Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(6); 2014 > Article
-
Brief Case Report
Indolent CD56-Positive Clonal T-Cell Lymphoproliferative Disease of the Stomach Mimicking Lymphomatoid Gastropathy - Mineui Hong, Won Seog Kim1, Young Hyeh Ko
-
Korean Journal of Pathology 2014;48(6):430-433.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.6.430
Published online: December 31, 2014
Department of Pathology, Hematology-Oncology Section, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
1Department of Medicine, Hematology-Oncology Section, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- Corresponding Author: Young Hyeh Ko, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea Tel: +82-2-3410-2762, Fax: +82-2-3410-0025, E-mail: yhko310@skku.edu
• Received: July 8, 2014 • Revised: September 4, 2014 • Accepted: September 12, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- A 41-year-old Korean woman without any symptoms was admitted to the hospital for her annual health checkup. Gastric endoscopy revealed diffuse mucosal atrophy, multiple ulcers, and ulcer scars up to 1.2 cm with surrounding mucosal nodularity on the body and proximal antrum. On sigmoidoscopy, no abnormal findings were observed. The chest and abdominal computed tomography (CT) showed mild and diffuse wall thickening of the stomach. On positron-emission tomography (PET), a focal increased 18F-2-fluoro-2-deoxy-D-glucose uptake in the lower body of the stomach was present. There was no organomegaly or significant lymphadenopathy.
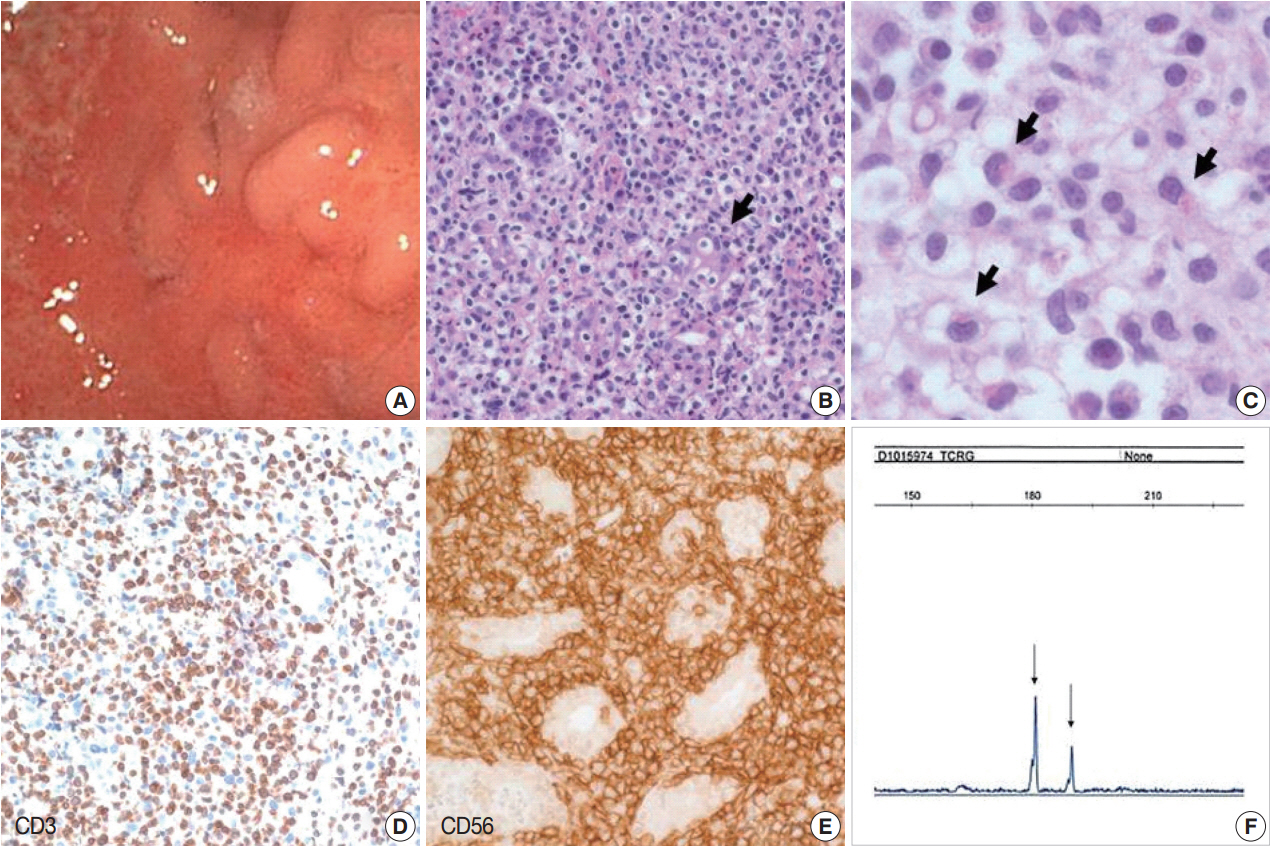
- Biopsies from the stomach revealed erosion of the mucosa with diffuse infiltration of small to medium atypical lymphoid cells in expanded lamina propria. Atypical lymphocytes had clear and abundant cytoplasm. The nuclei were round or indented with dispersed chromatin and inconspicuous nucleoli. Some of the atypical lymphocytes had eosinophilic cytoplasmic granules. The granules were less eosinophilic and finer than those of eosinophils. The gastric glands had been invaded by atypical cells leading to lymphoepithelial lesion-like appearance. Many plasma cells, eosinophils, and neutrophils were mixed with atypical lymphocytes. There was also focal necrosis. Angiocentric infiltration of atypical cells was not observed. Helicobacter pylori was seen on the surface of the mucosa. The atypical cells expressed cytoplasmic CD3, CD56, TIA-1, and granzyme B, but were negative for CD4, CD8, CD30, βF1, and TCRγ. The Ki-67 labeling index was low. EBER was not detected in any biopsies using in situ hybridization. Polymerase chain reaction analysis for TCRγ gene rearrangement revealed monoclonality (Fig. 1). Because the initial diagnosis was PTCL-NOS, Ann Arbor stage IE, the patient was treated with chemotherapy including cyclophosphamide, doxorubicin, vincristine, and prednisone for six cycles during 4 months. The patients achieved complete remission (CR) through three cycles of primary chemotherapy. Nine months after initial CR, the patient had no symptoms, but gastric biopsy revealed similar lesions in the lower body, antrum, and fundus. The patient postponed further therapy for 30 months and underwent regular follow-up with endoscopy and CT. During the 30 months, eight gastric biopsies revealed a persistent lesion, but there was no progression of the lesion in the stomach or to other sites. After 30 months, radiotherapy was given with disappearance of the infiltrate. However, 10 months later, a biopsy revealed reappearance of the infiltrate at the same site of the stomach. Subsequently, the patient was not been treated, but was checked by endoscopy and CT or PET. At 72 months after the first diagnosis, the patient still had localized gastric lesion, but no symptoms.
CASE REPORT
- Indolent T-cell LPD of the GI tract is a nonprogressive clonal T-cell LPD. Chemotherapy is not effective, with little or no response, and most patients have persistent disease localized to the GI tract. Among 22 cases reported so far, all patients were alive during follow-up except for two patients who died of disease at 176 months [7] and 132 months [8] after diagnosis because of progression to other organs, and large cell transformation, respectively. The clinical course of the present case was indolent. The lesion responded transiently to chemotherapy or radiotherapy, but relapsed shortly after. Multiple biopsies showed a persistent lesion for 6 years without progression.
- Abnormal infiltration of CD3+ CD56+ EBV-monoclonal T-lymphoid cells, which replaced the lamina propria of the gastric mucosa, led to the misdiagnosis of PTCL-NOS. CD56 is a marker of NK cells, but is expressed in various types of aggressive NK- or T-cell lymphomas of the GI tract including ENKL, EATL type II, and a subset of PTCL-NOS [2]. ENKL can be easily excluded because of EBV negativity in indolent T-cell LPD. In EATL type II, tumor cells are monomorphic and medium sized, and do not show eosinophilic granules of the cytoplasm as seen in the present case. In immunohistochemistry, tumor cells are usually CD4–CD8+CD56+, and less frequently CD4–CD8–CD56+. Because indolent T-cell LPD shows superficial infiltration of small, mature appearing lymphoid cells with a low Ki-67 labeling index of less than 10%, Ki-67 staining is important for excluding aggressive T-cell lymphoma such as PTCL-NOS or EATL [6].
- Histological and immunohistochemical findings of present case are very similar to those of lymphomatoid gastropathy. Atypical cells of lymphomatoid gastropathy are NK-cells with a CD4–CD5–CD8–CD56+TCRβF1–TCRδγ–TIA-1+GranzymeB+ immunophenotype and no clonality detected by TCR gene rearrangement [3,4]. In the present case, infiltrated cells were CD4–CD56+CD8–TCRβF1–TCRγ–TIA-1+, which might suggest NK cell origin, but gene rearrangement analysis demonstrated a T-cell lineage of atypical cells. Clinically, most patients with lymphomatoid gastropathy undergo spontaneous regression without treatment [3-5]. A few patients relapsed, but none of the patients died. Unlike lymphomatoid gastropathy, our patient had nonprogressive, but persistent disease. Treatment was not effective.
- The CD4–CD8–CD56+TCR-silent phenotype is uncommon among the indolent T-cell LPD of the GI tract reported thus far. In a large series of indolent T-cell LPDs reported by Perry et al. [6], the infiltrates comprised mainly of CD4–CD8+CD56–TCRβF1+ T-cells. There was one case showing a CD4–CD8–CD56–TCRβF1+ T-cell phenotype and one case showing a CD4+CD8–CD56–TCRβF1+ T-cell phenotype [6]. Other reports for indolent T-cell LPD have described cases with CD4+ T-cell phenotype [7,8]. Although atypical cells did not express TCR, one may raise the possibility of γδ T-cells or NKT-cells as the pathogenesis of our case. NKT-cells represent a minor subset of T-lymphocytes that share cell-surface proteins with conventional T-cells and NK-cells [9]. In the intestine, these cells are located among intraepithelial lymphocytes and within the lamina propria. The role of NKT-cells is related to mucosal immunity. A group of T-cells coexpress NK markers, so-called γδNKT-cells, constitute 1%–5% of the blood or peripheral organ lymphocytes. γδNKT-cells express a γδTCR instead of an αβTCR. These subsets can include CD4+, CD8+, CD4+/CD8+, and a minor subset (1%–2%) of double negative [10].
- In summary, this is the first case of indolent T-cell proliferative disease of the GI tract with expression of CD56, which needs to be distinguished from aggressive T- and NK-cell lymphoma and indolent lymphomatoid gastropathy. While the origin and function of CD4–CD8–CD56+TCR-silent T-cells remain to be explored, our case expands the immunophenotypic spectrum of indolent T-cell LPD.
DISCUSSION
Fig. 1.Indolent T-cell lymphoproliferative disease. (A) Gastroendoscopy reveals diffuse mucosal atrophy and nodular elevated lesions with ulcer. (B) The mucosa is infiltrated by small lymphoid cells that invaded the gastric glands. (C) Some of the atypical cells have eosinophilic cytoplasmic granules, which are described in lymphomatoid gastropathy. Immunohistochemically, lymphoid cells are CD3+ (D) and CD56+ (E). (F) BIOMED-II multiplex PCR for TCRγ gene rearrangement demonstrates clonal peaks.


- 1. Kim JM, Ko YH, Lee SS, et al. WHO classification of malignant lymphomas in Korea: report of the third nationwide study. Korean J Pathol 2011; 45: 254-60. Article
- 2. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haemtopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press, 2008.
- 3. Takeuchi K, Yokoyama M, Ishizawa S, et al. Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood 2010; 116: 5631-7. ArticlePubMedPDF
- 4. Mansoor A, Pittaluga S, Beck PL, Wilson WH, Ferry JA, Jaffe ES. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood 2011; 117: 1447-52. ArticlePubMedPMCPDF
- 5. Koh J, Go H, Lee WA, Jeon YK. Benign indolent CD56-positive NK-cell lymphoproliferative lesion involving gastrointestinal tract in an adolescent. Korean J Pathol 2014; 48: 73-6. ArticlePubMedPMC
- 6. Perry AM, Warnke RA, Hu Q, et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood 2013; 122: 3599-606. ArticlePubMedPMCPDF
- 7. Carbonnel F, d’Almagne H, Lavergne A, et al. The clinicopathological features of extensive small intestinal CD4 T cell infiltration. Gut 1999; 45: 662-7. ArticlePubMedPMC
- 8. Margolskee E, Jobanputra V, Lewis SK, Alobeid B, Green PH, Bhagat G. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One 2013; 8: e68343. ArticlePubMedPMC
- 9. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007; 25: 297-336. ArticlePubMed
- 10. Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol 2010; 11: 197-206. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Indolent T- and Natural Killer-Cell Lymphomas and Lymphoproliferative Diseases—Entities in Evolution
Chi Sing Ng
Lymphatics.2025; 3(4): 41. CrossRef - Case Report: Primary Indolent Epstein-Barr Virus-Positive T-Cell Lymphoproliferative Disease Involving the Central Nervous System
Kun Wang, Jinjian Li, Xuehui Zhou, Junhui Lv, Yirong Wang, Xinwei Li
Frontiers in Surgery.2022;[Epub] CrossRef - Indolent NK cell proliferative lesion mimicking NK/T cell lymphoma in the gallbladder
Su Hyun Hwang, Joon Seong Park, Seong Hyun Jeong, Hyunee Yim, Jae Ho Han
Human Pathology: Case Reports.2016; 5: 39. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Indolent CD56-Positive Clonal T-Cell Lymphoproliferative Disease of the Stomach Mimicking Lymphomatoid Gastropathy

Fig. 1. Indolent T-cell lymphoproliferative disease. (A) Gastroendoscopy reveals diffuse mucosal atrophy and nodular elevated lesions with ulcer. (B) The mucosa is infiltrated by small lymphoid cells that invaded the gastric glands. (C) Some of the atypical cells have eosinophilic cytoplasmic granules, which are described in lymphomatoid gastropathy. Immunohistochemically, lymphoid cells are CD3+ (D) and CD56+ (E). (F) BIOMED-II multiplex PCR for TCRγ gene rearrangement demonstrates clonal peaks.
Fig. 1.
Indolent CD56-Positive Clonal T-Cell Lymphoproliferative Disease of the Stomach Mimicking Lymphomatoid Gastropathy

 E-submission
E-submission