Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(3); 2016 > Article
-
Case Study
Rare Case of Anal Canal Signet Ring Cell Carcinoma Associated with Perianal and Vulvar Pagetoid Spread - Na Rae Kim, Hyun Yee Cho, Jeong-Heum Baek1, Juhyeon Jeong, Seung Yeon Ha, Jae Yeon Seok, Sung Won Park1, Sun Jin Sym2, Kyu Chan Lee3, Dong Hae Chung
-
Journal of Pathology and Translational Medicine 2016;50(3):231-237.
DOI: https://doi.org/10.4132/jptm.2015.08.08
Published online: October 8, 2015
Department of Pathology, Gachon University Gil Medical Center, Incheon, Korea
1Department of General Surgery, Gachon University Gil Medical Center, Incheon, Korea
2Division of Hematology and Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
3Department of Radiation Oncology, Gachon University Gil Medical Center, Incheon, Korea
- Corresponding Author: Dong Hae Chung, MD Department of Pathology, Gachon University Gil Medical Center, 21 Namdong-daero 774beon-gil, Namdong-gu, Incheon 21565, Korea Tel: +82-32-460-3866 Fax: +82-32-460-2394 E-mail: dhchung@gilhospital.com
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Primary Carcinomas of the Episiotomy Scar Site: A Systematic Literature Review
Andrea Palicelli, Federica Torricelli, Gabriele Tonni, Alessandra Bisagni, Eleonora Zanetti, Magda Zanelli, Venus Damaris Medina-Illueca, Beatrice Melli, Maurizio Zizzo, Andrea Morini, Maria Paola Bonasoni, Giacomo Santandrea, Giuseppe Broggi, Rosario Cal
Current Oncology.2025; 32(2): 65. CrossRef - Metastatic Carcinomas at the Episiotomy Site: A Systematic Literature Review
Andrea Palicelli, Gabriele Tonni, Federica Torricelli, Beatrice Melli, Vincenza Ylenia Cusenza, Sandra Martinelli, Eleonora Zanetti, Alessandra Bisagni, Magda Zanelli, Maria Paola Bonasoni, Teresa Rossi, Lucia Mangone, Venus Damaris Medina-Illueca, Mauriz
Cancers.2025; 17(17): 2801. CrossRef - A Case of Prostatic Signet-Ring Cell-like Carcinoma with Pagetoid Spread and Intraductal Carcinoma and Long-Term Survival: PD-L1 and Mismatch Repair System Proteins (MMR) Immunohistochemical Evaluation with Systematic Literature Review
Nektarios Koufopoulos, Argyro-Ioanna Ieronimaki, Andriani Zacharatou, Alina Roxana Gouloumis, Danai Leventakou, Ioannis Boutas, Dionysios T. Dimas, Adamantia Kontogeorgi, Kyparissia Sitara, Lubna Khaldi, Magda Zanelli, Andrea Palicelli
Journal of Personalized Medicine.2023; 13(6): 1016. CrossRef - Anal canal adenocarcinoma with neuroendocrine features accompanying secondary extramammary Paget disease, successfully treated with modified FOLFOX6: a case report
Masamichi Yamaura, Takeshi Yamada, Rei Watanabe, Hitomi Kawai, Suguru Hirose, Hiroki Tajima, Masashi Sato, Yuichi Uchida, Daisuke Suganuma, Yoshiyuki Yamamoto, Toshikazu Moriwaki, Ichinosuke Hyodo
BMC Cancer.2018;[Epub] CrossRef - Solitary left axillary lymph node metastasis after curative resection of carcinoma at the colostomy site: a case report
Ken Imaizumi, Shigenori Homma, Tadashi Yoshida, Tatsushi Shimokuni, Hideyasu Sakihama, Norihiko Takahashi, Hideki Kawamura, Emi Takakuwa, Akinobu Taketomi
Surgical Case Reports.2016;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


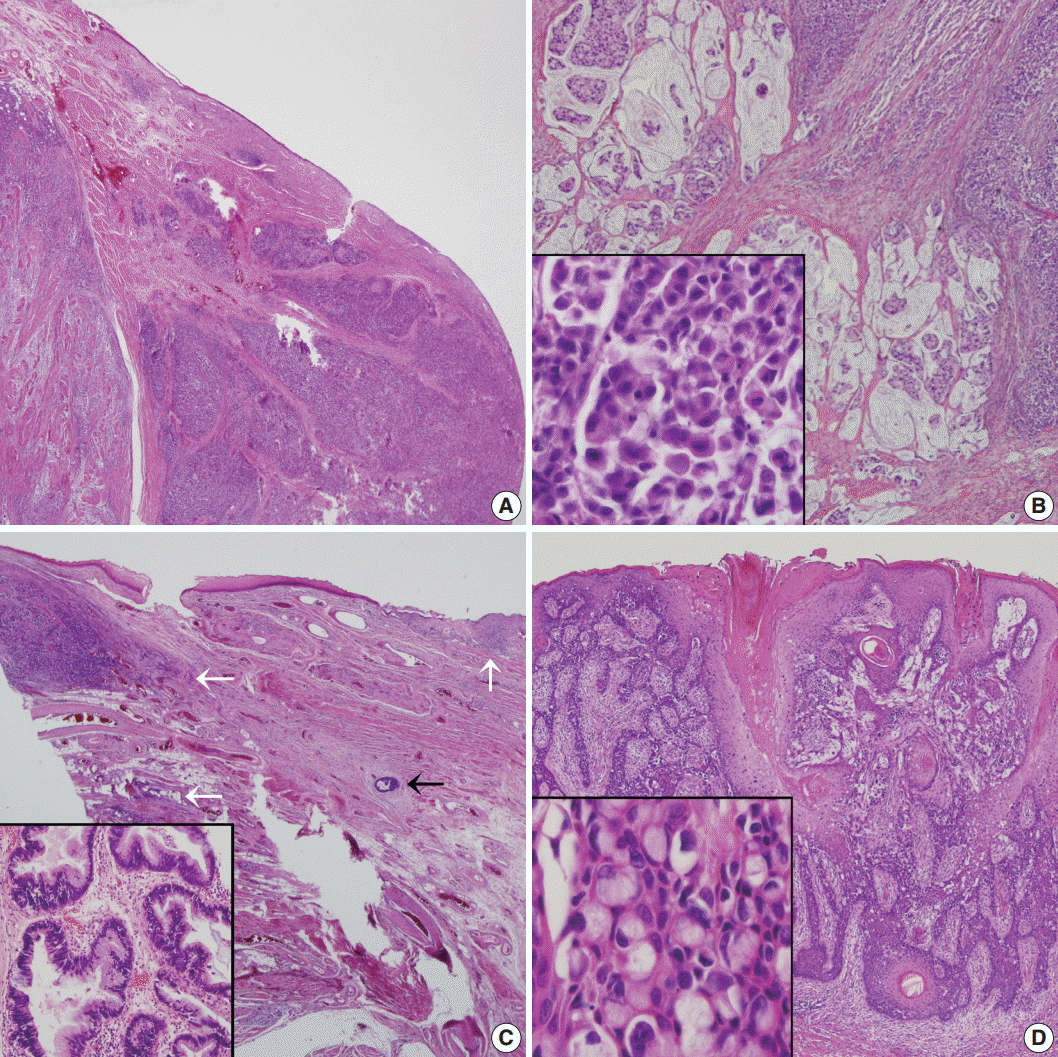
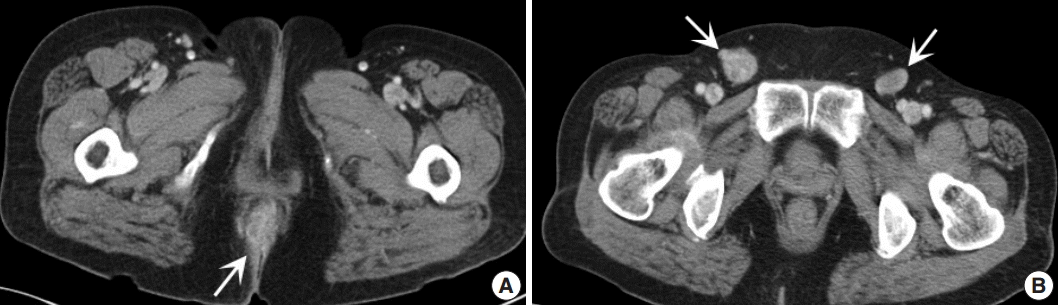
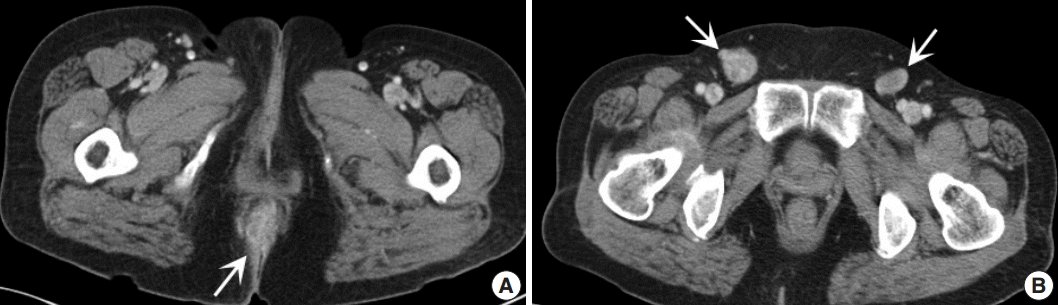
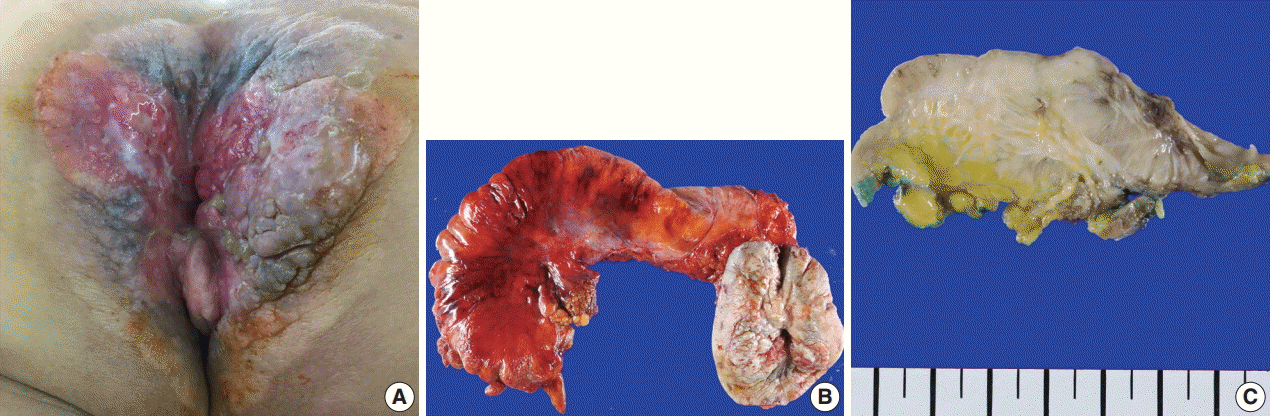
Fig. 1.
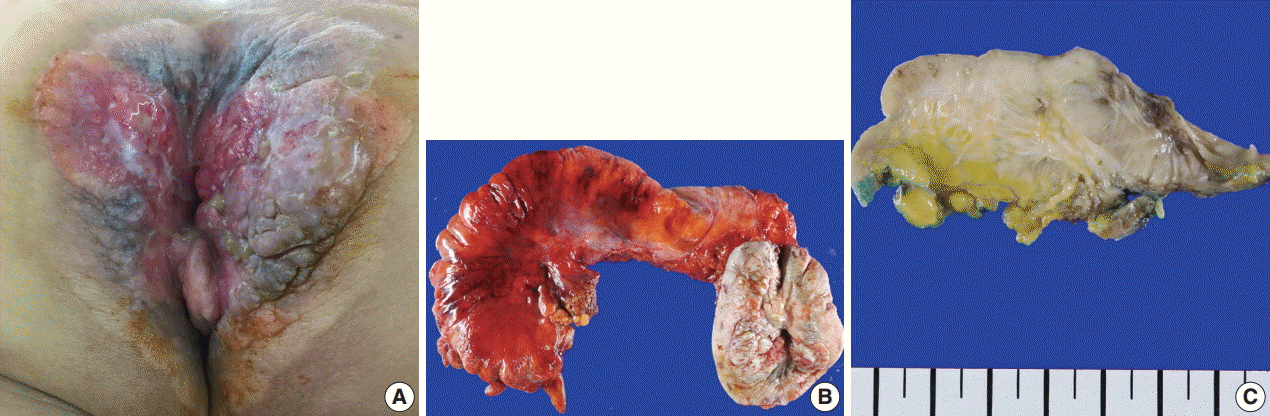
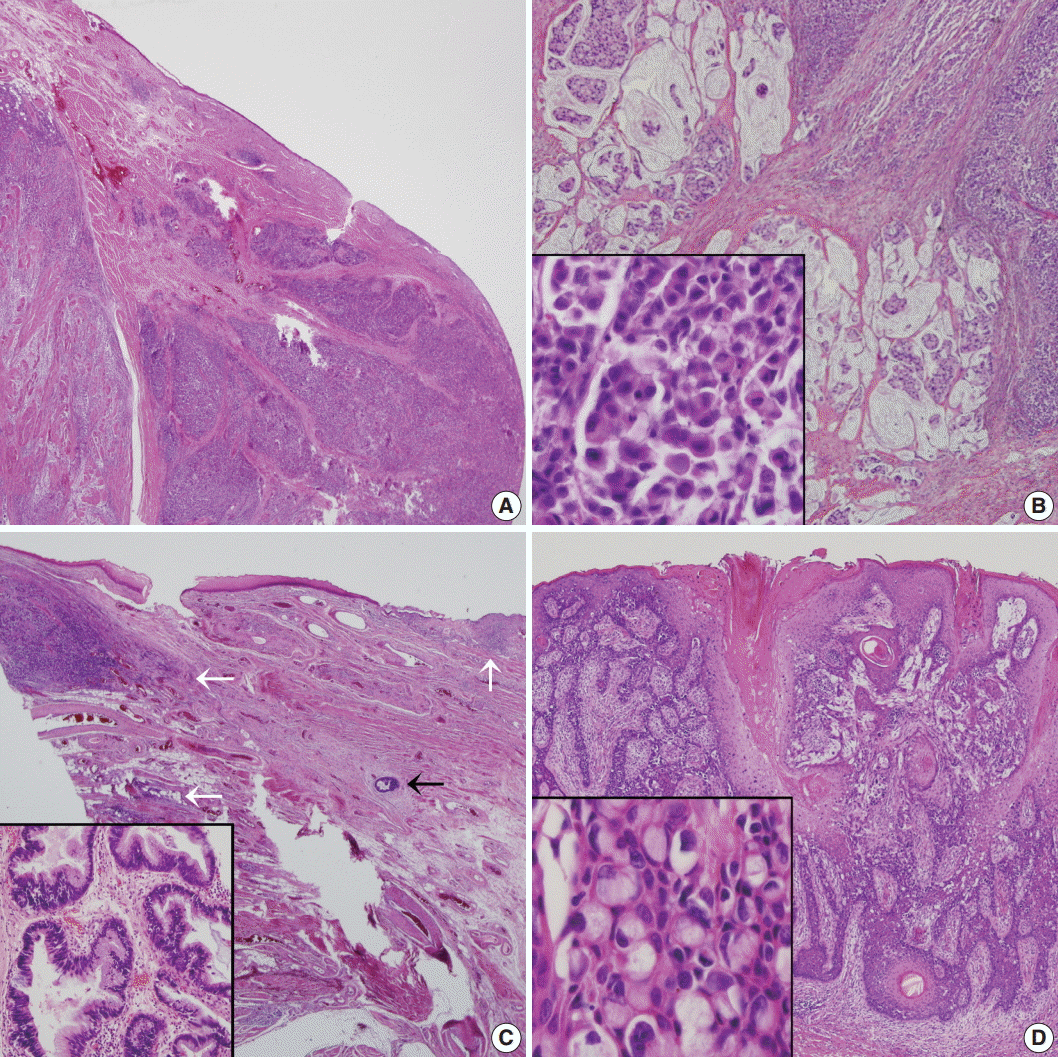
Fig. 2.
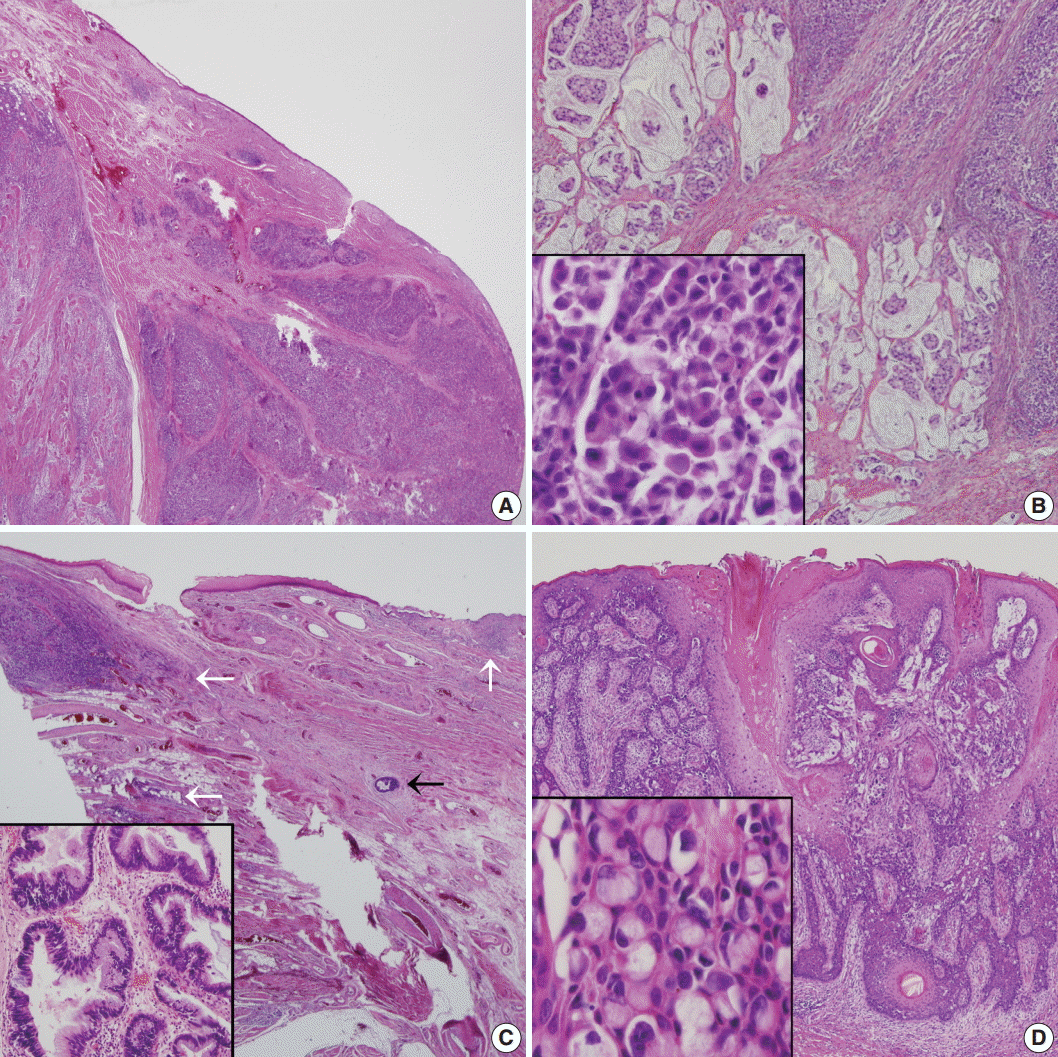
Fig. 3.
| Clone, dilution, company | Anal canal signet ring cell carcinoma | Pagetoid spread of vulvar and perianal skin | |
|---|---|---|---|
| CK7 | OV-TL 12/30; 1:100 dilution; Dako, Glostrup, Denmark | – | – |
| CK20 | Ks20.8; 1:100 dilution; Dako, Glostrup, Denmark | + | + |
| CK19 | A53-B/A2.26; 1:200 dilution; Thermo Scientific, Fremont, USA | + | + |
| CK5/6 | D5/16 B4; prediluted; Dako, Glostrup, Denmark | + weak | – |
| EGFR | SP84; 1:30 dilution; Novocastra, Newcastle upon Tyne, UK | + | + |
| CEA | Polyclonal; prediluted; Dako, Glostrup, Denmark | + | + |
| MOC-31 | MOC-31; 1:70 dilution; Novocastra, Newcastle upon Tyne, UK | + | + |
| CDX2 | AMT28; 1:50 dilution; Novocastra, Newcastle upon Tyne, UK | – | – |
| S-100 protein | Polyclonal; 1:1,200 dilution; Dako, Glostrup, Denmark | – | – |
| Synaptophysin | DAK-SYNAP; prediluted; Dako, Glostrup, Denmark | – | – |
| Chromogranin | polyclonal; prediluted; Dako, Glostrup, Denmark | – | – |
| CD56 | 123C3; prediluted; Dako, Glostrup, Denmark | – | – |
| HMB45 | HMB45; prediluted; Dako, Glostrup, Denmark | – | – |
| p53 | DO-7; 1:100 dilution; Dako, Glostrup, Denmark | + | + |
| Case No. | Author | Age(yr)/Sex | Presenting symptom | Gross and histopathology | Immunohistochemistry | TNM at the initial evaluation | Treatment | Clinical outcome (follow-up period) |
|---|---|---|---|---|---|---|---|---|
| 1 | Uchigasaki et al. (1992) [3] | 52/F | Anal ulcer and pruritus for 2 yr |
Anal canal SRCC progressed from adenocarcinoma in situ of anal glands or mucinous glands (1.5 cm), Pagetoid spread (7 cm) | pCEA+ mCEA+ mucicarmine+ PAS+ D-PAS-resistant | T1N3M0, stage IIIB | Topical 5-FU cream, EB, ASR | Recurrence with systemic metastasis and died (7 mo) |
| 2 | Morihisa et al. (1999) [7] |
75/F | Perianal erythema and erosion for 7 mo | Anal canal SRCC (size not described), Pagetoid spread (5 cm) | CEA+ mucicarmine+ PAS+ | N3M1, stage IV | Nephrostomy for ARF due to systemic metastasis | Systemic metastasis and died (4 mo) |
| 3 | Nagano et al. (1999) [8] | 46/F | Inguinal LN enlargement | Anal canal and rectal SRCC (3.5 cm), Pagetoid spread (7.5 cm) | Alcian blue+ PAS+ | T2N3M0, stage IIIB | APR, CTX (5-FU, LV) | NR (3 mo) |
| 4 | Naganuma et al. (2004) [9] | 85/F | Anal bleeding for 3 yr | Anal canal SRCC (unknown size of polyp, residual anal canal mass, 5 mm), Pagetoid spread (size not described) | CEA+ CA19-9+ CK20+ CK7– GCDFP-15– | N3M1, stage IV | Anal polypectomy with CTX (5-FU), RT | Systemic metastasis and died (25 mo) |
| 5 | Nishimura et al. (2004) [10] |
76/F | Anal erosion and etythema for 2 yr | Anal canal SRCC (size not described), Pagetoid spread (6 cm) | CK20+ GCDFP-15- | Not available |
APR, CTX (5-FU, cisplatin) | NR (5 mo) |
| 6 | Yoshitani et al. (2009) [11] | 50/M | Axillary and inguinal LN enlargement for 1 mo, erythematous perianal skin | Anal canal SRCC (size not precisely described; anal verge to dentate line), Pagetoid spread (size not described) | CK20+MUC-1+CK7- | N3M1, stage IV | CTX (bevacizumab/mFOLFOX6, FOLFIRI) | Alive with CR evaluated by PET-CT (6 mo) |
| 7 | Ikezawa et al. (2010) [12] | 53/M | Anal pruritus for 22 mo | Anal canal SRCC (1 cm), Pagetoid spread (2 cm) | pCEA+ CK20+ CK7– GCDFP-15– | T1N2M0, stage IIIB | APR, CTX (uracil-tegafur, LV, mFOLFOX6) | Recurrence with systemic metastasis and died (9 mo) |
| 8 | Ioannidis et al. (2012) [4] | 87/F | Anal discomfortness and pain for 8 mo | Anal canal SRCC (3 cm) | Not done | T2N0M0, stage II | Wide excision with chemotherapy (5-FU) and RT | NR (6yr) |
| 9 | Terada (2014) [5] | 49/M | Anal bleeding | Anal canal SRCC (< 2 cm) | AE1/3+ CK8+ CK19+ CEA+ CA19-9+ synaptophysin+ CDX2+ MUC1+ MUC2+ EMA+ CK7– CK20– CK5/6– MUC5AC– MUC6– CD56– NSE– chromogranin– vimentin– | T1N1M0, stage IIIA | Miles’ operation with LN dissection | Metastatic carcinomatosis and died (5 mo) |
| 10 | Kim et al. (2016) | 61/F | Erythematous perianal and vulvar skin for 3 yr, synchronous colon adenocarcinoma (0.2 cm) | Anal canal SRCC (4 cm), Pagetoid spread (8.2 cm) | pCEA+ CK20+ CK19+ MOC-31+ CDX2– CK7– HMB45– mucicarmine+ | T2N3M0, stage IIIB | Extended Miles’ operation and inguinal LN dissection, CTX (5-FU, MTC), RT (planned) | NR (2 mo) |
CK, cytokeratin; EGFR, epidermal growth factor receptor; CEA, carcinoembryonic antigen; CDX2, caudal-related homeobox gene nuclear transcription factor; HMB45, human melanoma black 45.
F, female; SRCC, signet ring cell carcinoma; pCEA, polyclonal carcinoembryonic antigen; mCEA, monoclonal carcinoembryonic antigen; PAS, periodic acid-Schiff; D-PAS, PAS diastase stain; 5-FU, 5-fluorouracil; EB, electron beam therapy; ASR, abdominosacral resection; CEA, carcinoembryonic antigen; ARF, acute renal failure; LN, lymph node; APR, abdominoperineal resection; CTX, chemotherapy; LV, leucovorin; NR, no recurrence; CA19-9, carbohydrate antigen 19-9; CK, cytokeratin; GCDFP-15, gross cystic disease fluid protein-15; RT, radiotherapy; M, male; mFOLFOX6, leucovorin, 5-FU, and oxaliplatin; FOLFIRI, irinotecan, 5-FU, and leucovorin; CR, complete remission; PET-CT, positron emission tomography-computed tomography; EMA, epithelial membrane antigen; NSE, neuron specific enolase; HMB-45, human melanoma black 45; MTC, mitomycin-C. This case was diagnosed as Paget’s disease 15 years before the anal canal SRCC; These articles were not available.

 E-submission
E-submission