Search
- Page Path
- HOME > Search
Original Articles
- Clinicopathologic characteristics of HER2-positive pure mucinous carcinoma of the breast
- Yunjeong Jang, Hera Jung, Han-Na Kim, Youjeong Seo, Emad Alsharif, Seok Jin Nam, Seok Won Kim, Jeong Eon Lee, Yeon Hee Park, Eun Yoon Cho, Soo Youn Cho
- J Pathol Transl Med. 2020;54(1):95-102. Published online November 13, 2019
- DOI: https://doi.org/10.4132/jptm.2019.10.24
- 10,837 View
- 293 Download
- 22 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Pure mucinous carcinoma (PMC) is a rare type of breast cancer, estimated to represent 2% of invasive breast cancer. PMC is typically positive for estrogen receptors (ER) and progesterone receptors (PR) and negative for human epidermal growth factor receptor 2 (HER2). The clinicopathologic characteristics of HER2-positive PMC have not been investigated.
Methods
Pathology archives were searched for PMC diagnosed from January 1999 to April 2018. Clinicopathologic data and microscopic findings were reviewed and compared between HER2-positive PMC and HER2-negative PMC. We also analyzed the differences in disease-free survival (DFS) and overall survival according to clinicopathologic parameters including HER2 status in overall PMC cases.
Results
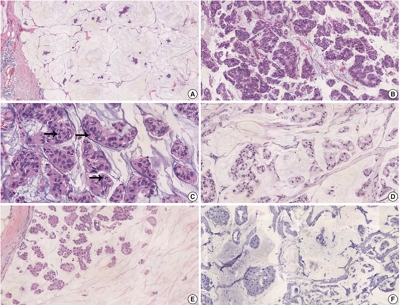
There were 21 HER2-positive cases (4.8%) in 438 PMCs. The average tumor size of HER2-positive PMC was 32.21 mm (± 26.55). Lymph node metastasis was present in seven cases. Compared to HER2-negative PMC, HER2-positive PMC presented with a more advanced T category (p < .001), more frequent lymph node metastasis (p = .009), and a higher nuclear and histologic grade (p < .001). Microscopically, signet ring cells were frequently observed in HER2-positive PMC (p < .001), whereas a micropapillary pattern was more frequent in HER2-negative PMC (p = .012). HER2-positive PMC was more frequently negative for ER (33.3% vs. 1.2%) and PR (28.6% vs. 7.2%) than HER2-negative PMC and showed a high Ki-67 labeling index. During follow-up, distant metastasis and recurrence developed in three HER2-positive PMC patients. Multivariate analysis revealed that only HER2-positivity and lymph node status were significantly associated with DFS.
Conclusions
Our results suggest that HER2-positive PMC is a more aggressive subgroup of PMC. HER2 positivity should be considered for adequate management of PMC. -
Citations
Citations to this article as recorded by- Mucin‐producing breast lesions: a practical approach to diagnosis
Sunayana Misra, Mihir Gudi, Kimberly H Allison, Edi Brogi, Cecily Quinn, Hannah Y Wen, Puay Hoon Tan
Histopathology.2026;[Epub] CrossRef - Clinicopathological characteristics of mucinous breast cancer: a retrospective analysis of a 6-years study from national cancer center in Vietnam
Thi Huyen Phung, Thanh Tung Pham, Huu Thang Nguyen, Dinh Thach Nguyen, Thanh Long Nguyen, Thi Hoai Hoang
Breast Cancer Research and Treatment.2025; 209(3): 667. CrossRef - Poor response of HER2-positive mucinous carcinomas of breast to neoadjuvant HER2-targeted therapy: A study of four cases
Min Han, Daniel Schmolze, Javier A. Arias-Stella, Christina H. Wei, Joanne Mortimer, Fang Fan
Annals of Diagnostic Pathology.2025; 74: 152396. CrossRef - Comprehensive Immunohistochemical Analysis of Mesonephric Marker Expression in Low-grade Endometrial Endometrioid Carcinoma
Yurimi Lee, Sangjoon Choi, Hyun-Soo Kim
International Journal of Gynecological Pathology.2024; 43(3): 221. CrossRef - Clinicopathological features and prognosis of mucinous breast carcinoma with a micropapillary structure
Beibei Yang, Menglu Shen, Bo Sun, Jing Zhao, Meng Wang
Thoracic Cancer.2024; 15(36): 2530. CrossRef - Pure Mucinous Carcinoma of the Breast: Radiologic-Pathologic Correlation
Cherie M Kuzmiak, Benjamin C Calhoun
Journal of Breast Imaging.2023;[Epub] CrossRef - Role of circ-FOXO3 and miR-23a in radiosensitivity of breast cancer
Elahe Abdollahi, Hossein Mozdarani, Behrooz Z. Alizadeh
Breast Cancer.2023; 30(5): 714. CrossRef - On Ultrasonographic Features of Mucinous Carcinoma with Micropapillary Pattern
Wei-Sen Yang, Yang Li, Ya Gao
Breast Cancer: Targets and Therapy.2023; Volume 15: 473. CrossRef - Spectrum of Mucin-containing Lesions of the Breast: Multimodality Imaging Review with Pathologic Correlation
Janice N. Thai, Melinda F. Lerwill, Shinn-Huey S. Chou
RadioGraphics.2023;[Epub] CrossRef - Mesonephric-like Adenocarcinoma of the Ovary: Clinicopathological and Molecular Characteristics
Hyun Hee Koh, Eunhyang Park, Hyun-Soo Kim
Diagnostics.2022; 12(2): 326. CrossRef - Alveolar Soft Part Sarcoma of the Uterus: Clinicopathological and Molecular Characteristics
Yurimi Lee, Kiyong Na, Ha Young Woo, Hyun-Soo Kim
Diagnostics.2022; 12(5): 1102. CrossRef - Metastasis of the Mucionous adenocarcinoma of breast to the mandibular gingiva: Rare case report
Ivana Mijatov, Aleksandra Fejsa Levakov, Aleksandar Spasić, Jelena Nikolić, Saša Mijatov
Medicine.2022; 101(38): e30732. CrossRef - Endometrioid Carcinomas of the Ovaries and Endometrium Involving Endocervical Polyps: Comprehensive Clinicopathological Analyses
Jihee Sohn, Yurimi Lee, Hyun-Soo Kim
Diagnostics.2022; 12(10): 2339. CrossRef - Serous Carcinoma of the Endometrium with Mesonephric-Like Differentiation Initially Misdiagnosed as Uterine Mesonephric-Like Adenocarcinoma: A Case Report with Emphasis on the Immunostaining and the Identification of Splice Site TP53 Mutation
Sangjoon Choi, Yoon Yang Jung, Hyun-Soo Kim
Diagnostics.2021; 11(4): 717. CrossRef - HER2 positive mucinous carcinoma of breast with micropapillary features: Report of a case and review of literature
Dinesh Chandra Doval, Rupal Tripathi, Sunil Pasricha, Pankaj Goyal, Chaturbhuj Agrawal, Anurag Mehta
Human Pathology: Case Reports.2021; 25: 200531. CrossRef - Carcinoma mucosecretor de mama HER2-positivo, un caso clínico
A.M. González Aranda, E. Martínez Gómez, A. Santana Costa, F. Arnanz Velasco, M.H. González de Diego, A. Zapico Goñi
Clínica e Investigación en Ginecología y Obstetricia.2021; 48(4): 100685. CrossRef - Clinicopathologic features of unexpectedly HER2 positive breast carcinomas: An institutional experience
Carissa LaBoy, Kalliopi P. Siziopikou, Lauren Rosen, Luis Z. Blanco, Jennifer L. Pincus
Pathology - Research and Practice.2021; 222: 153441. CrossRef - Mesonephric-like Differentiation of Endometrial Endometrioid Carcinoma: Clinicopathological and Molecular Characteristics Distinct from Those of Uterine Mesonephric-like Adenocarcinoma
Sujin Park, Go Eun Bae, Jiyoung Kim, Hyun-Soo Kim
Diagnostics.2021; 11(8): 1450. CrossRef - Mesonephric-like Adenocarcinoma of the Uterine Corpus: Comprehensive Immunohistochemical Analyses Using Markers for Mesonephric, Endometrioid and Serous Tumors
Hyunjin Kim, Kiyong Na, Go Eun Bae, Hyun-Soo Kim
Diagnostics.2021; 11(11): 2042. CrossRef
- Mucin‐producing breast lesions: a practical approach to diagnosis
- Evaluation of Pathologic Complete Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy: Experience in a Single Institution over a 10-Year Period
- Misun Choi, Yeon Hee Park, Jin Seok Ahn, Young-Hyuck Im, Seok Jin Nam, Soo Youn Cho, Eun Yoon Cho
- J Pathol Transl Med. 2017;51(1):69-78. Published online December 25, 2016
- DOI: https://doi.org/10.4132/jptm.2016.10.05
- 12,996 View
- 269 Download
- 23 Web of Science
- 23 Crossref
-
 Abstract
Abstract
 PDF
PDF - Background
Pathologic complete response (pCR) after neoadjuvant chemotherapy (NAC) has been associated with favorable clinical outcome in breast cancer patients. However, the possibility that the prognostic significance of pCR differs among various definitions has not been established. Methods: We retrospectively evaluated the pathologic response after NAC in 353 breast cancer patients and compared the prognoses after applying the following different definitions of pCR: ypT0/is, ypT0, ypT0/is ypN0, and ypT0 ypN0. Results: pCR was significantly associated with improved distant disease-free survival (DDFS) regardless of the definition (ypT0/is, p = .002; ypT0, p = .008; ypT0/is ypN0, p < .001; ypT0 ypN0, p = .003). Presence of tumor deposits of any size in the lymph nodes (LNs; ypN ≥ 0(i+)) was associated with worse DDFS (ypT0 ypN0 vs ypT0 ypN ≥ 0(i+), p = .036 and ypT0/is ypN0 vs ypT0/is ypN ≥ 0(i+), p = .015), and presence of isolated tumor cells was associated with decreased overall survival (OS; ypT0/is ypN0 vs ypT0/is ypN0(i+), p = .013). Residual ductal carcinoma in situ regardless of LN status showed no significant difference in DDFS or OS (DDFS: ypT0 vs ypTis, p = .373 and ypT0 ypN0 vs ypTis ypN0, p = .462; OS: ypT0 vs ypTis, p = .441 and ypT0 ypN0 vs ypTis ypN0, p = .758). In subsequent analysis using ypT0/is ypN0, pCR was associated with improved DDFS and OS in triple-negative tumors (p < .001 and p = .003, respectively). Conclusions: Based on our study results, the prognosis and rate of pCR differ according to the definition of pCR and ypT0/is ypN0 might be considered a more preferable definition of pCR. -
Citations
Citations to this article as recorded by- Differential prognostic value of residual nodal burden in breast cancer subtypes
Christine Hong Ngoc Che Thai, Selena J. An, Conner R. Haase, Julia M. Selfridge, Chris B. Agala, Philip M. Spanheimer
Breast Cancer Research and Treatment.2025; 209(2): 315. CrossRef - Neoadjuvant Chemotherapy for Early Breast Cancer: A Study on Response Rate and Toxicity
Matt Galloway, Paula Barlow, Jody Jordan, Edward Lo
Journal of Clinical Medicine.2025; 14(20): 7362. CrossRef - Association of residual ductal carcinoma in situ with breast cancer treatment outcomes after neoadjuvant chemotherapy according to hormone receptor status
Eunju Shin, Tae-Kyung Yoo, Jisun Kim, Il Yong Chung, Beom Seok Ko, Hee Jeong Kim, Jong Won Lee, Byung Ho Son, Sae Byul Lee
Discover Oncology.2024;[Epub] CrossRef - Efficacy of Mammographic Artificial Intelligence-Based Computer-Aided Detection in Predicting Pathologic Complete Response to Neoadjuvant Chemotherapy
Ga Eun Park, Bong Joo Kang, Sung Hun Kim, Han Song Mun
Life.2024; 14(11): 1449. CrossRef - Pathology after neoadjuvant treatment – How to assess residual disease
Giuseppe Viale, Nicola Fusco
The Breast.2022; 62: S25. CrossRef - Pathological examination of breast cancer samples before and after neoadjuvant therapy: recommendations from the Italian Group for the Study of Breast Pathology - Italian Society of Pathology (GIPaM-SIAPeC)
Nicola Fusco, Antonio Rizzo, Leopoldo Costarelli, Alfredo Santinelli, Bruna Cerbelli, Cristian Scatena, Ettore Macrì, Francesca Pietribiasi, Giulia d’Amati, Anna Sapino, Isabella Castellano
Pathologica.2022; 114(2): 104. CrossRef - Pathological complete response as a surrogate to improved survival in human epidermal growth factor receptor-2-positive breast cancer: systematic review and meta-analysis
Matthew G. Davey, Ferdia Browne, Nicola Miller, Aoife J. Lowery, Michael J. Kerin
BJS Open.2022;[Epub] CrossRef - Neoadjuvant therapy with doxorubicin-cyclophosphamide followed by weekly paclitaxel in early breast cancer: a retrospective analysis of 200 consecutive patients treated in a single center with a median follow-up of 9.5 years
Lisi M. Dredze, Michael Friger, Samuel Ariad, Michael Koretz, Bertha Delgado, Ruthy Shaco-Levy, Margarita Tokar, Michael Bayme, Ravit Agassi, Maia Rosenthal, Victor Dyomin, Olga Belochitski, Shai Libson, Tamar Mizrahi, David B. Geffen
Breast Cancer Research and Treatment.2022; 193(3): 597. CrossRef - “No Ink on Tumor” in Breast-Conserving Surgery after Neoadjuvant Chemotherapy
Giulia Atzori, Marco Gipponi, Chiara Cornacchia, Raquel Diaz, Marco Sparavigna, Maurizio Gallo, Tommaso Ruelle, Federica Murelli, Simonetta Franchelli, Francesca Depaoli, Daniele Friedman, Piero Fregatti
Journal of Personalized Medicine.2022; 12(7): 1031. CrossRef - Machine Learning Models and Multiparametric Magnetic Resonance Imaging for the Prediction of Pathologic Response to Neoadjuvant Chemotherapy in Breast Cancer
Carmen Herrero Vicent, Xavier Tudela, Paula Moreno Ruiz, Víctor Pedralva, Ana Jiménez Pastor, Daniel Ahicart, Silvia Rubio Novella, Isabel Meneu, Ángela Montes Albuixech, Miguel Ángel Santamaria, María Fonfria, Almudena Fuster-Matanzo, Santiago Olmos Antó
Cancers.2022; 14(14): 3508. CrossRef - Applying artificial intelligence technology to assist with breast cancer diagnosis and prognosis prediction
Meredith A. Jones, Warid Islam, Rozwat Faiz, Xuxin Chen, Bin Zheng
Frontiers in Oncology.2022;[Epub] CrossRef - Chemotherapy response score as a prognostic tool in patients with advanced stage endometrial carcinoma treated with neoadjuvant chemotherapy
Ina Jani, Ricardo R Lastra, Katherine S Brito, Chuanhong Liao, Isabel Lazo, Nita Karnik Lee, S Diane Yamada, Katherine C Kurnit
International Journal of Gynecological Cancer.2021; 31(6): 852. CrossRef - Application of neoadjuvant chemotherapy combined with anlotinib in occult breast cancer: A case report and review of literature
Yu Zhang, Di Wu, Bo Zhao, Xue-Liang Tian, Tian-Cheng Yao, Feng Li, Wei-Fang Liu, Ai-Ping Shi
World Journal of Clinical Cases.2021; 9(4): 919. CrossRef - Pathologic Complete Response and Its Impact on Breast Cancer Recurrence and Patient’s Survival after Neoadjuvant Therapy: A Comprehensive Meta-Analysis
Hui Liu, Liqiong Lv, Hui Gao, Ming Cheng, Tao Huang
Computational and Mathematical Methods in Medicine.2021; 2021: 1. CrossRef - Impact of Surgical Margins in Breast Cancer After Preoperative Systemic Chemotherapy on Local Recurrence and Survival
K. Wimmer, M. Bolliger, Z. Bago-Horvath, G. Steger, D. Kauer-Dorner, R. Helfgott, C. Gruber, F. Moinfar, M. Mittlböck, F. Fitzal
Annals of Surgical Oncology.2020; 27(5): 1700. CrossRef - Predictive factors for omitting lymphadenectomy in patients with node‐positive breast cancer treated with neo‐adjuvant systemic therapy
Sergi Fernandez‐Gonzalez, Catalina Falo, Maria J. Pla, Paula Verdaguer, Diana Nuñez, Anna Guma, Teresa Soler, Andrea Vethencourt, Silvia Vázquez, Maria Eulalia Fernandez‐Montoli, Miriam Campos, Sonia Pernas, Miguel Gil, Jordi Ponce, Amparo Garcia‐Tejedor
The Breast Journal.2020; 26(5): 888. CrossRef - Is There a Role for Post-Mastectomy Radiotherapy for T1-2N1 Breast Cancers With Node-Positive Pathology After Patients Become Node-Negative Pathology Following Neoadjuvant Chemotherapy?
Qian Wang, Jingjing Zhao, Xiaowei Han, Puchun Er, Xiangying Meng, Jinyan Shi, Huiru Sun, Jingyang Zhu, Li Zhu, Shikai Wu, Wencheng Zhang, Bing Sun
Frontiers in Oncology.2020;[Epub] CrossRef - Prognostic role of microRNA 182 and microRNA 18a in locally advanced triple negative breast cancer
Rajat Bajaj, Rupal Tripathi, T. S. Sridhar, Aruna Korlimarla, Kumardeep Dutta Choudhury, Moushumi Suryavanshi, Anurag Mehta, Dinesh Chandra Doval, Elda Tagliabue
PLOS ONE.2020; 15(11): e0242190. CrossRef - Association of Pathologic Complete Response with Long-Term Survival Outcomes in Triple-Negative Breast Cancer: A Meta-Analysis
Min Huang, Joyce O'Shaughnessy, Jing Zhao, Amin Haiderali, Javier Cortés, Scott D. Ramsey, Andrew Briggs, Peter Hu, Vassiliki Karantza, Gursel Aktan, Cynthia Z. Qi, Chenyang Gu, Jipan Xie, Muhan Yuan, John Cook, Michael Untch, Peter Schmid, Peter A. Fasch
Cancer Research.2020; 80(24): 5427. CrossRef - Multiparametric MR imaging to assess response following neoadjuvant systemic treatment in various breast cancer subtypes: Comparison between different definitions of pathologic complete response
G Santamaría, X Bargalló, S Ganau, I Alonso, M Muñoz, M Mollà, PL Fernández, A Prat
European Journal of Radiology.2019; 117: 132. CrossRef - Prognostic significance of residual nodal burden using lymph node ratio in locally advanced breast cancer after neoadjuvant chemotherapy
Reshu Agarwal, Arun Philip, Keechilat Pavithran, Anupama Rajanbabu, Gaurav Goel, DK Vijaykumar
Indian Journal of Cancer.2019; 56(3): 228. CrossRef - Application of neoadjuvant chemotherapy in occult breast cancer
Haisong Yang, Ling Li, Mengmeng Zhang, Shiyong Zhang, Shu Xu, Xiaoxia Ma
Medicine.2017; 96(40): e8200. CrossRef - Wnt7a Deficiency Could Predict Worse Disease-Free and Overall Survival in Estrogen Receptor-Positive Breast Cancer
Kijong Yi, Kyueng-Whan Min, Young Chan Wi, Yeseul Kim, Su-Jin Shin, Min Sung Chung, Kiseok Jang, Seung Sam Paik
Journal of Breast Cancer.2017; 20(4): 361. CrossRef
- Differential prognostic value of residual nodal burden in breast cancer subtypes

 E-submission
E-submission

 First
First Prev
Prev



