Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 49(2); 2015 > Article
-
Original Article
Image-Guided Fine Needle Cytology with Aspiration Versus Non-Aspiration in Retroperitoneal Masses: Is Aspiration Necessary? - Rajiv Kumar Misra, Shaila Mitra, Rishav Kumar Jain1, Shilpa Vahikar, Archana Bundela, Purak Misra2
-
Journal of Pathology and Translational Medicine 2015;49(2):129-135.
DOI: https://doi.org/10.4132/jptm.2015.01.28
Published online: March 12, 2015
Department of Pathology, B.R.D. Medical College, Gorakhpur, India
1Department of Radiology, B.R.D. Medical College, Gorakhpur, India
2Apollo Hospital, New Delhi, India
- Corresponding Author: Shaila Mitra, M.D. Department of Pathology, B.R.D Medical College, Gorakhpur, 273013 Uttar Pradesh, India Tel: +91-7379651447 Fax: +91-0551-2501736 E-mail: shaila.prasad14@yahoo.co.in
© 2015 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- Although using fine needle cytology with aspiration (FNC-A) for establishing diagnoses in the retroperitoneal region has shown promise, there is scant literature supporting a role of non-aspiration cytology (FNC-NA) for this region. We assessed the accuracy and reliability of FNC-A and FNC-NA as tools for preoperative diagnosis of retroperitoneal masses and compared the results of both techniques with each other and with histopathology.
-
Methods:
- Fifty-seven patients with retroperitoneal masses were subjected to FNC-A and FNC-NA. Smears were stained with May-Grunwald Giemsa and hematoxylin and eosin stain. An individual slide was objectively analysed using a point scoring system to enable comparison between FNC-A and FNC-NA.
-
Results:
- By FNC-A, 91.7% accuracy was obtained in cases of retroperitoneal lymph node lesions followed by renal masses (83.3%). The diagnostic accuracy of other sites by FNC-A varied from 75.0%–81.9%. By FNC-NA, 93.4% diagnostically accurate results were obtained in the kidney, followed by 75.0% in adrenal masses. The diagnostic accuracy of other sites by FNC-NA varied from 66.7%–72.8%.
-
Conclusions:
- Although both techniques have their own advantages and disadvantages, FNC-NA may be a more efficient adjuvant method of sampling in retroperitoneal lesions.
- The study was carried out in the Department of Pathology from August 2010 to July 2013 on 57 patients with retroperitoneal masses on ultrasound (USG). After proper workup, including detailed clinical history and examination, FNC-A was performed using a 9 cm, 22–24 gauge spinal needle attached to a 20-mL syringe. FNC-NA was performed with a 22–24 gauge spinal needle without a syringe. Fine needle aspiration sampling was performed as described previously [3].
- FNC-NA was performed by holding the needle directly with the finger tips and inserting it into the target lesion with USG guidance. After reaching the site, the stylet was removed and the needle was moved back and forth in various directions at different depths. Removal of the stylet at this stage avoids contamination of diagnostic material with other tissues of the needle track. The needle was then taken out from the site and connected to a syringe filled with air. Cellular material was then expelled onto a glass slide. Uniform and thinly spread smears were obtained with the superimposition technique [1].
- Both cytotechniques were done at the same time and slides were made by a single operator, avoiding bias in all stages of sampling from patient examination to slide fixation. Smears were stained with May-Grunwald Giemsa and hematoxylin and eosin stain. An individual slide was objectively analysed using a point scoring system [4] to enable comparison between FNC-A and FNC-NA techniques as shown in Table 1. On the basis of the five criteria tabulated, a cumulative score between 0–10 points was allocated to each fine needle specimen, which was then categorized as unsuitable for cytodiagnosis (score, 0–2), suitable for cytodiagnosis (score, 3–6) or diagnostically superior (score, 7–10). Accuracy of the cytological diagnoses was assessed by two different pathologists through comparison with the histological diagnosis All the results so obtained were interpreted statistically using the student’s t-test.
MATERIALS AND METHODS
- Fifty-seven cases of retroperitoneal lesions were studied in patients ranging from 6–80 years of age. Most patients (56.1%) were 50–60 years old; 37 patients (64.9%) were male and 20 (35.0%) were female.
- Thirty cases (52.6%) were from the kidney followed by 12 cases (21.0%) from retroperitoneal lymph nodes. Eleven cases (19.2%) were from soft tissues and miscellaneous organs, while only four cases (7.0%) were from the adrenal glands.
- Among 30 cases of renal masses, eight (14.0%) were polycystic kidney disease, 16 cases (28.0%) were renal cell carcinoma and six (10.5%) were neuroblastoma. Out of twelve cases of retroperitoneal lymphadenopathy, tuberculous lymphadenitis was found in six cases (10.5%), while non-Hodgkin lymphoma and metastatic seminoma were found in three cases each (5.2%). Out of 11 cases (19.2%) of soft tissue tumours, six cases (10.5%) were diagnosed as liposarcoma, three cases (5.2%) as malignant fibrous histiocytoma, and two cases (3.5%) as fibrosarcoma. All four cases (7.0%) of adrenal mass were pheochromocytoma. The relatively high incidence of neuroblastoma does not reflect the epidemiological incidence of this area, because our center is a referral center and caters to the referred patients from eastern parts of India and Nepal. Although polycystic kidney does not present as a renal mass, a provisional diagnosis of cystic renal lesion was rendered in radiological workup. Therefore, cytological examination was performed to arrive at an accurate diagnosis and to differentiate among cystic lesions such as benign renal cysts, cystic renal cell carcinoma and polycystic kidney.
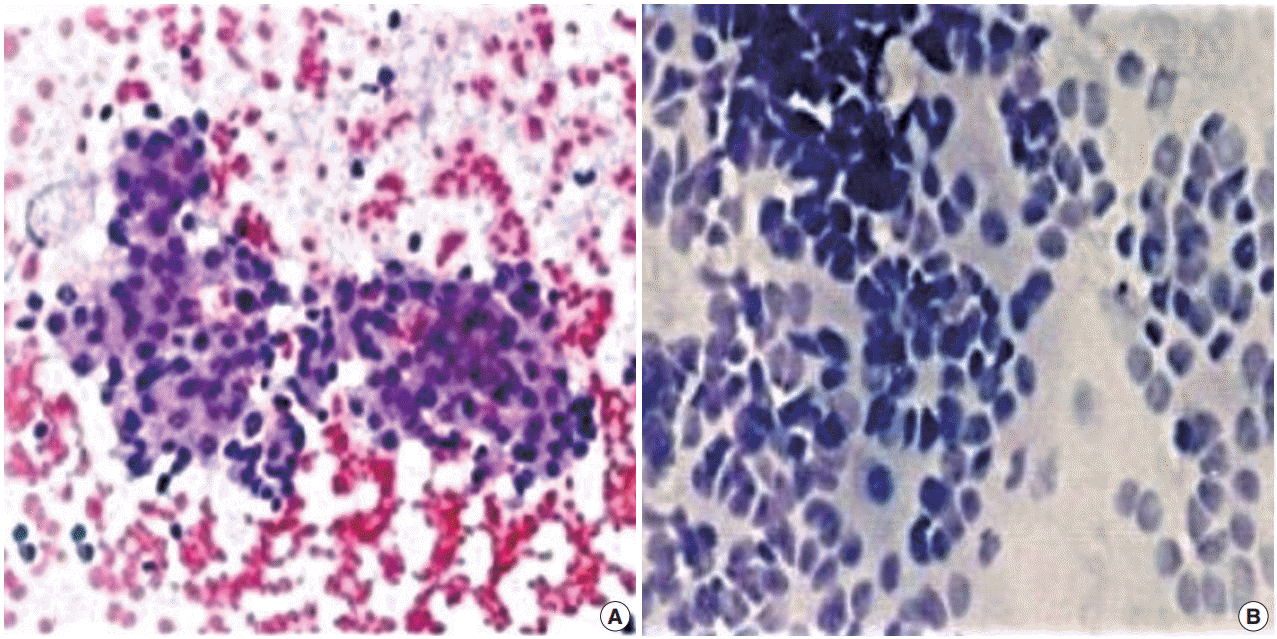
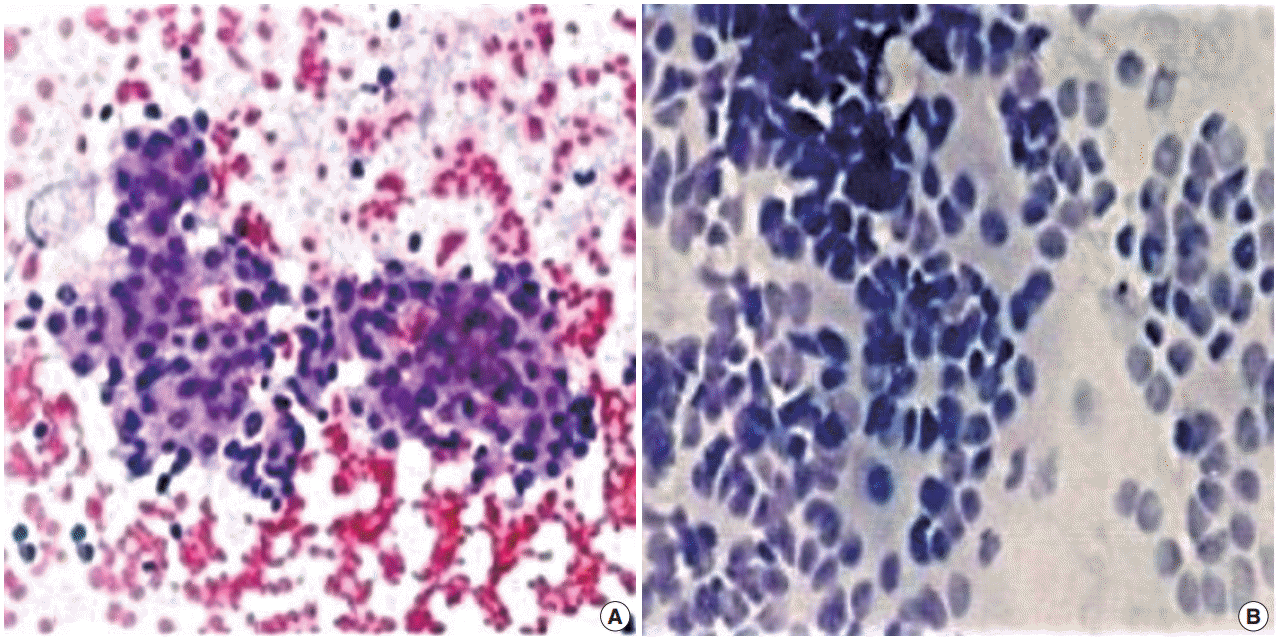
- FNC-A had more blood contamination than FNC-NA smears in all cases and the difference between the techniques was statistically significant in all cases except adrenal masses (Table 2, Fig. 1).
- FNC-A smears revealed more dislodged cellular material across the slides than FNC-NA smears but statistical superiority was seen only for retroperitoneal lymph nodes (p<.01) (Table 2).
- Cellular degeneration was greater in FNC-A in all cases, but this difference was statistically significant for kidney (p=.03) and miscellaneous groups (p=.04) only.
- Greater trauma was observed in FNC-A smears as evidenced by increased blood contamination, clumping of cells, and shrinkage artefacts along with chromatin smearing and smudging while cellular preservation was better in FNC-NA.
- When comparing the architectural arrangements of cells in smears obtained by both techniques, such as rosette formations in neuroblastoma, dissociated cells in lymphoma, glandular tissue fragments in adenocarcinoma, and papillary fragments in papillary tumors, the difference was statistically insignificant in all cases except the kidneys where FNC-NA was superior to FNC-A (p<.01).
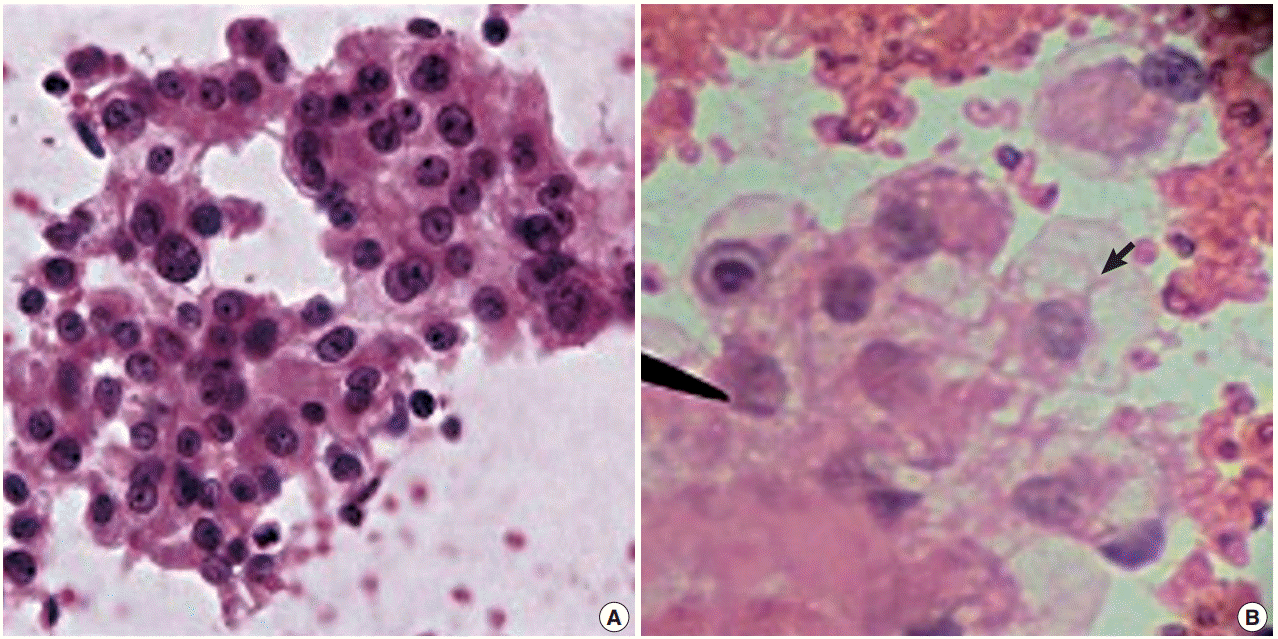
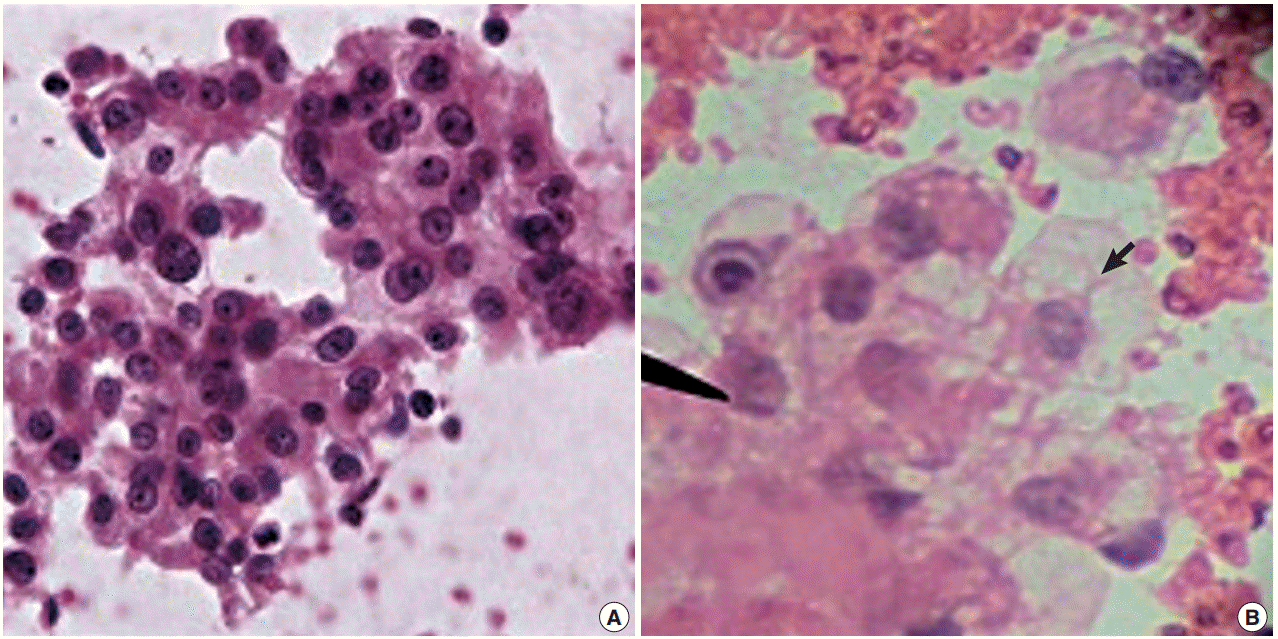
- There was a statistically insignificant difference in sampling technique score in all cases except in the kidney, where the FNCNA score was statistically significant (p<.01) (Table 2, Fig. 2).
- Smears obtained by FNC-A and FNC-NA techniques were then categorized on the basis of scores obtained (Table 3). FNCA produced a greater number of diagnostically adequate smears (31 cases, 54.4%) than FNC-NA (20 cases, 35.1%) (p=.03). FNC-NA provided more diagnostically “superior quality smears” (27 cases, 47.4%) than FNC-A (20 cases, 35.1%), but the difference was statistically insignificant (p=.18). Diagnosis could be made in 51 cases (89.4%) by FNC-A as compared to 47 cases (82.4%) by FNC-NA when combining superior and diagnostic quality scores. FNC-NA had more smears showing inadequate material for diagnosis (10 cases, 17.5%) than FNC-A (6 cases, 10.5%).
- The overall diagnostic accuracy was 82.4% in FNC-NA and 84.2% in FNC-A (p=.08). Accuracy of 91.7% was obtained in retroperitoneal lymph node lesions while 83.3% accuracy was obtained in renal masses by FNC-A. The diagnostic accuracy of other sites by FNC-A varied from 75.0%–81.9%. Diagnostically accurate results of 93.4% were obtained in the kidney and 75.0% diagnostically accurate results were obtained in adrenal masses by FNC-NA. The diagnostic accuracy of other sites by FNC-NA varied from 66.7%–72.8%, with lowest being in the retroperitoneal lymph nodes (Table 4).
RESULTS
- FNC-A is widely accepted as the primary method for diagnosis of palpable masses [5]. In 1930, Martin and Ellis [3] first presented a tumor diagnosis by needle aspiration and termed it “aspiration biopsy.” Franzen et al. [6] in 1955 introduced a special syringe holder and thus improved the technique [7,8].
- FNC-NA was developed in France by Brifford et al. [1] in 1982. It avoids aspiration and relies on capillary pressure to suck cells inside the needle core. The French authors termed this technique as “cytopuncture.” It has been shown that with the application of an objective scoring system, FNC-NA produces a comparable cellular yield, and has a similar diagnostic accuracy to the classic fine needle aspiration technique [9-11]. Many studies have proved that FNC-NA seems to be better for diagnosing malignant lesions while FNC-A appeared better for diagnosing benign lesions [12-14]. Malignant cells, being fragile, are more prone to degeneration and trauma of suction. The application of suction to draw cells through a fine needle traumatizes fragile cells, resulting in artifacts that can lead to diagnostic error. They opined that FNC-NA was more patient friendly, gave more cellular yield with less blood contamination and improved quality of the smears. FNC-A was considered as the procedure of choice for cystic lesions as the fluid could be collected for cytological evaluation. According to them better diagnostic results could be obtained if both the techniques are used together [9,11-15].
- The function of negative pressure is not to tear the cells from the tissue but to hold the tissue against the sharp cutting edge of the needle. Santos and Leiman [16] explained the scientific basis of the FNC-NA technique. This technique which employs the insertion of a fine needle into a lesion without attachment of a syringe, depends on the property of capillary tension in a narrow channel (outer diameter of needle, 0.6 mm). A fluid in a narrow channel is governed by the formula h=2T/pgr, where h is the height attained, T is the surface tension of the fluid, p is the density of that fluid, g is the gravity and r is the radius. They performed a more exhaustive comparative analysis using both FNC-A and FNC-NA techniques on 50 thyroid lesions. In their study, diagnostically superior material was obtained in 22 (44.0%) of the non-aspiration samples versus four of aspiration samples (8.0%) (p=.0033). This is probably because in FNC-NA, concentrated cellular material that was less distorted by blood had better preservation of architecture and excellent picture quality.
- Zajdela et al. [17] 1987, studied a large series of mammary tumors in order to compare the results of FNC-A with those of FNC-NA. In their study FNC-A was employed in all the cases prior to 1981, and after that FNC-NA was used. Therefore, these techniques were not used together on the same tumours or patient populations. With FNC-NA, more precise entry into the mass was possible and this is particularly important in locations like the orbit and thyroid, to avoid injury to the eyeball and trachea [18].
- Other workers [19-21] tried to explain the reason for the lesser degree of blood contamination by FNC-NA. They reported that this could be because the specimen is obtained by a spontaneous capillary action without much trauma to tissues. Thus, it gives a clean and clear picture to the cytopathologist. In FNCA, significant quantity of blood is aspirated, especially in vascular organs.
- Cellular yield was more or less comparable for both techniques except in the kidneys, where FNC-NA was significantly better. USG-guided percutaneous FNC-A of renal masses was first reported by Kristensen et al. [21]. The present findings are consistent with findings of Renshaw et al. [22]. However, Mair et al. [4] and Zajdela et al. [17] did not find any significant difference in the smears prepared by both techniques. Jayaram and Gupta [23] observed that cellularity was higher in aspiration smears than in non-aspiration smears in goiters. Zhou et al. [24] mentioned that FNC-A may be more suitable than FNC-NA for sampling nodules that measure from 5.1 to 10.0 mm and >20.0 mm. Stewart et al. [25] directly compared FNC-A and needle core biopsy in 141 patients undergoing image guided sampling of abdominal lesions and noticed that FNC-A cytology was more sensitive and accurate than biopsy.
- Available literature on cellular trauma, degeneration and retention of architecture revealed less cellular degeneration and cellular trauma in FNC-NA as compared to FNC-A [14,15]. The high suction pressure that is maintained during FNC-A causes some increase in cellular trauma [26,27]. In FNC-NA, since suction pressure is not used, concentrated cellular material shows better preservation of the architecture with less traumatic distortion and less contamination by blood. FNC-NA smears showed better retention of architecture and excellent picture quality, whereas FNC-A smears had good quantity of material. Ghosh et al. [28] also observed the same findings in their study of FNC-NA on thyroid lesions. Better preservation of architecture and excellent picture quality was the only parameter in which FNC-NA scored much better than FNC-A, as reported by other authors [14,15,18,28].
- Low cellularity and fibrous lesions appear to be a main cause of FNC-NA failure. However Zajdela et al. [17] found that the frequency of insufficient cellular yield was less by FNC-NA (5.5%) than FNC-A (6.0%).
- In this study, non-aspiration sampling was used as the first sampling method in all cases. This design was based on the interventional radiology literature, which suggests that if both methods are to be used, the less traumatic method (i.e., non-aspiration sampling) must precede the negative pressure method (FNC-A) to ensure the initial samples will contain less bloody material and be more amenable to rapid staining and analysis. The drawback of using this technique was that initial biopsies with non-aspiration sampling may have caused tissue damage and bleeding, putting subsequently collected FNC-A specimens at a disadvantage.
- In most cases, sufficient diagnostic material was dislodged over the slide by FNC-A, thereby increasing its diagnostic accuracy (p=.03). Out of 57 cases of retroperitoneal lumps, diagnosis was made in greater number of cases by FNC-A compared to FNC-NA, while the quality of smears was superior in FNCNA.
- We used a long, 24-gauge lumbar puncture needle of 24-gauge instead of a short hypodermic needles with good results, as cells are detached by the cutting edge of the needle and conducted into the lumen by capillary force. The caliber of the needle is more important than the length as noted by the physical principle that ascent of fluid into a narrow channel is governed by the formula h=2T/pgr.
- In retroperitoneal masses, USG-guided FNC-NA may be a more efficient adjuvant method of sampling. Non-aspiration (FNC-NA) provides “superb quality” of smears with superior diagnostic value and is less traumatic, simple, and easy to perform with better patient compliance. It also produces better quality of cellularity and less field obscurity by blood, and allows for much better control of the needle while in the lesion. In addition, direct contact with the needle allows a more sensitive fingertip feeling of the consistency of the tumor tissue during sampling. Preferably, FNC-NA should be performed initially, followed by FNC-A in order to attain a clear and accurate cytological diagnosis. In highly cellular lesions where abundant material was obtained, FNC-NA was most likely to be diagnostically superior, although FNC-A can also diagnose most lesions. In less cellular lesions, however, FNC-A was most likely to be diagnostically superior to FNC-NA. In addition, simple benign lesion or abscesses can be drained by aspiration for therapeutic purposes.
DISCUSSION
- 1. Briffod M, Gentile A, Hebert H. Cytopuncture in the follow-up of breast carcinoma. Acta Cytol 1982; 26: 195-200. PubMed
- 2. Zajdela A, de Maublanc MA, Schlienger P, Haye C. Cytologic diagnosis of orbital and periorbital palpable tumors using fine-needle sampling without aspiration. Diagn Cytopathol 1986; 2: 17-20. ArticlePubMed
- 3. Martin HE, Ellis EB. Biopsy by needle puncture and aspiration. Ann Surg 1930; 92: 169-81. ArticlePubMedPMC
- 4. Mair S, Dunbar F, Becker PJ, Du Plessis W. Fine needle cytology: is aspiration suction necessary? A study of 100 masses in various sites. Acta Cytol 1989; 33: 809-13. PubMed
- 5. Orell SR, Sterrett GF, Whitaker D. Fine needle aspiration cytology. 4th ed. Philadelphia: Elsevier Churchill Livingstone, 2005; 125-64.
- 6. Franzen S, Giertz G, Zajicek J. Cytological diagnosis of prostatic tumours by transrectal aspiration biopsy: a preliminary report. Br J Urol 1960; 32: 193-6. ArticlePubMed
- 7. Zajicek J, Franzén S, Jakobsson P, Rubio C, Unsgaard B. Aspiration biopsy of mammary tumors in diagnosis and research: a critical review of 2,200 cases. Acta Cytol 1967; 11: 169-75. PubMed
- 8. Kate MS, Kamal MM, Bobhate SK, Kher AV. Evaluation of fine needle capillary sampling in superficial and deep-seated lesions. An analysis of 670 cases. Acta Cytol 1998; 42: 679-84. PubMed
- 9. Kamal MM, Arjune DG, Kulkarni HR. Comparative study of fine needle aspiration and fine needle capillary sampling of thyroid lesions. Acta Cytol 2002; 46: 30-4. ArticlePubMed
- 10. Gadkari RU, Pangarkar M, Dandige S, Munshi M, Kher A. Efficacy of fine needle capillary sampling in the diagnosis of stage III and IV cervical carcinoma. Acta Cytol 1999; 43: 114-6. ArticlePubMed
- 11. Pothier DD, Narula AA. Should we apply suction during fine needle cytology of thyroid lesions? A systematic review and meta-analysis. Ann R Coll Surg Engl 2006; 88: 643-5. ArticlePubMedPMC
- 12. Suen KC, Quenville NF. Fine needle aspiration biopsy of the thyroid gland: a study of 304 cases. J Clin Pathol 1983; 36: 1036-45. ArticlePubMedPMC
- 13. Jayaram N, Chetan M, Prasad SR, Ramaprasad AV. Thyroiditis: thyroid function and cytologic correlation: a study of 66 cases. J Cytol 1996; 13: 21-4. Article
- 14. Raghuveer CV, Leekha I, Pai MR, Adhikari P. Fine needle aspiration cytology versus fine needle sampling without aspiration: a prospective study of 200 cases. Indian J Med Sci 2002; 56: 431-9. PubMed
- 15. Pinki P, Alok D, Ranjan A, Chand MN. Fine needle aspiration cytology versus fine needle capillary sampling in cytological diagnosis of thyroid lesion. Iran J Pathol 2015; 10: 47-53. PubMedPMC
- 16. Santos JE, Leiman G. Nonaspiration fine needle cytology: application of a new technique to nodular thyroid disease. Acta Cytol 1988; 32: 353-6. PubMed
- 17. Zajdela A, Zillhardt P, Voillemot N. Cytological diagnosis by fine needle sampling without aspiration. Cancer 1987; 59: 1201-5. ArticlePubMed
- 18. Rajasekhar A, Sundaram C, Chowdhary T, Charanpal M, Ratnakar KS. Diagnostic utility of fine-needle sampling without aspiration: a prospective study. Diagn Cytopathol 1991; 7: 473-6. ArticlePubMed
- 19. Rizvi SA, Husain M, Khan S, Mohsin M. A comparative study of fine needle aspiration cytology versus non-aspiration technique in thyroid lesions. Surgeon 2005; 3: 273-6. ArticlePubMed
- 20. Haddadi-Nezhad S, Larijani B, Tavangar SM, Nouraei SM. Comparison of fine-needle-nonaspiration with fine-needle-aspiration technique in the cytologic studies of thyroid nodules. Endocr Pathol 2003; 14: 369-73. ArticlePubMed
- 21. Kristensen JK, Bartels E, Jorgensen HE. Percutaneous renal biopsy under the guidance of ultrasound. Scand J Urol Nephrol 1974; 8: 223-6. ArticlePubMed
- 22. Renshaw AA, Granter SR, Cibas ES. Fine-needle aspiration of the adult kidney. Cancer 1997; 81: 71-88. ArticlePubMed
- 23. Jayaram G, Gupta B. Nonaspiration fine needle cytology in diffuse and nodular thyroid lesions: a study of 220 cases. Acta Cytol 1991; 35: 789-90. PubMed
- 24. Zhou JQ, Zhang JW, Zhan WW, et al. Comparison of fine-needle aspiration and fine-needle capillary sampling of thyroid nodules: a prospective study with emphasis on the influence of nodule size. Cancer Cytopathol 2014; 122: 266-73. ArticlePubMed
- 25. Stewart CJ, Coldewey J, Stewart IS. Comparison of fine needle aspiration cytology and needle core biopsy in the diagnosis of radiologically detected abdominal lesions. J Clin Pathol 2002; 55: 93-7. ArticlePubMedPMC
- 26. Hamburger JI, Hamburger SW. Fine needle biopsy of thyroid nodules: avoiding the pitfalls. N Y State J Med 1986; 86: 241-9. PubMed
- 27. Lowhagen T, Sprenger E. Cytologic presentation of thyroid tumors in aspiration biopsy smear. A review of 60 cases. Acta Cytol 1974; 18: 192-7. PubMed
- 28. Ghosh A, Misra RK, Sharma SP, Singh HN, Chaturvedi AK. Aspiration vs nonaspiration technique of cytodiagnosis: a critical evaluation in 160 cases. Indian J Pathol Microbiol 2000; 43: 107-12. PubMed
REFERENCES
Figure & Data
References
Citations

- A comparison of cytologic quality in fine‐needle specimens obtained with and without aspiration from superficial lymph nodes in the dog
V. Karakitsou, M. M. Christopher, E. Meletis, P. Kostoulas, D. Pardali, C. K. Koutinas, M. E. Mylonakis
Journal of Small Animal Practice.2022; 63(1): 16. CrossRef - A comparison of cytological quality between fine‐needle aspiration and non‐aspiration techniques for obtaining ultrasound‐guided samples from canine and feline lymph nodes
James Whitlock, Olivier Taeymans, Paola Monti
Veterinary Record.2021;[Epub] CrossRef - A randomised controlled comparison of aspiration and non-aspiration fine-needle techniques for obtaining ultrasound-guided cytological samples from canine livers
K.L. Fleming, E.J. Howells, E.J. Villiers, T.W. Maddox
The Veterinary Journal.2019; 252: 105372. CrossRef - Minimally invasive biopsy in retroperitoneal tumors (Review)
Radu Marcu, Camelia Diaconu, Traian Constantin, Bogdan Socea, Florentina Ionita‑Radu, Dan Mischianu, Ovidiu Bratu
Experimental and Therapeutic Medicine.2019;[Epub] CrossRef - For diagnosis of liver masses, fine-needle aspiration versus needle core biopsy: which is better?
Liye Suo, Ruby Chang, Vijayalakshmi Padmanabhan, Shilpa Jain
Journal of the American Society of Cytopathology.2018; 7(1): 46. CrossRef - Fine needle aspiration in retroperitoneal lesions
Parikshaa Gupta, Arvind Rajwanshi, Raje Nijhawan, Radhika Srinivasan, Nalini Gupta, Uma Nahar Saikia, Pranab Dey
APMIS.2017; 125(1): 16. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Criteria | Description | Point score | |
|---|---|---|---|
| Background, blood clot | Large amount | Great compromise to diagnosis | 0 |
| Large amount | Diagnosis still possible | 0.5 | |
| Moderate | Diagnosis possible | 1 | |
| Moderate | Diagnosis evident | 1.5 | |
| Minimal | Excellent quality | 2 | |
| Amount of cellular material | Absent | Diagnosis not possible | 0 |
| Minimal | Diagnosis still possible | 0.5 | |
| Moderate | Sufficient for diagnosis | 1 | |
| Moderate to abundant | Diagnosis evident | 1.5 | |
| Abundant | Diagnosis simple, excellent quality | 2 | |
| Degree of cellular degeneration | Marked | Diagnosis impossible | 0 |
| Marked | Diagnosis still possible | 0.5 | |
| Moderate | Diagnosis possible | 1 | |
| Moderate | Diagnosis evident | 1.5 | |
| Minimal | Diagnosis easy | 2 | |
| Degree of cellular trauma | Marked | Diagnosis impossible | 0 |
| Marked | Diagnosis still possible | 0.5 | |
| Moderate | Diagnosis possible | 1 | |
| Moderate | Diagnosis evident | 1.5 | |
| Minimal | Diagnosis easy | 2 | |
| Retention of appropriate architectures | Minimal to absent | Diagnosis impossible | 0 |
| Minimal | Diagnosis still possible | 0.5 | |
| Moderate | Some preservation | 1 | |
| Follicles, papillae, acini, flat sheets, syncitia, single cells, etc. | |||
| Diagnosis evident | |||
| Moderate | Excellent architectural display closely reflecting histological diagnosis | 1.5 | |
| Excellent | 2 |
| Site | Background, blood clot | Amount of cellular material | Degeneration | Cell trauma | Maintenance of architectural/cellular arrangement | Average |
|---|---|---|---|---|---|---|
| Kidney (n=30) | ||||||
| FNC-NA | 1.630 ± 0.556 | 1.060 ± 0.365 | 1.580 ± 0.648 | 1.360 ± 0.614 | 1.260 ± 0.520 | 6.530 ± 1.846 |
| FNC-A | 1.160 ± 0.580 | 1.260 ± 0.520 | 1.000 ± 0.574 | 1.300 ± 0.534 | 0.520 ± 0.210 | 5.090 ± 1.246 |
| p-value | <.01 | .09 | .03 | .31 | <.01 | <.01 |
| Adrenal (n=4) | ||||||
| FNC-NA | 1.00 ± 0.707 | 0.75 ± 0.830 | 1.020 ± 0.72 | 1.000 ± 0.707 | 0.980 ± 0.707 | 2.000 ± 1.590 |
| FNC-A | 0.75 ± 0.830 | 0.99 ± 0.810 | 0.680 ± 0.789 | 0.750 ± 0.830 | 0.980 ± 0.707 | 1.500 ± 1.660 |
| p-value | .66 | .69 | .55 | .25 | >.99 | .68 |
| RPLN (n=12) | ||||||
| FNC-NA | 1.33 ± 0.346 | 0.580 ± 0.3 | 1.200 ± 0.484 | 1.600 ± 0.648 | 0.916 ± 0.493 | 5.960 ± 2.780 |
| FNC-A | 0.916 ± 0.277 | 1.290 ± 0.62 | 1.023 ± 0.348 | 1.000 ± 0.578 | 0.916 ± 0.493 | 6.500 ± 2.160 |
| p-value | <.01 | <.01 | .11 | .03 | >.99 | .60 |
| Miscellaneous (n=11) | ||||||
| FNC-NA | 1.020 ± 0.417 | 0.660 ± 0.486 | 1.020 ± 0.417 | 1.020 ± 0.319 | 0.630 ± 0.298 | 6.360 ± 1.846 |
| FNC-A | 0.59 ± 0.298 | 1.000 ± 0.574 | 0.590 ± 0.312 | 0.520 ± 0.312 | 0.997 ± 0.660 | 5.090 ± 1.246 |
| p-value | <.01 | .13 | .04 | .001 | .05 | .07 |
| Total (n=57) | ||||||
| FNC-NA | 1.105 ± 0.325 | 1.139 ± 0.464 | 1.233 ± 0.426 | 1.267 ± 0.455 | 1.067 ± 0.456 | 5.833 ± 1.403 |
| FNC-A | 1.102 ± 0.425 | 1.161 ± 0.611 | 0.911 ± 0.339 | 0.642 ± 0.321 | 1.170 ± 0.488 | 4.884 ± 1.146 |
| p-value | .26 | .82 | <.01 | <.01 | .15 | <.01 |
| Quality of smear | FNC-A | FNC-NA | p-value |
|---|---|---|---|
| Superior (7-10) | 20 (35.1) | 27 (47.4) | .18 |
| Diagnostic (3-6) | 31 (54.4) | 20 (35.1) | .03 |
| Superior+Diagnostic (3-10) | 51 (89.4) | 47 (82.4) | .28 |
| Insufficient (0-2) | 6 (10.5) | 10 (17.5) | - |
| Site | Histopathology obtained | Diagnostic accuracy |
p-value | |
|---|---|---|---|---|
| FNC-NA | FNC-A | |||
| Kidney | 30 | 28 (93.4) | 25 (83.3) | .42 |
| Adrenal | 4 | 3 (75.0) | 3 (75.0) | >.99 |
| RPLN | 12 | 8 (66.7) | 11 (91.6) | .31 |
| Miscellaneous | 11 | 8 (72.8) | 9 (81.9) | >.05 |
| Total | 57 | 47 (82.4) | 48 (84.2) | .80 |
FNC-NA, fine needle cytology with non-aspiration; FNC-A, fine needle cytology with aspiration; RPLN, retroperitoneal lymph node.
Values are presented as number (%). FNC-A, fine needle cytology with aspiration; FNC-NA, fine needle cytology with non-aspiration.
Values are presented as number (%). FNC-NA, fine needle cytology with non-aspiration; FNC-A, fine needle cytology with aspiration; RPLN, retroperitoneal lymph node.

 E-submission
E-submission