Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(5); 2014 > Article
-
Brief Case Report
The Limitations of Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology in the Diagnosis of Pancreatic Serous Cystadenoma: A Brief Case Report - Heae Surng Park, Sun Och Yoon, Beom Jin Lim, Joo Hee Kim1, Soon Won Hong
-
Korean Journal of Pathology 2014;48(5):405-408.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.5.405
Published online: October 27, 2014
Departments of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
1Departments of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author: Soon Won Hong, M.D. Department of Pathology, Gangnam Severance Hospital, 211 Eonju-ro, Gangnam-gu, Seoul 135-720, Korea Tel: +82-2-2019-3543, Fax: +82-2-3463-2103, E-mail: soonwonh@yuhs.ac
• Received: March 25, 2014 • Revised: June 27, 2014 • Accepted: July 1, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 8,213 Views
- 63 Download
- 1 Scopus
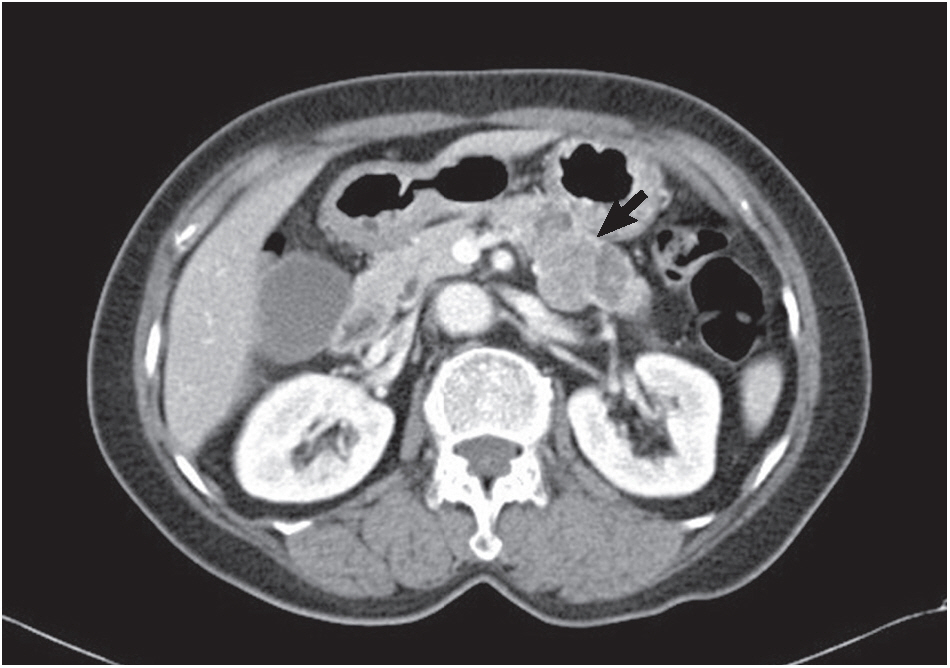
- A 74-year-old woman complaining of dyspepsia was referred to the hospital. An abdominal computed tomography scan revealed a 5-cm-sized mass in the body of the pancreas (Fig. 1) without upstream pancreatic duct dilatation or enlarged regional lymph nodes. The mass was mixed solid, macrocystic, and microcystic in nature. The large tumor size, macrocystic portion (suspicion for mucin pool), and solid components implied the possibility of pancreatic malignancy of an unusual cell type. Screening laboratory results for serum amylase, lipase, and tumor markers (carcinoembryonic antigen [CEA] and carbohydrate antigen 19-9) were normal. Upper gastrointestinal endoscopy revealed diffuse chronic gastritis. A microcystic pancreatic mass with solid components was found during an EUS examination. An EUS-guided FNA biopsy of the pancreatic lesion was performed using a 22-gauge needle. Papanicolaou-stained slides including conventional smears and a ThinPrep (Hologic Inc., Bedford, MA, USA) preparation were made.
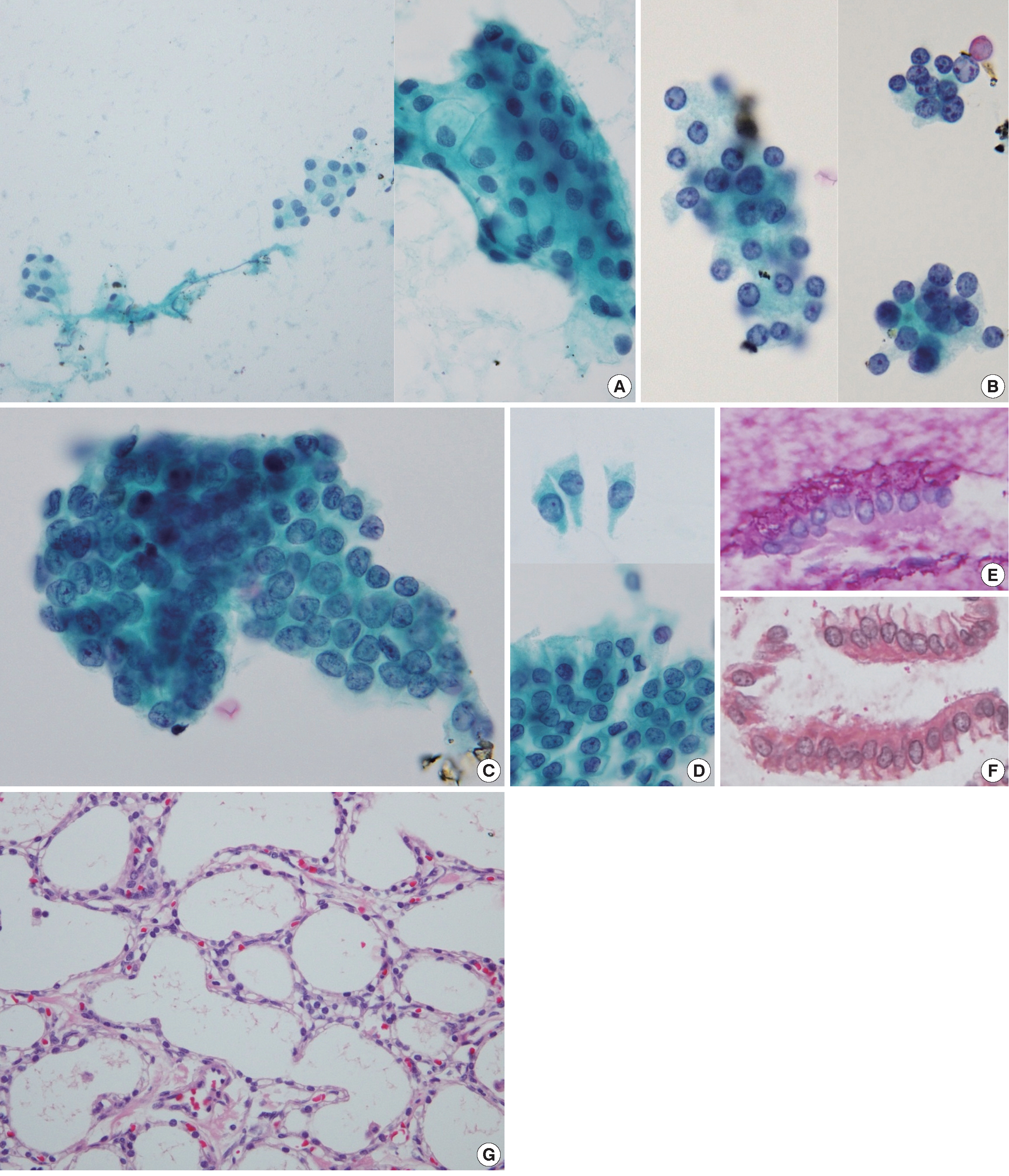
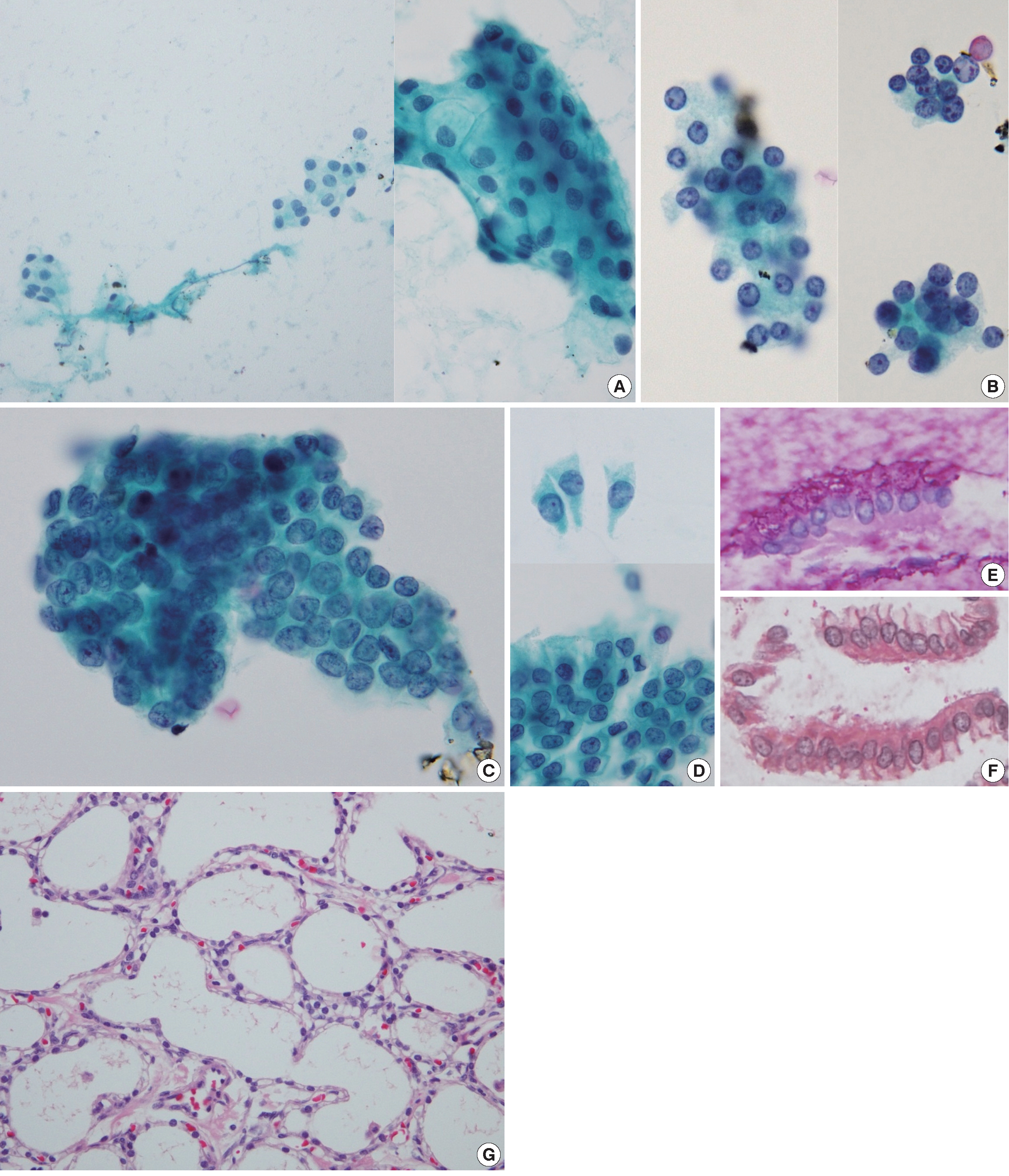
- The aspiration smear revealed low cellularity with a clean background devoid of mucin. A few small collections of cuboidal epithelial cells with uniform round nuclei and pale cytoplasm were observed (Fig. 2A). The nuclear membranes were smooth, the chromatin was finely granular, and the nucleoli were small. The nuclear-cytoplasmic ratio was low. In a liquid-based preparation, two different cell populations were identified. The first population was composed of loose cell clusters of cuboidal cells with small, round nuclei and undefined cell borders (Fig. 2B). Sheets of atypical cells with disorderly arranged nuclei, occasional nuclear overlapping and nuclear grooves comprised the second cell population (Fig 2C). The cell borders were relatively well defined. These atypical cells were regarded as low-grade adenocarcinoma cells during the initial cytological diagnosis, but they were identified as gastric epithelial cells with degenerating atypia during retrospective review. Epithelial sheets with uniform chromatin and nucleoli, an adequate amount of cytoplasm, and prominent cytoplasmic borders enable us to determine the benign feature of these cells. In addition, those cells had transitional morphologic features from normal gastric surface epithelium observed in both the ThinPrep and cell block preparations (Fig. 2D). The gastric foveolar epithelium was arranged in a sheet-like structure composed of tall columnar cells with distinct cytoplasmic borders. The apical cytoplasm contained neutral mucin that was positive with periodic acid-Schiff (Fig. 2E) and negative with mucicarmine (Fig. 2F) stain. Singly occurring abnormal cells were not found.
- The patient underwent distal pancreatectomy. Pancreatic tissue cross-sections revealed a sponge-like cystic mass, measuring 4.9×4.3×2.2 cm in size. The cystic spaces were filled with serous fluid. The cystic mass was composed of numerous small cysts lined with a single layer of cuboidal cells with clear cytoplasm (Fig. 2G). Nuclear atypia and mitosis were absent. A final diagnosis of pancreatic microcystic SCA was made.
CASE REPORT
- Pancreatic cystic neoplasms include SCA, mucinous cystic neoplasm (MCN), intraductal papillary mucinous neoplasm (IPMN), solid pseudopapillary tumor, cystic neuroendocrine tumor, and ductal adenocarcinoma with cystic change. SCA is considered to be benign and patients presenting with this tumor do not require follow-up or treatment unless they are symptomatic. Mucinous cystic tumors such as MCN and IPMN have malignant potential and therefore, surgical resection of these types of tumors is generally recommended. For the proper management of pancreatic cystic tumors, an accurate preoperative diagnosis is critical.
- Pancreatic SCA has some typical imaging findings: polycystic, honeycomb, and oligocystic (or macrocystic) patterns. However, various atypical features on cross-sectional imaging including solid variant, unilocular cystic tumors, interval growth, giant tumors with ductal dilatation, intratumoral hemorrhages, and disseminated forms may lead to an incorrect diagnosis[1]. In such cases, EUS can provide an alternative high-resolution image. In addition, EUS allows EUS-guided FNA of the lesion for cytological evaluation and CEA analysis of the cyst fluid. Compared with the final surgical diagnosis, diagnostic accuracy of EUS FNA in differentiating among pancreatic cystic neoplasms has been reported to be between 55% and 97%[2-6].
- The diagnostic value of FNA in SCA is controversial. Earlier studies only consider single case reports or discuss only a few cases, resulting in variable diagnostic accuracy (from 10% to 100%). Recently, there have been several systematic reviews regarding EUS FNA findings of SCAs that were confirmed postoperatively. Collins[7] demonstrated that out of 17 surgically verified SCAs, not a single case had a definitive preoperative cytodiagnosis of SCA. Huang et al.[8] reported that the diagnostic accuracy of EUS FNA in the diagnosis of SCA was 17%. Belsley et al.[9] showed that 1 out of 9 cases (11.1%) was initially diagnosed as “consistent with SCA” using EUS FNA.
- A cytological diagnosis of SCA can be made if benign, cuboidal epithelial cells containing intracytoplasmic glycogen devoid of a thick mucinous background are identified. The low sensitivity of EUS-guided FNA in the diagnosis of SCA can be attributed to several reasons. First, the aspirate smear usually contains small numbers of neoplastic cells owing to the cystic nature of this tumor. Belsley et al.[9] reported that in histologically proven SCA cases, none of the EUS-guided aspirates contained typical glycogenated serous epithelium. Collins[7] showed that 20% of cases contained lesional cells. Second, the SCA cells are bland, so there may be a tendency to overlook these cells. Third, gastrointestinal contamination can lead to an erroneous diagnosis of a mucinous cystic tumor or well-differentiated ductal adenocarcinoma. Mucosal sampling was reported in 41.2%[7] and 52.4%[9] in two studies regarding EUS-guided FNA. Fourth, variable nuclear atypia can be seen in SCA cytology[8]. Huang et al.[8] reported that 5 out of 26 FNA cases presented with focal nuclear atypia and that 1 case presented with a focally prominent nuclear pleomorphism, overlap, and hyperchromasia due to degenerated cellular change.
- In the present case, the aspirate smear included some SCA cells despite the low cellularity of the FNA sample. As previously described, the SCA cells had small, monomorphous, round nuclei and formed loose clusters with a rounded group pattern[7-9]. SCA cells appear benign, but they can be differentiated from pancreatic ductal or gastrointestinal epithelia because SCA cells are more disarrayed in their arrangement whereas pancreatic ductal or gastrointestinal epithelia are arranged in evenly-spaced, honeycomb-type sheets. Gastric mucosal sampling was also identified in ThinPrep and cell block preparations of this case. Some gastric foveolar cells showed atypical features such as nuclear overlapping and nuclear membrane irregularity, which resulted in the initial misdiagnosis of pancreatic adenocarcinoma. Well-differentiated adenocarcinoma of the pancreas has deceptively bland cytomorphology and may be misinterpreted as gastrointestinal contamination, as gastrointestinal sampling may be misinterpreted as adenocarcinoma[10]. The important diagnostic features of well-differentiated ductal adenocarcinoma include altered polarity, anisonucleosis of 4:1, and hypochromatic nuclei. Another diagnostic clue in ductal adenocarcinoma is that there are usually a few scattered, obviously malignant cells. Although atypical cells in this case have nuclear grooves and overlapping, the nuclei were not pleomorphic, chromatin was finely granular, and no overtly malignant cells were present.
- Unequivocal, preoperative diagnosis of SCA using EUS FNA is quite challenging. Neoplastic cells of SCA are usually aspirated in small numbers during the procedure and have benign cytomorphology. Possible gastrointestinal contamination in EUS-guided FNA samples from the pancreas should be considered to prevent the misinterpretation that normal gastric or intestinal epithelial cells are atypical cells, which may result in a misdiagnosis. Familiarity with the cytological features of both SCA and normal gastrointestinal epithelium may help improve the diagnostic accuracy of SCA using EUS-guided FNA samples.
DISCUSSION
Fig. 1.Abdominal computed tomography shows a solid and cystic mass (arrow) with focal enhancement in the body of the pancreas.
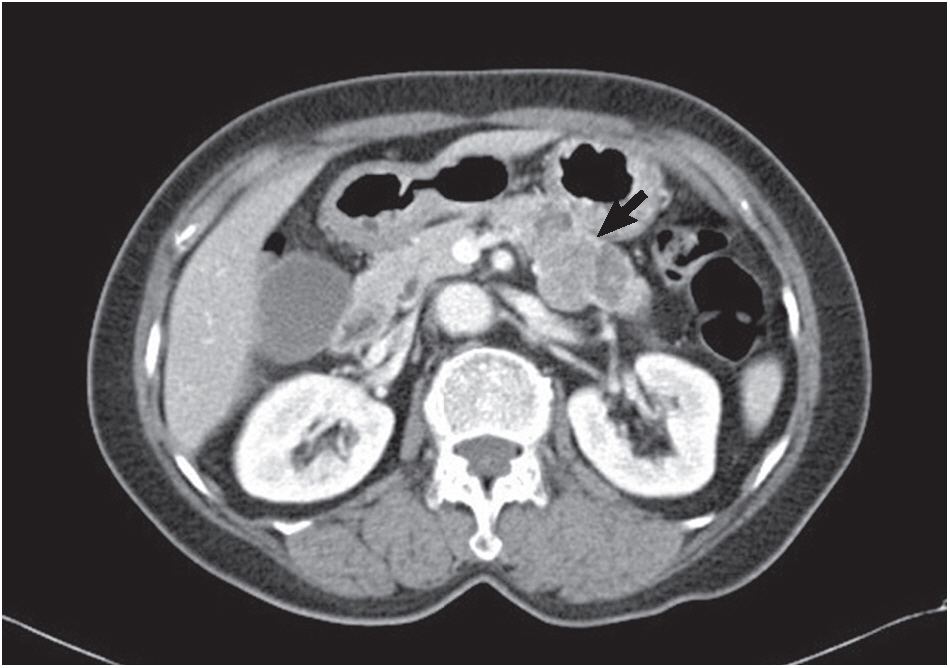

Fig. 2.Cytohistologic findings. Some neoplastic cells are identified in a retrospective review. (A) In the smear preparation, small collections of benign cuboidal epithelial cells are found. (B) In a ThinPrep slide, several loose clusters of cuboidal cells with small, round nuclei are present. Sheets of atypical cells with occasional nuclear overlapping and nuclear grooves are also present in a ThinPrep slide (C), which is proven to be degenerated gastric mucosa epithelium. Gastric mucosal sampling is observed in both the ThinPrep and cell block preparations. (D) The gastric surface epithelium is arranged in a sheet-like structure composed of tall columnar cells with distinct cytoplasmic borders. The apical cytoplasm contains neutral mucin as confirmed by periodic acid–Schiff (E) and mucicarmine (F) staining of the cell block preparation. On histological examination, the cystic mass consists of numerous small cysts lined with a single layer of cuboidal cells with clear cytoplasm (G).

- 1. Choi JY, Kim MJ, Lee JY, et al. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol 2009; 193: 136-42. ArticlePubMed
- 2. Brandwein SL, Farrell JJ, Centeno BA, Brugge WR. Detection and tumor staging of malignancy in cystic, intraductal, and solid tumors of the pancreas by EUS. Gastrointest Endosc 2001; 53: 722-7. ArticlePubMed
- 3. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126: 1330-6. ArticlePubMed
- 4. Frossard JL, Amouyal P, Amouyal G, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol 2003; 98: 1516-24. ArticlePubMed
- 5. Moparty B, Logrono R, Nealon WH, et al. The role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in distinguishing pancreatic cystic lesions. Diagn Cytopathol 2007; 35: 18-25. ArticlePubMed
- 6. Sedlack R, Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc 2002; 56: 543-7. ArticlePubMed
- 7. Collins BT. Serous cystadenoma of the pancreas with endoscopic ultrasound fine needle aspiration biopsy and surgical correlation. Acta Cytol 2013; 57: 241-51. ArticlePubMedPDF
- 8. Huang P, Staerkel G, Sneige N, Gong Y. Fine-needle aspiration of pancreatic serous cystadenoma: cytologic features and diagnostic pitfalls. Cancer 2006; 108: 239-49. ArticlePubMed
- 9. Belsley NA, Pitman MB, Lauwers GY, Brugge WR, Deshpande V. Serous cystadenoma of the pancreas: limitations and pitfalls of endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer 2008; 114: 102-10. ArticlePubMed
- 10. Nawgiri RS, Nagle JA, Wilbur DC, Pitman MB. Cytomorphology and B72.3 labeling of benign and malignant ductal epithelium in pancreatic lesions compared to gastrointestinal epithelium. Diagn Cytopathol 2007; 35: 300-5. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
The Limitations of Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology in the Diagnosis of Pancreatic Serous Cystadenoma: A Brief Case Report


Fig. 1. Abdominal computed tomography shows a solid and cystic mass (arrow) with focal enhancement in the body of the pancreas.
Fig. 2. Cytohistologic findings. Some neoplastic cells are identified in a retrospective review. (A) In the smear preparation, small collections of benign cuboidal epithelial cells are found. (B) In a ThinPrep slide, several loose clusters of cuboidal cells with small, round nuclei are present. Sheets of atypical cells with occasional nuclear overlapping and nuclear grooves are also present in a ThinPrep slide (C), which is proven to be degenerated gastric mucosa epithelium. Gastric mucosal sampling is observed in both the ThinPrep and cell block preparations. (D) The gastric surface epithelium is arranged in a sheet-like structure composed of tall columnar cells with distinct cytoplasmic borders. The apical cytoplasm contains neutral mucin as confirmed by periodic acid–Schiff (E) and mucicarmine (F) staining of the cell block preparation. On histological examination, the cystic mass consists of numerous small cysts lined with a single layer of cuboidal cells with clear cytoplasm (G).
Fig. 1.
Fig. 2.
The Limitations of Endoscopic Ultrasound-Guided Fine Needle Aspiration Cytology in the Diagnosis of Pancreatic Serous Cystadenoma: A Brief Case Report

 E-submission
E-submission