Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(2); 2015 > Article
-
Review
Role of Osteal Macrophages in Bone Metabolism - Sun Wook Cho
-
Journal of Pathology and Translational Medicine 2015;49(2):102-104.
DOI: https://doi.org/10.4132/jptm.2015.02.02
Published online: March 12, 2015
Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- Corresponding Author: Sun Wook Cho, M.D., Ph.D. Department of Internal Medicine, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 110-744, Korea Tel: +82-2-2072-2114 Fax: +82-2-762-2292 E-mail: swchomd@gmail.com
• Received: January 31, 2015 • Accepted: February 2, 2015
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Macrophages have been shown to have pleiotropic functions in various pathophysiologies, especially in terms of anti-inflammatory and regenerative activity. Recently, the novel functions of bone marrow resident macrophages (called osteal macrophages) were intensively studied in bone development, remodeling and tissue repair processes. This review discusses the current evidence for a role of osteal macrophages in bone modeling, remodeling, and fracture healing processes.
- Evidence suggests that macrophages participate in pleiotropic aspects of bone metabolism. Macrophages have been involved in vascular calcification in ectopic bone formation. Co-culture of macrophages with calcifying vascular cells [6] or human vascular smooth muscle cells [7] enhanced alkaline phosphatase activity and mineralization potential. Tumor necrosis factor [6,7] and oncostatin M [7] have been suggested as molecular mediators of macrophage-derived vascular calcification. In addition, depletion of macrophages reduced osteophyte formation in osteoarthritic models [8-10], and macrophages have been implicated in the sites of pathologic bone loss in inflammatory bone disorders [11,12]. These observations suggest that macrophages play a critical role in bone formation and mineralization.
- Depletion of macrophages in primary calvarial osteoblast cultures in vitro has been shown to delay osteogenic differentiation and mineralization [4,13]. In the macrophage fas-induced apoptosis (Mafia) transgenic mouse model, short-term depletion of macrophages with treatment of a synthetic ligand in vivo showed a quantitative reduction of bone formation sites in endocortical bones [4,13]. In a recent study, in vivo long-term depletion of macrophages in young (3–21 days) and adult (16–22 months) Mafia mice demonstrated an osteopenic phenotypes with suppressed serum bone turnover markers [5]. The anabolic actions of PTH in bone were markedly reduced in this model [5]. This study reinforced the hypothesis that osteal macrophages play a pivotal role in bone anabolism. Another independent study with lysozyme M-deficient mice also showed that pre-natal macrophage depletion led to early skeletal growth retardation and progressive osteoporosis [14]. The latter two studies clearly showed that functional osteoclasts were not significantly affected in these macrophage-deficient models [5,14]. Taken together, these results suggest that osteal macrophages play an essential role in normal bone development and remodeling, especially through anabolic actions.
- One of the critical concerns with osteal macrophages is how to distinguish bone marrow resident macrophages from osteoclasts, since they share the same precursors. Studies of Mafia mice [5] or lysozyme M-deficient mice [14] showed functionally active osteoclast activities, while macrophages were remarkably depleted, but still there were possible impacts of subtle changes in osteoclasts on overall bone metabolism. A recent study showed more clear evidence supporting the independent presence of functioning osteal macrophages apart from osteoclasts. CCL5- deficient mice showed decreased F4/80-positive macrophages at the endocortical bone surface, following reduced bone formation compared to the wild-type mice [15]. Osteoclastogenesis was enhanced in this model [15]. More studies with a bone marrowspecific macrophage depletion model are needed.
OSTEAL MACROPHAGES IN BONE FORMATION
- Fracture healing is composed of inflammation and bone repair processes, including endochondral ossification. Previous studies have demonstrated that macrophages are present during multiple stages of fracture healing, and produce mesenchymal growth factors [16]. Macrophages are also associated with more stable callus formation and healthy union [17].
- Keeping pace with the studies of osteal macrophages in bone metabolism, several groups have extensively studied how osteal macrophages participate in fracture healing of bone. Both systemic and local depletions of macrophages delayed fracture healing and impaired woven bone formation, while treatment of colony stimulating factor 1 increased macrophage recruitment into the injury sites and supported woven bone formation [18]. This study showed that macrophages were essential for collagen type I-positive matrix formation and bone mineralization [18]. Similarly, an independent study also showed that depletion of macrophages during fracture repair, even after several days later to fracture, led to impaired bone union with incomplete callus formation accompanied with more fibrotic changes. They observed that macrophages were also involved in promoting the osteogenic differentiation of marrow mesenchymal progenitor cells [14]. Moreover, a recent study clarified that inflammatory M1-macrophages (F4/80+ Mac-2+ ) played a crucial role in the initiation of early inflammation, and both inflammatory (F4/80+ Mac-2+ ) and resident (F4/80+ Mac-2- ) macrophages derived anabolic signals for endochondral callus formations in murine fracture models [19]. Taken together, accumulating evidence suggests that macrophages and their specific molecular mediators contribute to fracture healing in a phase-specific polarization-dependent manner.
OSTEAL MACROPHAGES IN BONE REPAIR
- To date, osteal macrophages have been considered a third cellular component, in addition to osteoblasts and osteoclasts. Macrophages construct a cellular canopy structure over bone remodeling sites, coordinate osteoclast-to-osteoblast coupling, and drive anabolic cytokines for bone formation. Macrophages also create a regenerative microenvironment in the fracture healing processes. In addition, macrophages might play a role in bone and marrow interactions especially at the osteoblastic stem cell niche. Targeting osteal macrophages or their molecular mediators could be potent therapeutic challenges for developing anabolic therapies for bone disease. Further studies are needed to develop specific targets that could be distinguished from osteoclast or inflammatory macrophages.
CONCLUSIONS AND FUTURE DIRECTIONS
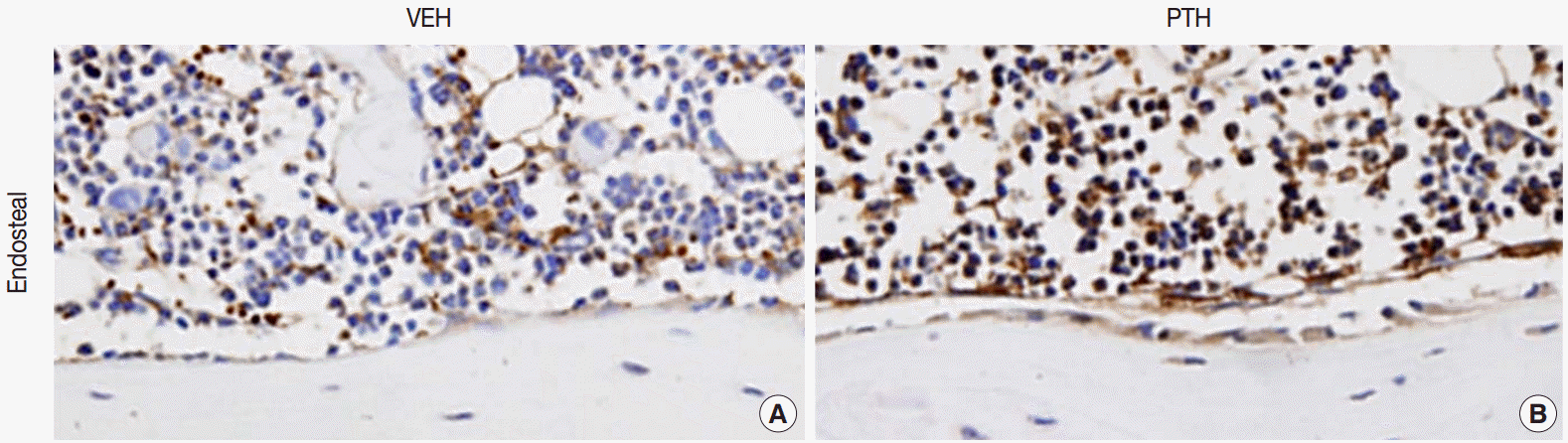
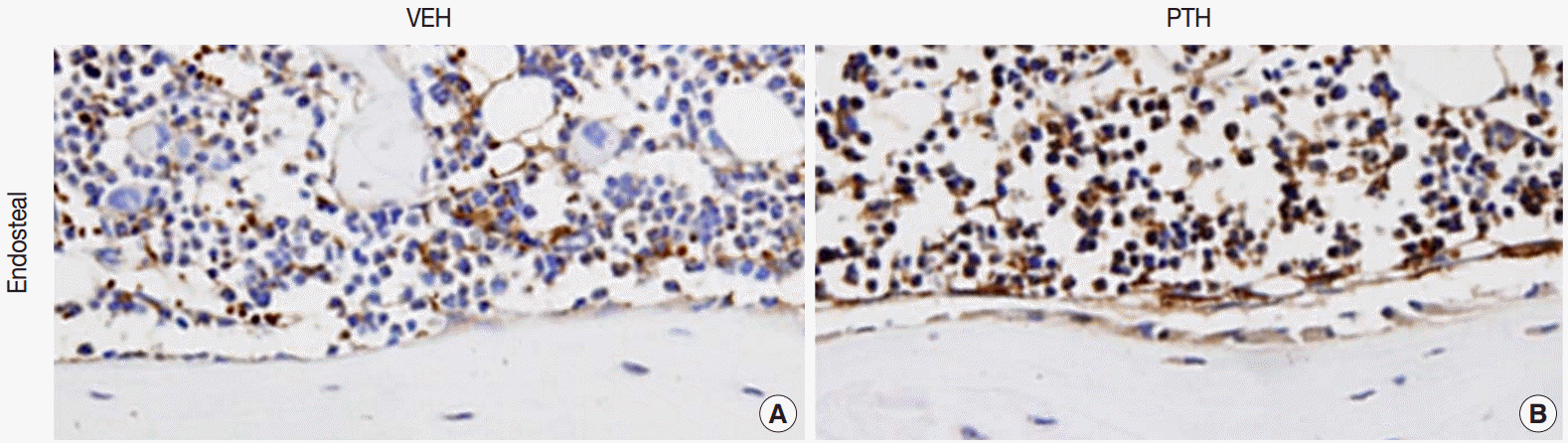
Fig. 1.Osteal macrophages at the bone formation sites of murine bones. F4/80-positive osteal macrophages create a canopy-like structure over the bone remodeling site. Compare to the vehicle (VEH) treatment (A), administration of parathyroid hormones (PTH) enhances bone formation, resulting in cuboidal-changes of osteoblasts and increased recruitment of osteal macrophages at bone remodeling sites (B) [5].

- 1. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11: 723-37. ArticlePubMedPMCPDF
- 2. Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 1981; 11: 805-15. ArticlePubMed
- 3. Lean JM, Matsuo K, Fox SW, et al. Osteoclast lineage commitment of bone marrow precursors through expression of membrane-bound TRANCE. Bone 2000; 27: 29-40. ArticlePubMed
- 4. Chang MK, Raggatt LJ, Alexander KA, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008; 181: 1232-44. ArticlePubMedPDF
- 5. Cho SW, Soki FN, Koh AJ, et al. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A 2014; 111: 1545-50. ArticlePubMedPMC
- 6. Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation 2002; 105: 650-5. ArticlePubMed
- 7. Shioi A, Katagi M, Okuno Y, et al. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res 2002; 91: 9-16. PubMed
- 8. van Lent PL, Blom AB, van der Kraan P, et al. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum 2004; 50: 103-11. PubMed
- 9. Blom AB, van Lent PL, Holthuysen AE, et al. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 2004; 12: 627-35. ArticlePubMed
- 10. Kamekura S, Hoshi K, Shimoaka T, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage 2005; 13: 632-41. ArticlePubMed
- 11. Kaneko M, Tomita T, Nakase T, et al. Expression of proteinases and inflammatory cytokines in subchondral bone regions in the destructive joint of rheumatoid arthritis. Rheumatology (Oxford) 2001; 40: 247-55. ArticlePubMed
- 12. Haynes DR, Hay SJ, Rogers SD, Ohta S, Howie DW, Graves SE. Regulation of bone cells by particle-activated mononuclear phagocytes. J Bone Joint Surg Br 1997; 79: 988-94. ArticlePubMed
- 13. Pettit AR, Chang MK, Hume DA, Raggatt LJ. Osteal macrophages: a new twist on coupling during bone dynamics. Bone 2008; 43: 976-82. ArticlePubMed
- 14. Vi L, Baht GS, Whetstone H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 2014 Dec 8 [Epub] http://dx.doi.org/10.1002/jbmr.2422. ArticlePDF
- 15. Wintges K, Beil FT, Albers J, et al. Impaired bone formation and increased osteoclastogenesis in mice lacking chemokine (C-C motif)ligand 5 (Ccl5). J Bone Miner Res 2013; 28: 2070-80. ArticlePubMedPDF
- 16. Bourque WT, Gross M, Hall BK. Expression of four growth factors during fracture repair. Int J Dev Biol 1993; 37: 573-9. ArticlePubMedPDF
- 17. Hankemeier S, Grassel S, Plenz G, Spiegel HU, Bruckner P, Probst A. Alteration of fracture stability influences chondrogenesis, osteogenesis and immigration of macrophages. J Orthop Res 2001; 19: 531-8. ArticlePubMed
- 18. Alexander KA, Chang MK, Maylin ER, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 2011; 26: 1517-32. ArticlePubMedPDF
- 19. Raggatt LJ, Wullschleger ME, Alexander KA, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol 2014; 184: 3192-204. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- 3D printed composite scaffold accelerates bone regeneration by modulating immunity and promoting angiogenesis
Yiye Fan, Jiaxin Yao, Wan Liu, Lebin Wang, Jing Yang, Xiaoyan Zheng, Junfeng Hui, Daidi Fan
Journal of Materials Science & Technology.2026; 240: 1. CrossRef - Transcriptomic profiling reveals a dramatic inflammatory shift in osteal macrophages during colitis-induced osteoporosis
Ryota Suzuki, Liyile Chen, Tsutomu Endo, Taiki Tokuhiro, Masaya Nakajo, Yuki Ogawa, Hend Alhasan, Taku Ebata, Daisuke Takahashi, Ken Kadoya, Masahiko Takahata, Norimasa Iwasaki, M. Alaa Terkawi
Inflammation Research.2025;[Epub] CrossRef - Rescuing Mitochondrial Dysfunction in Macrophages Prevents Osteonecrosis of the Jaw in Anti‐Resorptive Therapy
Hang Zhang, Xin Shen, Haiyang Liu, Xinxi Yuan, Mumin Cao, Xuepeng Lv, Ziji Ling, Songsong Guo, Rongyao Xu, Xiang Li, Hongbing Jiang
Advanced Science.2025;[Epub] CrossRef - Surgical stress induced tumor immune suppressive environment
Fan Yang, Qing Hua, Xiaoyan Zhu, Pingbo Xu
Carcinogenesis.2024; 45(4): 185. CrossRef - A Systematic Review and Meta-Analysis of the Outcomes of Reconstruction with Vascularised vs Non-Vascularised Bone Graft after Surgical Resection of Primary Malignant and Non-Malignant Bone Tumors
R. PATEL, G. MCCONAGHIE, M. M. KHAN, W. GIBSON, R. SINGH, R. BANERJEE
Acta chirurgiae orthopaedicae et traumatologiae Cechoslovaca.2024; 91(3): 143. CrossRef - Macrophage Polarization during MRONJ Development in Mice
A. Soundia, N. Elzakra, D. Hadaya, I. Gkouveris, O. Bezouglaia, S. Dry, T. Aghaloo, S. Tetradis
Journal of Dental Research.2024; 103(9): 899. CrossRef - 3D printing of gear-inspired biomaterials: Immunomodulation and bone regeneration
Xiaopeng Yu, Yufeng Wang, Meng Zhang, Hongshi Ma, Chun Feng, Bingjun Zhang, Xin Wang, Bing Ma, Qingqiang Yao, Chengtie Wu
Acta Biomaterialia.2023; 156: 222. CrossRef - Origin, production and molecular determinants of macrophages for their therapeutic targeting
Sangita Chowdhury, Arun K. Trivedi
Cell Biology International.2023; 47(1): 15. CrossRef - The Macrophage’s Role on Bone Remodeling and Osteogenesis: a Systematic Review
João Maria Orvalho, Juliana Campos Hasse Fernandes, Rogerio Moraes Castilho, Gustavo Vicentis Oliveira Fernandes
Clinical Reviews in Bone and Mineral Metabolism.2023; 21(1-4): 1. CrossRef - Neglected immunoregulation: M2 polarization of macrophages triggered by low‐dose irradiation plays an important role in bone regeneration
Shaoqing Chen, Su Ni, Chun Liu, Mu He, Yiwen Pan, Pengfei Cui, Cheng Wang, Xinye Ni
Journal of Cellular and Molecular Medicine.2023; 27(8): 1095. CrossRef - Insight into the effect of biomaterials on osteogenic differentiation of mesenchymal stem cells: A review from a mitochondrial perspective
Ziyi Feng, Meiqi Jin, Junzhi Liang, Junning Kang, Huazhe Yang, Shu Guo, Xiaoting Sun
Acta Biomaterialia.2023; 164: 1. CrossRef - Nano wear particles and the periprosthetic microenvironment in aseptic loosening induced osteolysis following joint arthroplasty
Yu Xie, Yujie Peng, Guangtao Fu, Jiewen Jin, Shuai Wang, Mengyuan Li, Qiujian Zheng, Feng-Juan Lyu, Zhantao Deng, Yuanchen Ma
Frontiers in Cellular and Infection Microbiology.2023;[Epub] CrossRef - Integrated computational and in vivo models reveal Key Insights into macrophage behavior during bone healing
Etienne Baratchart, Chen Hao Lo, Conor C. Lynch, David Basanta, Dominik Wodarz
PLOS Computational Biology.2022; 18(5): e1009839. CrossRef - Strategies of Macrophages to Maintain Bone Homeostasis and Promote Bone Repair: A Narrative Review
Yingkun Hu, Jinghuan Huang, Chunying Chen, Yi Wang, Zhuowen Hao, Tianhong Chen, Junwu Wang, Jingfeng Li
Journal of Functional Biomaterials.2022; 14(1): 18. CrossRef - Macrophages and Stem Cells—Two to Tango for Tissue Repair?
Emilia Manole, Cristina Niculite, Ioana Maria Lambrescu, Gisela Gaina, Octavian Ioghen, Laura Cristina Ceafalan, Mihail Eugen Hinescu
Biomolecules.2021; 11(5): 697. CrossRef - Bone remodeling stages under physiological conditions and glucocorticoid in excess: Focus on cellular and molecular mechanisms
V. V. Povoroznyuk, N. V. Dedukh, M. A. Bystrytska, V. S. Shapovalov
Regulatory Mechanisms in Biosystems.2021; 12(2): 212. CrossRef - Menaquinone-7 Supplementation Improves Osteogenesis in Pluripotent Stem Cell Derived Mesenchymal Stem Cells
Asim Cengiz Akbulut, Grzegorz B. Wasilewski, Nikolas Rapp, Francesco Forin, Heike Singer, Katrin J. Czogalla-Nitsche, Leon J. Schurgers
Frontiers in Cell and Developmental Biology.2021;[Epub] CrossRef - The Effects of Biomaterial Implant Wear Debris on Osteoblasts
Li Zhang, El-Mustapha Haddouti, Kristian Welle, Christof Burger, Dieter C. Wirtz, Frank A. Schildberg, Koroush Kabir
Frontiers in Cell and Developmental Biology.2020;[Epub] CrossRef Local Cellular Responses to Metallic and Ceramic Nanoparticles from Orthopedic Joint Arthroplasty Implants
Li Zhang, El-Mustapha Haddouti, Kristian Welle, Christof Burger, Koroush Kabir, Frank A Schildberg
International Journal of Nanomedicine.2020; Volume 15: 6705. CrossRef- Mesenchymal stem cell-macrophage crosstalk and bone healing
Jukka Pajarinen, Tzuhua Lin, Emmanuel Gibon, Yusuke Kohno, Masahiro Maruyama, Karthik Nathan, Laura Lu, Zhenyu Yao, Stuart B. Goodman
Biomaterials.2019; 196: 80. CrossRef - Inflammation, mesenchymal stem cells and bone regeneration
Hongrui Liu, Dongfang Li, Yi Zhang, Minqi Li
Histochemistry and Cell Biology.2018; 149(4): 393. CrossRef - Inflammatory and degenerative phases resulting from anterior cruciate rupture in a non‐invasive murine model of post‐traumatic osteoarthritis
Sophie J. Gilbert, Cleo S. Bonnet, Paulina Stadnik, Victor C. Duance, Deborah J. Mason, Emma J. Blain
Journal of Orthopaedic Research.2018; 36(8): 2118. CrossRef - M2 macrophages are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury: a retrospective cohort study
Ran Zhang, Yi Liang, Shuxiang Wei
Journal of Orthopaedic Surgery and Research.2018;[Epub] CrossRef - Digesting the role of bone marrow macrophages on hematopoiesis
Esther Heideveld, Emile van den Akker
Immunobiology.2017; 222(6): 814. CrossRef - Concise Review: Stem Cells in Osteoimmunology
Fernando A. Fierro, Jan A. Nolta, Iannis E. Adamopoulos
Stem Cells.2017; 35(6): 1461. CrossRef - Aging, inflammation, stem cells, and bone healing
Emmanuel Gibon, Laura Lu, Stuart B. Goodman
Stem Cell Research & Therapy.2016;[Epub] CrossRef - The roles of immune cells in bone healing; what we know, do not know and future perspectives
Jehan J. El-Jawhari, Elena Jones, Peter V. Giannoudis
Injury.2016; 47(11): 2399. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Role of Osteal Macrophages in Bone Metabolism

Fig. 1. Osteal macrophages at the bone formation sites of murine bones. F4/80-positive osteal macrophages create a canopy-like structure over the bone remodeling site. Compare to the vehicle (VEH) treatment (A), administration of parathyroid hormones (PTH) enhances bone formation, resulting in cuboidal-changes of osteoblasts and increased recruitment of osteal macrophages at bone remodeling sites (B) [5].
Fig. 1.
Role of Osteal Macrophages in Bone Metabolism

 E-submission
E-submission