Search
- Page Path
- HOME > Search
Case Report
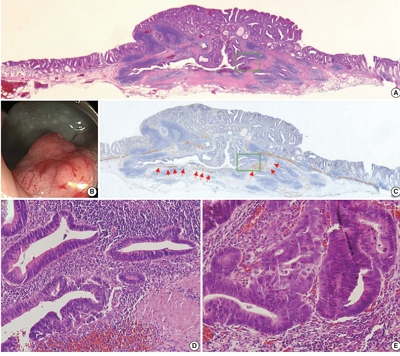
- Solitary Peutz-Jeghers type harmartomatous polyp in duodenum with gastric foveolar epithelium: a case report
- Eugene Choi, Junghwan Lee, Youngsoo Park
- J Pathol Transl Med. 2023;57(2):128-131. Published online January 10, 2023
- DOI: https://doi.org/10.4132/jptm.2022.11.07
- 5,307 View
- 190 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF - Peutz-Jeghers type hamartomatous polyp is known to be associated with Peutz-Jeghers syndrome, which shows characteristic multiple hamartomatous polyp involvement in the gastrointestinal tract, combined with mucocutaneous symptom, familial history of Peutz- Jeghers syndrome or STK11/LTB1 mutation. However, some cases showing histologic appearance of the polyps discovered in Peutz- Jeghers syndrome while lacking other diagnostic criteria of the syndrome have been reported, and these are called solitary Peutz- Jeghers type polyps. Herein, we report a case of solitary Peutz-Jeghers type polyp covered with heterotopic epithelium. The patient was 47-year-old female without any mucocutaneous symptoms nor familial history of Peutz-Jeghers syndrome. Microscopic examination revealed Peutz-Jeghers type hamartomatous polyp in duodenum covered with gastric type foveolar epithelium. Considering the definition of hamartomatous polyp, which is, the abnormal overgrowth of the indigenous epithelial component, the histological feature of current case is noteworthy in a point that it shows proliferation of heterotopic component, rather than the indigenous component.
-
Citations
Citations to this article as recorded by- A Solitary Peutz-Jeghers Hamartomatous Polyp in the Gastric Body: A Case Report
Noelia Madera, Noemí Acevedo, Carmen González-Peralta, Rafael Castro, Vismelis Mezquita-Luna
Cureus.2024;[Epub] CrossRef
- A Solitary Peutz-Jeghers Hamartomatous Polyp in the Gastric Body: A Case Report
Review
- Evolving pathologic concepts of serrated lesions of the colorectum
- Jung Ho Kim, Gyeong Hoon Kang
- J Pathol Transl Med. 2020;54(4):276-289. Published online June 26, 2020
- DOI: https://doi.org/10.4132/jptm.2020.04.15
- 18,486 View
- 846 Download
- 36 Web of Science
- 36 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Here, we provide an up-to-date review of the histopathology and molecular pathology of serrated colorectal lesions. First, we introduce the updated contents of the 2019 World Health Organization classification for serrated lesions. The sessile serrated lesion (SSL) is a new diagnostic terminology that replaces sessile serrated adenoma and sessile serrated polyp. The diagnostic criteria for SSL were revised to require only one unequivocal distorted serrated crypt, which is sufficient for diagnosis. Unclassified serrated adenomas have been included as a new category of serrated lesions. Second, we review ongoing issues concerning the morphology of serrated lesions. Minor morphologic variants with distinct molecular features were recently defined, including serrated tubulovillous adenoma, mucin-rich variant of traditional serrated adenoma (TSA), and superficially serrated adenoma. In addition to intestinal dysplasia and serrated dysplasia, minimal deviation dysplasia and not otherwise specified dysplasia were newly suggested as dysplasia subtypes of SSLs. Third, we summarize the molecular features of serrated lesions. The critical determinant of CpG island methylation development in SSLs is patient age. Interestingly, there may be ethnic differences in BRAF/KRAS mutation frequencies in SSLs. The molecular pathogenesis of TSAs is divided into KRAS and BRAF mutation pathways. SSLs with MLH1 methylation can progress into favorable prognostic microsatellite instability-positive (MSI+)/CpG island methylator phenotype-positive (CIMP+) carcinomas, whereas MLH1-unmethylated SSLs and BRAF-mutated TSAs can be precursors of poor-prognostic MSI−/CIMP+ carcinomas. Finally, based on our recent data, we propose an algorithm for stratifying risk subgroups of non-dysplastic SSLs.
-
Citations
Citations to this article as recorded by- Predominant Serrated Molecular Signature in Postcolonoscopy Colorectal Cancer: A Systematic Review and Meta-Analysis
Jen-Hao Yeh, Sin-Hua Moi, Chia-Chi Chen, Chao-Wen Hsu, Wen-Shuo Yeh, Tzu-Ning Tseng, Chuan-Pin Lin, Yu-Peng Liu, Jaw-Yuan Wang
American Journal of Gastroenterology.2026; 121(1): 122. CrossRef - Re-evaluating post-polypectomy surveillance: The role of non-invasive modalities in colorectal cancer prevention
Ethna McFerran, Damian McKay, Maurice B. Loughrey, Mark Lawler, Stephen T. McSorley
Best Practice & Research Clinical Gastroenterology.2026; : 102092. CrossRef - Clinical and endoscopic characteristics of colorectal traditional serrated adenomas with dysplasia/adenocarcinoma in a Korean population
Ki-Hyun Kim, Eun Myung, Hyung Hoon Oh, Chan-Muk Im, Young-Eun Seo, Je-Seong Kim, Chae-June Lim, Ga-Ram You, Sung-Bum Cho, Wan-Sik Lee, Myung-Giun Noh, Kyung-Hwa Lee, Young-Eun Joo
World Journal of Gastrointestinal Oncology.2025;[Epub] CrossRef - MicroRNA: role in macrophage polarisation and colorectal cancer pathogenesis
Haihong Lin, Jun Zhou, Ying He, Yifan Zhu, Puwen Chen, Hongwei Yan, Junyun Huang, Ersheng Gong, Xiaoling Wang
Frontiers in Cell and Developmental Biology.2025;[Epub] CrossRef - Submucosal fibrosis in large colorectal serrated lesions in cases receiving endoscopic submucosal dissection
Erik Manriquez-Alegria, Naohisa Yoshida, Reo Kobayashi, Naoto Iwai, Ken Inoue, Osamu Dohi, Lucas Cardoso, Hideyuki Konishi
Therapeutic Advances in Gastroenterology.2025;[Epub] CrossRef - Navigating the Colorectal Cancer Maze: Unveiling Pathways To Diagnosis, Management, Pathophysiology and Prevention
Khalid Ali Mohammed Al Kamzari, Constantina Constantinou
Current Oncology Reports.2025; 27(10): 1115. CrossRef - Fosl1 is a transcriptional effector of BRAFV600E-driven intestinal tumorigenesis
Zakia Alam, Rebecca Nightingale, Analia Lesmana, Cheng Liu, Laura J. Jenkins, Mark F. Richardson, Lawrence Croft, Ian Y. Luk, Camilla M. Reehorst, Fiona Chionh, Natalia Vukelic, Faiza Basheer, Eugene Tulchinsky, Joshua Badshah, Troy Dumenil, Latifa Bakiri
iScience.2025; 28(11): 113875. CrossRef - Sessile Serrated Lesions in Inflammatory Bowel Disease: Hidden Players in Colitis-Associated Colorectal Cancer?
Roberto de Sire, Diletta De Deo, Miriana Mercurio, Gianluca Franchellucci, Giulio Calabrese, Livio Bonacci, Mauro Sollai Pinna, Cristina Bezzio, Alessandro Armuzzi, Cesare Hassan, Alessandro Repici, Fabiana Castiglione, Sandro Ardizzone, Roberta Maselli
Journal of Clinical Medicine.2025; 14(22): 8042. CrossRef - Histologic Reappraisal and Evaluation of MLH1 Protein Expression in Sessile Serrated Lesions of the Proximal Colon
Priscilla de Sene Portel Oliveira, Miriam Aparecida da Silva Trevisan, Rita Barbosa de Carvalho, Rita de Cássia Perina Martins, João José Fagundes, Claudio Saddy Rodrigues Coy, Ashwini Esnakula
Gastroenterology Research and Practice.2025;[Epub] CrossRef - Superiority of excellent over good bowel preparation for proximal serrated polyp detection in a fecal immunochemical test-based screening cohort
Stefano Fantasia, Stefano Kayali, Pablo Cortegoso Valdivia, Stefano Andreotti, Daniele Macchi, Giorgio Nervi, Nico Pagano, Luigi Laghi
Gastrointestinal Endoscopy.2025;[Epub] CrossRef - Impact of AI-aided colonoscopy in clinical practice: a prospective randomised controlled trial
Johanna Schöler, Marko Alavanja, Thomas de Lange, Shunsuke Yamamoto, Per Hedenström, Jonas Varkey
BMJ Open Gastroenterology.2024; 11(1): e001247. CrossRef - The histologic features, molecular features, detection and management of serrated polyps: a review
Jin-Dong Wang, Guo-Shuai Xu, Xin-Long Hu, Wen-Qiang Li, Nan Yao, Fu-Zhou Han, Yin Zhang, Jun Qu
Frontiers in Oncology.2024;[Epub] CrossRef - Serrated polyps <10 mm cannot reliably be characterized by i-Scan without magnification at routine colonoscopy
Sabrina G.G. TESTONI, Chiara NOTARISTEFANO, Giuliano F. BONURA, Maria NAPOLITANO, Dario ESPOSITO, Edi VIALE, Lorella FANTI, Francesco AZZOLINI, Giulia M. CAVESTRO, PierAlberto TESTONI
Minerva Gastroenterology.2024;[Epub] CrossRef - Interobserver variability in the histopathological classification and grading of dysplasia in elevated colon lesions in the city of Lima
Guido Gallegos-Serruto, Aldo Gutiérrez, César Chian García, Isthvan Torres Perez
Revista de Gastroenterología del Perú.2024; 44(3): 239. CrossRef - Comparison of adenoma detection rate and proximal serrated polyp detection rate and their effect on post-colonoscopy colorectal cancer mortality in screening patients
Jasmin Zessner-Spitzenberg, Elisabeth Waldmann, Lena Jiricka, Lisa-Maria Rockenbauer, Anna Hinterberger, Jeremy Cook, Arno Asaturi, Aleksandra Szymanska, Barbara Majcher, Michael Trauner, Monika Ferlitsch
Endoscopy.2023; 55(05): 434. CrossRef - The yield of dysplasia and serrated lesions in a single-centre tertiary inflammatory bowel disease cohort
Fiona Yeaman, Lena Thin
Therapeutic Advances in Gastroenterology.2023;[Epub] CrossRef -
The BEETS (JACCRO CC-18) Trial: An Observational and Translational Study of
BRAF
-Mutated Metastatic Colorectal Cancer
Chiaki Inagaki, Ryo Matoba, Satoshi Yuki, Manabu Shiozawa, Akihito Tsuji, Eisuke Inoue, Kei Muro, Wataru Ichikawa, Masashi Fujii, Yu Sunakawa
Future Oncology.2023; 19(17): 1165. CrossRef - A retrospective analysis of the histology of resected polyps and colonoscopy quality parameters in Belgium
E Macken, S Van Dongen, G Van Hal
Acta Gastro Enterologica Belgica.2023; 86(2): 277. CrossRef - Prognostic Biomarkers of Cell Proliferation in Colorectal Cancer (CRC): From Immunohistochemistry to Molecular Biology Techniques
Aldona Kasprzak
Cancers.2023; 15(18): 4570. CrossRef - Assimilating Epigenetics and Transcriptomics for the Identification
of Prognostic Novel Biomarkers and Imminent Targets in
Colorectal Carcinoma with Therapeutic Potential
Suman Kumar Ray, Sukhes Mukherjee
Current Molecular Medicine.2023; 23(8): 784. CrossRef - Multitarget Stool RNA Test for Colorectal Cancer Screening
Erica K. Barnell, Elizabeth M. Wurtzler, Julie La Rocca, Thomas Fitzgerald, Jessica Petrone, Yansheng Hao, Yiming Kang, Faith L. Holmes, David A. Lieberman
JAMA.2023; 330(18): 1760. CrossRef - Microbiome in Colonic Carcinogenesis
Jun Sun, Yinglin Xia
Comprehensive Physiology.2023; 13(3): 4685. CrossRef - Impact of comprehensive optical diagnosis training using Workgroup serrAted polypS and Polyposis classification on detection of adenoma and sessile serrated lesion
Jooyoung Lee, Jung Ho Bae, Su Jin Chung, Hae Yeon Kang, Seung Joo Kang, Min‐Sun Kwak, Ji Yeon Seo, Ji Hyun Song, Sun Young Yang, Jong In Yang, Seon Hee Lim, Jeong Yoon Yim, Joo Hyun Lim, Goh Eun Chung, Eun Hyo Jin, Ji Min Choi, Yoo Min Han, Joo Sung Kim
Digestive Endoscopy.2022; 34(1): 180. CrossRef - Clinicopathological and molecular analyses of hyperplastic lesions including microvesicular variant and goblet cell rich variant hyperplastic polyps and hyperplastic nodules—Hyperplastic nodule is an independent histological entity
Noriyuki Uesugi, Yoichi Ajioka, Tomio Arai, Yoshihito Tanaka, Tamotsu Sugai
Pathology International.2022; 72(2): 128. CrossRef - Comprehensive clinicopathologic, molecular, and immunologic characterization of colorectal carcinomas with loss of three intestinal markers, CDX2, SATB2, and KRT20
Ji Ae Lee, Mi-Kyoung Seo, Seung-Yeon Yoo, Nam-Yun Cho, Yoonjin Kwak, Kyoungbun Lee, Jung Ho Kim, Gyeong Hoon Kang
Virchows Archiv.2022; 480(3): 543. CrossRef - Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis
Martino Mezzapesa, Giuseppe Losurdo, Francesca Celiberto, Salvatore Rizzi, Antonio d’Amati, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo
International Journal of Molecular Sciences.2022; 23(8): 4461. CrossRef - Arterial stiffness is associated with high-risk colorectal adenomas and serrated lesions: A cross-sectional study in a Taiwanese population
Hung-Yu Chen, Wen-Huang Lee, Hung-Lung Hsu, Yu-Tsung Chou, Fei-Lin Su, I-Hsuan Wu, Ting-Hsing Chao
Journal of Cardiology.2022; 80(2): 139. CrossRef - Morphological and molecular characterization of colorectal sessile serrated lesions with dysplasia
Filippo Cappello, Valentina Angerilli, Luca Dal Santo, Giada Munari, Marianna Sabbadin, Marcello Lo Mele, Gianmaria Pennelli, Claudio Luchini, Paola Parente, Stefano Lazzi, Matteo Fassan
Pathology - Research and Practice.2022; 240: 154214. CrossRef - Serrated polyposis: an overview
Jonathan Fawkes
Gastrointestinal Nursing.2022; 20(9): 24. CrossRef - Sessile serrated lesion presenting as large pedunculated polyp in the rectum: A case report
Shin Ju Oh, Jung-Wook Kim, Chi Hyuk Oh
Medicine.2022; 101(51): e32287. CrossRef - WHICH LESIONS ARE AT HIGHER RISK OF DEVELOPING COLORECTAL CARCINOMAS: SUPERFICIALLY ELEVATED SERRATED LESIONS OR DEPRESSED LESIONS?
Artur Adolfo PARADA, Filadelfio Euclydes VENCO, Miguel Reynaldo VARCA-NETO, Roberto EL IBRAHIM, Paula Bechara POLETTI, Helcio Pedrosa BRITO, Heloisa de Fátima SARE, Osvaldo MALAFAIA
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2022;[Epub] CrossRef - WNT5a in Colorectal Cancer: Research Progress and Challenges
Guangshun Sun, Liangliang Wu, Guoqiang Sun, Xuesong Shi, Hongyong Cao, Weiwei Tang
Cancer Management and Research.2021; Volume 13: 2483. CrossRef - Endoscopic diagnosis for colorectal sessile serrated lesions
Toshihiro Nishizawa, Shuntaro Yoshida, Akira Toyoshima, Tomoharu Yamada, Yoshiki Sakaguchi, Taiga Irako, Hirotoshi Ebinuma, Takanori Kanai, Kazuhiko Koike, Osamu Toyoshima
World Journal of Gastroenterology.2021; 27(13): 1321. CrossRef - NTRK oncogenic fusions are exclusively associated with the serrated neoplasia pathway in the colorectum and begin to occur in sessile serrated lesions
Jung Ho Kim, Jeong Hoon Hong, Yoon‐La Choi, Ji Ae Lee, Mi‐kyoung Seo, Mi‐Sook Lee, Sung Bin An, Min Jung Sung, Nam‐Yun Cho, Sung‐Su Kim, Young Kee Shin, Sangwoo Kim, Gyeong Hoon Kang
The Journal of Pathology.2021; 255(4): 399. CrossRef - Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps
Bob Chen, Cherie’ R. Scurrah, Eliot T. McKinley, Alan J. Simmons, Marisol A. Ramirez-Solano, Xiangzhu Zhu, Nicholas O. Markham, Cody N. Heiser, Paige N. Vega, Andrea Rolong, Hyeyon Kim, Quanhu Sheng, Julia L. Drewes, Yuan Zhou, Austin N. Southard-Smith, Y
Cell.2021; 184(26): 6262. CrossRef - Molecular Insights Into Colorectal Carcinoma
Domenika Ortiz Requena, Monica Garcia-Buitrago
Archives of Medical Research.2020; 51(8): 839. CrossRef
- Predominant Serrated Molecular Signature in Postcolonoscopy Colorectal Cancer: A Systematic Review and Meta-Analysis
Original Article
- Colorectal epithelial neoplasm associated with gut-associated lymphoid tissue
- Yo Han Jeon, Ji Hyun Ahn, Hee Kyung Chang
- J Pathol Transl Med. 2020;54(2):135-145. Published online January 29, 2020
- DOI: https://doi.org/10.4132/jptm.2019.11.06
- 10,080 View
- 256 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Colorectal epithelial neoplasm extending into the submucosal gut-associated lymphoid tissue (GALT) can cause difficulties in the differential diagnosis. Regarding GALT-associated epithelial neoplasms, a few studies favor the term “GALT carcinoma” while other studies have mentioned the term “GALT-associated pseudoinvasion/epithelial misplacement (PEM)”.
Methods
The clinicopathologic characteristics of 11 cases of colorectal epithelial neoplasm associated with submucosal GALT diagnosed via endoscopic submucosal dissection were studied.
Results
Eight cases (72.7%) were in males. The median age was 59 years, and age ranged from 53 to 73. All cases had a submucosal tumor component more compatible with GALT-associated PEM. Eight cases (72.7%) were located in the right colon. Ten cases (90.9%) had a non-protruding endoscopic appearance. Nine cases (81.8%) showed continuity between the submucosal and surface adenomatous components. Nine cases showed (81.8%) focal defects or discontinuation of the muscularis mucosae adjacent to the submucosal GALT. No case showed hemosiderin deposits in the submucosa or desmoplastic reaction. No case showed single tumor cells or small clusters of tumor cells in the submucosal GALT. Seven cases (63.6%) showed goblet cells in the submucosa. No cases showed oncocytic columnar cells lining submucosal glands.
Conclusions
Our experience suggests that pathologists should be aware of the differential diagnosis of GALT-associated submucosal extension by colorectal adenomatous neoplasm. Further studies are needed to validate classification of GALT-associated epithelial neoplasms. -
Citations
Citations to this article as recorded by- Redefining GALT-associated carcinoma: a distinct subtype of colorectal adenocarcinoma
Jennifer Fallas, Marianna Arvanitaki, Sophie Lecomte, Jean-Yves Bonnet, Sarah De Clercq, Audrey Verrellen, Nicky D’Haene, María Gómez Galdón, Laurine Verset
Virchows Archiv.2026;[Epub] CrossRef - Family adenomatous polyposis come across dome type adenocarcinoma: a case report and literature review
Ying-Ying Chang, Xiao-Long Zhang, Yao-Hui Wang, Ting-Sheng Ling
Diagnostic Pathology.2025;[Epub] CrossRef - Radiation-induced injury and the gut microbiota: insights from a microbial perspective
Qiaoli Wang, Guoqiang Xu, Ouying Yan, Shang Wang, Xin Wang
Therapeutic Advances in Gastroenterology.2025;[Epub] CrossRef
- Redefining GALT-associated carcinoma: a distinct subtype of colorectal adenocarcinoma
Case Study
- A Case of Giant Colonic Muco-submucosal Elongated Polyps Associated with Intussusception
- Joo Heon Kim, Seung Yun Lee, Je Ho Jang, Hyun Young Han, Dong Wook Kang
- J Pathol Transl Med. 2016;50(6):474-478. Published online May 23, 2016
- DOI: https://doi.org/10.4132/jptm.2016.04.27
- 11,695 View
- 133 Download
- 6 Web of Science
- 8 Crossref
-
 Abstract
Abstract
 PDF
PDF - Colonic muco-submucosal elongated polyp (CMSEP), a newly categorized non-neoplastic colorectal polyp, is a pedunculated and elongated polyp composed of normal mucosal and submucosal layers without any proper muscle layer. We herein report a giant variant of CMSEP associated with intussusception in the rectosigmoid colon, with a review of the literature. A 48-year-old woman underwent a laparoscopic low anterior resection due to multiple large submucosal polypoid masses associated with intussusception. Grossly, the colonic masses were multiple pedunculated polyps with a long stalk and branches ranging in size from a few millimeters to 14.0 cm in length. Microscopically, there was no evidence of hyperplasia, atypia, or active inflammation in the mucosa. The submucosal layers were composed of edematous and fibrotic stroma with fat tissue, dilated vessels, and lymphoid follicles.
-
Citations
Citations to this article as recorded by- Unusually rapid growth of a duodenal muco-submucosal elongated polyp: A case report
Yi Yang, Ding-Fu Zhong
World Journal of Gastrointestinal Surgery.2025;[Epub] CrossRef - Multiple enteric muco-submucosal elongated polyps causing intussusception
Atsuki Taniguchi, Izuru Endo, Takeyoshi Nishiyama, Nobuyuki Watanabe, Osamu Yoshida, Hiroaki Asano, Masatoshi Kubo, Tetsunobu Udaka
Clinical Journal of Gastroenterology.2024; 17(1): 41. CrossRef - Intussusception due to a Muco-submucosal Elongated Polyp in the Small Intestine—A Case Report—
Hiroki ISHIGE, Ken IMAIZUMI, Takumu FUKASAWA, Keiichiro ITO, Hiroyuki KASAJIMA, Satoru MUNAKATA, Norihiko SHIMOYAMA, Kazuaki NAKANISHI
Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).2024; 85(6): 744. CrossRef - Jejunal Intussusception Caused by Enteric Muco-submucosal Elongated Polyp: A Case Report
Young Min Jo
Soonchunhyang Medical Science.2024; 30(2): 60. CrossRef - Jejunal intussusception and perforation due to enteric muco-submucosal elongated polyp: a case report and literature review
Ryosuke Kikuchi, Shigenobu Emoto, Hiroaki Nozawa, Kazuhito Sasaki, Koji Murono, Shinya Abe, Hirofumi Sonoda, Aya Shinozaki-Ushiku, Soichiro Ishihara
Surgical Case Reports.2023;[Epub] CrossRef - A stalk with no polyp—A muco‐submucosal elongated polyp in the duodenum
Neil O’Morain, Ciaran McCloskey, Sinead Flanagan, Glen Doherty
United European Gastroenterology Journal.2023; 11(4): 392. CrossRef - Duodenal Worm-Like Polyp
Pan Pan, Guoshan Zhang, Xiao Cui, Liang Liu
Digestive Diseases and Sciences.2023; 68(12): 4275. CrossRef - Colonic Mucosubmucosal Elongated Polyp in the Sigmoid Colon on Surveillance Colonoscopy
Xiaowen Fan, Melissa Hershman, Gabriel Levi, Ilan Weisberg
ACG Case Reports Journal.2019; 6(6): e00110. CrossRef
- Unusually rapid growth of a duodenal muco-submucosal elongated polyp: A case report
Original Article
- Investigation of the Roles of Cyclooxygenase-2 and Galectin-3 Expression in the Pathogenesis of Premenopausal Endometrial Polyps
- Esin Kasap, Serap Karaarslan, Esra Bahar Gur, Mine Genc, Nur Sahin, Serkan Güclü
- J Pathol Transl Med. 2016;50(3):225-230. Published online April 16, 2016
- DOI: https://doi.org/10.4132/jptm.2016.03.08
- 9,197 View
- 84 Download
- 4 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
The pathogenesis and etiology of endometrial polyps has not been elucidated. In this study, we aimed to examine the pathogenic mechanisms of endometrial polyp development using immunohistochemistry. We evaluated the expression of galectin-3 and cyclooxgenase-2 (COX-2) during the menstrual cycle in premenopausal women with endometrial polyps or normal endometrium.
Methods
Thirty-one patients with endometrial polyps and 50 healthy control patients were included in this study. The levels of expression of COX-2 and galectin-3 were studied by immunohistochemistry.
Results
The percentage of COX-2–positive cells and the intensity of COX-2 staining in the endometrium did not vary during the menstrual cycle either in the control group or in patients with endometrial polyps. However, expression of galectin-3 was significantly lower in endometrial polyps and during the proliferative phase of the endometrium compared with the secretory phase.
Conclusions
Our data suggests that the pathogenesis of endometrial polyps does not involve expression of COX-2 or galectin-3. -
Citations
Citations to this article as recorded by- Abnormal expression of Hippo–YAP1 signalling pathway and progesterone resistance mechanism in endometrial polyps
Xinyu Yu, Weijia Kong, Kaiyue Shang, Hongxin Xing, Wenjing Sun, Qianqian Li, Hui Zhang
Journal of Obstetrics and Gynaecology.2025;[Epub] CrossRef - Research Progress in the Treatment of Endometrial Polyps
秀芬 蔡
Advances in Clinical Medicine.2024; 14(01): 1772. CrossRef - ER and COX2 expression in endometrial hyperplasia processes
Nataliia Tsyndrenko, Mykola Lyndіn, Kateryna Sikora, Andrew Awuah Wireko, Toufik Abdul-Rahman, Nataliia Hyriavenko, Anatolii Romaniuk
Medicine.2023; 102(33): e34864. CrossRef - Novel microarchitecture of human endometrial glands: implications in endometrial regeneration and pathologies
Nicola Tempest, Christopher J Hill, Alison Maclean, Kathleen Marston, Simon G Powell, Hannan Al-Lamee, Dharani K Hapangama
Human Reproduction Update.2022; 28(2): 153. CrossRef - Variances in the Level of COX-2 and iNOS in Different Grades of Endometrial Cancer
Marcin Oplawski, Konrad Dziobek, Nikola Zmarzły, Beniamin O. Grabarek, Robert Kiełbasiński, Przemysław Kieszkowski, Piotr Januszyk, Karol Talkowski, Michał Schweizer, Piotr Kras, Andrzej Plewka, Dariusz Boroń
Current Pharmaceutical Biotechnology.2020; 21(1): 52. CrossRef
- Abnormal expression of Hippo–YAP1 signalling pathway and progesterone resistance mechanism in endometrial polyps
Case Reports
- Isolated Polypoid Ganglioneuroma in the Rectum.
- Se Hoon Kim, Chang Hwan Choi, Yong Han Paik, Won Ho Kim, Hoguen Kim
- Korean J Pathol. 2001;35(4):344-346.
- 2,380 View
- 42 Download
-
 Abstract
Abstract
 PDF
PDF - Gastrointestinal ganglioneuroma is a rare benign neoplasm, composed of ganglion cells, nerve fibers, and supporting cells. Ganglioneuromas are presented as isolated polypoid ganglioneuroma, ganglioneuromatous polyposis, and diffuse ganglioneuromas. We have experienced a case of an isolated ganglioneuromatous polyp in the rectum. The patient was a 58-year-old female who had experienced low abdominal discomfort and tenesmus for 6 to 7 months. Colonoscopic examination revealed a polypoid tumor in the rectum. Microscopically, the tumor showed cystic glands, expanded lamina propria, and smooth surface epithelium. Many proliferated ganglion cells with nerve fibers were evident in the lamina propria which was extended to the submucosa.
- Multiple Localized Hyperplastic Gastropathy: Report of A Case with A Special Reference to its Growth Pattern.
- Jung Ran Kim, Yong Il Kim
- Korean J Pathol. 1989;23(1):154-159.
- 1,919 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - We present a case of localized mucosal hyperplasia of the stomach. The resected stomach contained four large, short stalked polyps, three of which were located in the anterior wall of body and the other in the posterior wall. In addition, numerous small sessile polyps were also scattered in the anterior and posterior fundic walls. Microscopically, the abnormally thick mucosa, carrying with it the muscularis mucosae and a thin core of loose fibrous tissue comprised the polyps by intraluminal infolding of widening of mucosal area. Abundant vasculature of the rugal pattern was prominent in the submucosa. The above findings suggest that the histogenesis of the polyps is related to both hyperplastic thickening and widening of mucosal areas in rugal pattern in the background of inverted distribution pattern of intestinal metaplasia.

 E-submission
E-submission



 First
First Prev
Prev



