgkin and Reed-Sternberg cells, in abundant reactive and inflammatory background including lymphocytes, plasma cells and histiocytes (Fig. 2B). The atypical large lymphoid cells were positive for CD30, CD15, and PAX5 (Fig. 2C, D), and negative for CD3 and CD20. These cells were negative for Epstein-Barr encoding region in situ hybridization. The histologic diagnosis, even conjunction with immunohistochemical stainings, was consistent with classical HL, nodular sclerosis type. Adjacent lung parenchyma was unremarkable with fibrinous exudates and foamy histiocytes in the air spaces at the edge of mass (Fig. 2A). The mediastinal lymph node was biopsied and exhibited the same histologic findings of classical HL. Segmental resection of the small bowel was histologically compatible with CD, and there was no evidence of malignancy. A tuberculosis–polymerase chain reaction study was negative.
- Consequently, the patient was referred to oncology. A bone marrow smear and biopsy for staging were negative. The patient was started on chemotherapy with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD). Adalimumab infusion was immediately discontinued; however, the patient continued to receive oral mesalazine to control CD. At five-month follow-up after one cycle of ABVD, the patient showed a good response, with complete resolution of the lung mass and lymphadenopathy on F-fluorodeoxyglucose–positron emission tomography/computed tomography.
CASE REPORT
- IBD-affected patients treated with immunosuppressive agents such as thiopurine (azathioprine) and anti-TNF-α (infliximab, adalimumab, etc.) are at increased risk of malignant lymphoma[1]. Recently, some cases of classical HL arising in the setting of iatrogenic immunodeficiency have been reported; these diseases usually present in lymph nodes and are commonly associated with Epstein-Barr virus (EBV) infection[4]. The most common subtypes are diffuse large B cell lymphoma, follicular lymphoma and Hodgkin lymphoma[1]. Cases of primary intestinal HL in CD patients are commonly reported[5], but only a few isolated cases of HL in CD patients have been described in extra-intestinal sites, such as the cervical and supraclavicular lymph nodes[2,3].
- The mechanism underlying the association between CD and lymphoma remains unclear. Many patients with CD receive a combination of several immunomodulating agents, which complicates the interpretation of the role of any specific agent. Treatment with immunosuppressive agents, including TNF-α antagonists, changes the immunologic state of patients, and thus may lead to the development of lymphoma[6]. Although most reported cases are NHL, a few cases of HL have been described in patients who received TNF-α antagonist therapy[7]. The patient described in that study received two cycles of adalimumab eight weeks before he was diagnosed with HL. The interval between the initiation of therapy with TNF-α antagonists and the development of lymphoma, including HL, was very short (median, 8 weeks) in that study[7]; therefore, we suggest that in this case, the pathogenesis of HL may have involved the patient’s treatment with TNF-α antagonist.
- Azathioprine (thiopurine) has been used for therapeutic purposes to maintain remission in patients with CD. The association between azathioprine and an increased risk of lymphoma in patients with IBD was also reported[8]. In our case, the patient had a four-year history of CD and received azathioprine therapy with prednisone and mesalazine. The interval between his initial treatment with azathioprine and the development of HL was about four years. Farrell et al.[8] reported a patient with NHL that was detected after two years of mesalazine, prednisolone and azathioprine treatment for CD. Although, it is still controversial whether azathioprine is related to the pathogenesis of lymphoma in patients with IBD, the possibility of lymphoma should always be considered in treating CD.
- Primary intestinal HL, as a consequence of underlying CD, may have a strong association with EBV. EBV-associated HL usually involves the colorectal regions[5]. In our case, there was no evidence of an EBV infection in surgical specimens or blood serologic evaluations. Dahhan et al.[2] reported that EBV was positive only in blood serology, and absent in both bone marrow and lymph node biopsy specimens, in primary HL in a CD patient. EBV-related lymphomas are associated with methotrexate, suggesting that defective immunosurveillance is important in lymphomagenesis. However, the development of lymphoma related to cyclophosphamide/azathioprine was EBV-negative in over 75% of cases, and had frequent p15 and p16 methylation; this could suggest that direct drug-induced mutagenesis may be involved in the pathogenesis of lymphoma[9].
- Therefore, our case of EBV-negative HL may be explained by other possible causes, such as molecular alteration and/or mutation.
- In conclusion, many factors may influence the development of lymphoma in patients with CD, and we were not able to clearly define the role of any specific immunomodulating drug in lymphomagenesis in this particular case. Although the patient’s CD and associated treatment were of relatively short duration, the cumulative dose of azathioprine over four years and recent additional therapy of TNF-α antagonist may have influenced the pathogenesis of HL. Thus, in treating patients with CD using immunosuppressive therapy, it is important to closely follow patients and be aware of this potential complication.
DISCUSSION
Fig. 1.Computed tomography scans of the right lower lobe of the lung reveal a round mass approximately 3 cm in diameter.
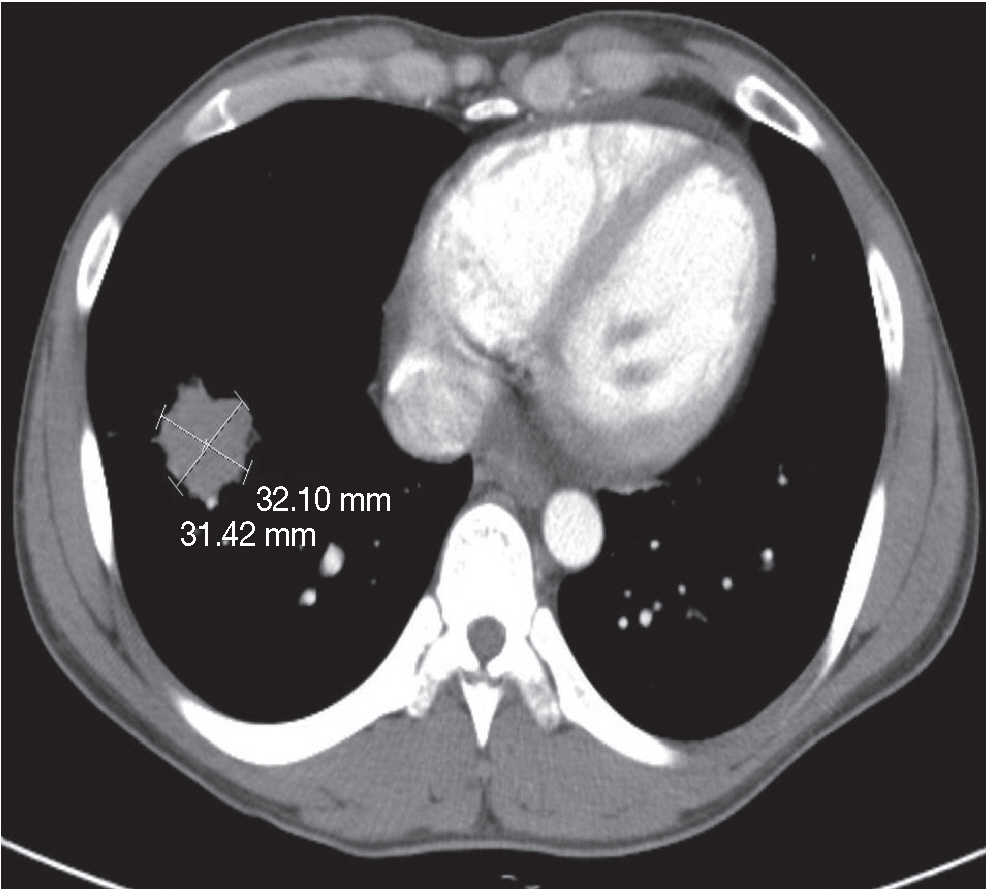

Fig. 2.The wedge resection specimen of the lung mass shows classic signs of Hodgkin lymphoma. (A) At low power, the neoplastic mass exhibits a dense cellular area with a nodular pattern separated by fibrous bands. (B) Reed-Sternberg cells are scattered in a background of small lymphocytes. Hodgkin and Reed-Sternberg cells are positive for CD30 (C) and PAX5 (D).

- 1. Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol 2011; 106: 2146-53. ArticlePubMedPDF
- 2. Dahhan T, Al Kahtani K, Bakshi N, Abouzied ME, Helmy A. Extra-intestinal Hodgkin’s lymphoma in a Crohn’s disease patient on long-term azathioprine and infliximab therapy. Trop Gastroenterol 2010; 31: 51-3. PubMed
- 3. Calvo-Villas JM, Ramirez Sanchez MJ, Cuesta Tovar J, García C. Extraintestinal Hodgkin’s disease in a patient with Crohn’s disease. South Med J 2003; 96: 632.Article
- 4. Loo EY, Medeiros LJ, Aladily TN, et al. Classical Hodgkin lymphoma arising in the setting of iatrogenic immunodeficiency: a clinicopathologic study of 10 cases. Am J Surg Pathol 2013; 37: 1290-7. PubMed
- 5. Bai M, Katsanos KH, Economou M, et al. Rectal Epstein-Barr virus-positive Hodgkin’s lymphoma in a patient with Crohn’s disease: case report and review of the literature. Scand J Gastroenterol 2006; 41: 866-9. ArticlePubMed
- 6. Smedby KE, Baecklund E, Askling J. Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Biomarkers Prev 2006; 15: 2069-77. ArticlePubMedPDF
- 7. Brown SL, Greene MH, Gershon SK, Edwards ET, Braun MM. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 2002; 46: 3151-8. ArticlePubMed
- 8. Farrell RJ, Ang Y, Kileen P, et al. Increased incidence of non-Hodgkin’s lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut 2000; 47: 514-9. ArticlePubMedPMC
- 9. Au WY, Ma ES, Choy C, et al. Therapy-related lymphomas in patients with autoimmune diseases after treatment with disease-modifying anti-rheumatic drugs. Am J Hematol 2006; 81: 5-11. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- The Complex Relationship between Mechanisms Underlying Inflammatory Bowel Disease, Its Treatment, and the Risk of Lymphomas: A Comprehensive Review
Katarzyna Stasik, Rafał Filip
International Journal of Molecular Sciences.2024; 25(8): 4241. CrossRef - Extra-intestinal malignancies in inflammatory bowel diseases: An update with emphasis on MDCT and MR imaging features
A. Dohan, S.A. Faraoun, M. Barral, Y. Guerrache, M. Boudiaf, X. Dray, C. Hoeffel, M. Allez, O. Farges, L. Beaugerie, T. Aparicio, P. Marteau, E.K. Fishman, O. Lucidarme, C. Eveno, M. Pocard, R. Dautry, P. Soyer
Diagnostic and Interventional Imaging.2015; 96(9): 871. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Pulmonary Hodgkin Lymphoma in a Patient with Crohn’s Disease


Fig. 1. Computed tomography scans of the right lower lobe of the lung reveal a round mass approximately 3 cm in diameter.
Fig. 2. The wedge resection specimen of the lung mass shows classic signs of Hodgkin lymphoma. (A) At low power, the neoplastic mass exhibits a dense cellular area with a nodular pattern separated by fibrous bands. (B) Reed-Sternberg cells are scattered in a background of small lymphocytes. Hodgkin and Reed-Sternberg cells are positive for CD30 (C) and PAX5 (D).
Fig. 1.
Fig. 2.
Pulmonary Hodgkin Lymphoma in a Patient with Crohn’s Disease

 E-submission
E-submission