Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 50(3); 2016 > Article
-
Original Article
Interobserver Agreement on Pathologic Features of Liver Biopsy Tissue in Patients with Nonalcoholic Fatty Liver Disease - Eun Sun Jung1,2, Kyoungbun Lee1,3, Eunsil Yu1,4, Yun Kyung Kang1,5, Mee-Yon Cho1,6, Joon Mee Kim1,7, Woo Sung Moon1,8, Jin Sook Jeong1,9, Cheol Keun Park1,10, Jae-Bok Park1,11, Dae Young Kang1,12, Jin Hee Sohn1,13, So-Young Jin1,14
-
Journal of Pathology and Translational Medicine 2016;50(3):190-196.
DOI: https://doi.org/10.4132/jptm.2016.03.01
Published online: April 18, 2016
1Gastrointestinal Pathology Study Group of Korean Society of Pathologist, Seoul, Korea
2Department of Pathology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
3Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
5Department of Pathology, Inje University Seoul Paik Hospital, Seoul, Korea
6Department of Pathology, Yonsei University Wonju College of Medicine, Wonju, Korea
7Department of Pathology, Inha University Hospital, Incheon, Korea
8Department of Pathology, Chonbuk National University Medical School, Jeonju, Korea
9Department of Pathology, Dong-A University College of Medicine, Busan, Korea
10Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
11Department of Pathology, Daegu Catholic University College of Medicine, Daegu, Korea
12Department of Pathology, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea
13Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
14Department of Pathology, Soon Chun Hyang University Seoul Hospital, Seoul, Korea
- Corresponding Author: So-Young Jin, MD Department of Pathology, Soon Chun Hyang University Seoul Hospital, 59 Daesagwan-ro, Yongsan-gu, Seoul 04401, Korea Tel: +82-2-709-9424 Fax: +82-2-709-9441 E-mail: jin0924@schmc.ac.kr
© 2016 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background:
- The histomorphologic criteria for the pathological features of liver tissue from patients with non-alcoholic fatty liver disease (NAFLD) remain subjective, causing confusion among pathologists and clinicians. In this report, we studied interobserver agreement of NAFLD pathologic features and analyzed causes of disagreement.
-
Methods:
- Thirty-one cases of clinicopathologically diagnosed NAFLD from 10 hospitals were selected. One hematoxylin and eosin and one Masson’s trichrome-stained virtual slide from each case were blindly reviewed with regard to 12 histological parameters by 13 pathologists in a gastrointestinal study group of the Korean Society of Pathologists. After the first review, we analyzed the causes of disagreement and defined detailed morphological criteria. The glass slides from each case were reviewed a second time after a consensus meeting. The degree of interobserver agreement was determined by multi-rater kappa statistics.
-
Results:
- Kappa values of the first review ranged from 0.0091–0.7618. Acidophilic bodies (k = 0.7618) and portal inflammation (k = 0.5914) showed high levels of agreement, whereas microgranuloma (k = 0.0984) and microvesicular fatty change (k = 0.0091) showed low levels of agreement. After the second review, the kappa values of the four major pathological features increased from 0.3830 to 0.5638 for steatosis grade, from 0.1398 to 0.2815 for lobular inflammation, from 0.1923 to 0.3362 for ballooning degeneration, and from 0.3303 to 0.4664 for fibrosis.
-
Conclusions:
- More detailed histomorphological criteria must be defined for correct diagnosis and high interobserver agreement of NAFLD.
- Thirty-one patients with clinically and pathologically diagnosed NAFLD from 10 hospitals (Daegu Catholic University Medical Center, Dong-A University Hospital, Samsung Medical Center, Seoul National University Hospital, Inje University Seoul Paik Hospital, Seoul St. Mary’s Hospital, Soon Chun Hyang University Seoul Hospital, Wonju Severance Christian Hospital, Inha University Hospital, Chungnam National University Hospital) were selected. Selection criteria were clinical NAFLD (nonalcoholic, serologically negative for viral and autoimmune markers, abnormal levels of liver enzymes such as aspartate aminotransferase and alanine aminotransferase) and age ≥19 years. Cirrhosis cases were included, and cases of drug and toxic injury conditions were excluded. Fifty-one liver biopsies from 10 hospitals were collected. Among them, 31 biopsies (≥1.5 cm in length and ≥16 G needle size) were selected. One hematoxylin and eosin (H&E)- and one Masson’s trichrome–stained slide were selected from each of the 31 cases. The biopsy specimens were anonymized and randomized by a researcher not involved in the study. All selected slides were scanned by a virtual slide scanning system (3DHistotech Ltd., Budapest, Hungary) at Asan Medical Center in Seoul.
- The following 12 NAFLD pathologic features were selected: steatosis grade, steatosis location, microvesicular steatosis, fibrosis stage, lobular inflammation, microgranuloma, large lipogranuloma, portal inflammation, ballooning degeneration, acidophilic bodies, Mallory’s hyaline, and glycogenated nuclei. Each parameter was reviewed and scored using the detailed scoring criteria shown in Table 1.
- One H&E- and one Masson’s trichrome–stained virtual slide from each case were reviewed for the 12 parameters. Reviews were performed blindly by 13 pathologists from a gastrointestinal study group of the Korean Society of Pathologists. The degree of interobserver agreement for the first review was analyzed by multi-rater Kappa statistics.
- The results were shared with all 13 pathologists, and a consensus meeting was held after the first review to analyze the reasons for disagreement and to define the morphologic criteria in more detail.
- After the consensus meeting, a second review of the 12 pathological parameters was performed using glass slides from each of the 31 cases. The degree of interobserver agreement after the second review was analyzed by multi-rater Kappa statistics and compared with the results of the first review.
- The Institutional Review Board of Seoul St. Mary’s Hospital approved this study (KIRB-00562_5-001).
MATERIALS AND METHODS
- Kappa values of interobserver agreement for the first review ranged from 0.0091 to 0.7618 (Table 2). The order of agreement, according to the kappa value, was acidophilic bodies (k=0.7618), portal inflammation (k =0.5914), large lipogranuloma (k=0.4822), Mallory’s hyaline (k=0.4603), steatosis grade (k=0.3830), steatosis location (k=0.3388), fibrosis (k=0.3303), glycogenated nuclei (0.3218), ballooning degeneration (k=0.1923), lobular inflammation (0.1398), microgranuloma (0.0984), and microvesicular fatty change (0.0091). The kappa values of the four major pathologic features (steatosis grade, portal inflammation, ballooning degeneration, and fibrosis) were measured as 0.3829, 0.5913, 0.1923, and 0.3303, respectively. In particular, ballooning degeneration (k=0.1923), which is an important feature for diagnosis of NASH, showed a low level of agreement.
- Kappa values of interobserver agreement for the second review ranged from 0.1199 to 0.7386 (Table 2). The order of kappa values for interobserver agreement after the second review were portal inflammation (k=0.7386), acidophilic bodies (k=0.6493), steatosis grade (k=0.5638), Mallory’s hyaline (k=0.5236), large lipogranuloma (k=0.5004), fibrosis (k=0.4664), steatosis location (k=0.4502), glycogenated nuclei (k=0.3846), ballooning degeneration (k=0.3362), microvesicular fatty change (k=0.2916), lobular inflammation (k=0.2815), and microgranuloma (k=0.1199). The kappa values of interobserver agreement increased for all parameters except acidophilic bodies. Microvesicular steatosis demonstrated the largest improvement (k=0.0091 to 0.2916), and microgranuloma the smallest (k=0.0984 to 0.1199). All kappa values of the four major pathological features increased as follows: steatosis grade from k=0.3830 to 0.5638, portal inflammation from k=0.5914 to 0.7386, ballooning degeneration from k=0.1923 to 0.3362, and fibrosis from k=0.3303 to 0.4664.
RESULTS
- Since the first description of NASH by Ludwig et al. in 1980 [18], several NAFLD scoring schemes have been proposed [16,17,19,20]. Among them, the NAFLD activity score (NAS) proposed by Kleiner et al. [17] is the most well-known and popular system. Their proposed NAS system is based on agreement data and a multiple regression analysis of the [14] histological features of steatosis grade, steatosis location, microvesicular steatosis, fibrosis, lobular inflammation, microgranuloma, large lipogranuloma, portal inflammation, ballooning degeneration, acidophilic bodies, pigmented macrophages, megamitochondria, Mallory’s hyaline, and glycogenated nuclei. The NAS is defined as the unweighted sum of the scores for steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2) and ranges from 0 to 8. Fibrosis was not included as an NAS component. Kleiner et al. [17] reported that the interobserver agreement values for the four major features were 0.79 for steatosis grade, 0.45 for lobular inflammation, 0.56 for ballooning degeneration, and 0.84 for fibrosis. The agreement for other histologic features ranged from k=0.15 to 0.58. However, the histomorphological features of some parameters remain ambiguous, contributing to low interobserver agreement. We studied interobserver agreement among 13 pathologists for each of the 12 well-known parameters and analyzed the reasons for disagreement. At the first circulation of slides, we reviewed each case without consensus to identify current discrepancies in diagnostic criteria. The kappa values in this review ranged widely from 0.0091 to 0.7618. Kappa values of the four major pathological features at the first review were 0.3830 for steatosis grade, 0.1398 for lobular inflammation, 0.1923 for ballooning degeneration, and 0.3303 for fibrosis, lower than those of Kleiner et al. [17].
- After the first review, we discussed several points of debate surrounding the definition of each parameter. As a result, we identified several details regarding steatosis grade, lobular inflammation, ballooning degeneration, fibrosis, Mallory’s body, and microvesicular fatty change and recommend the following:
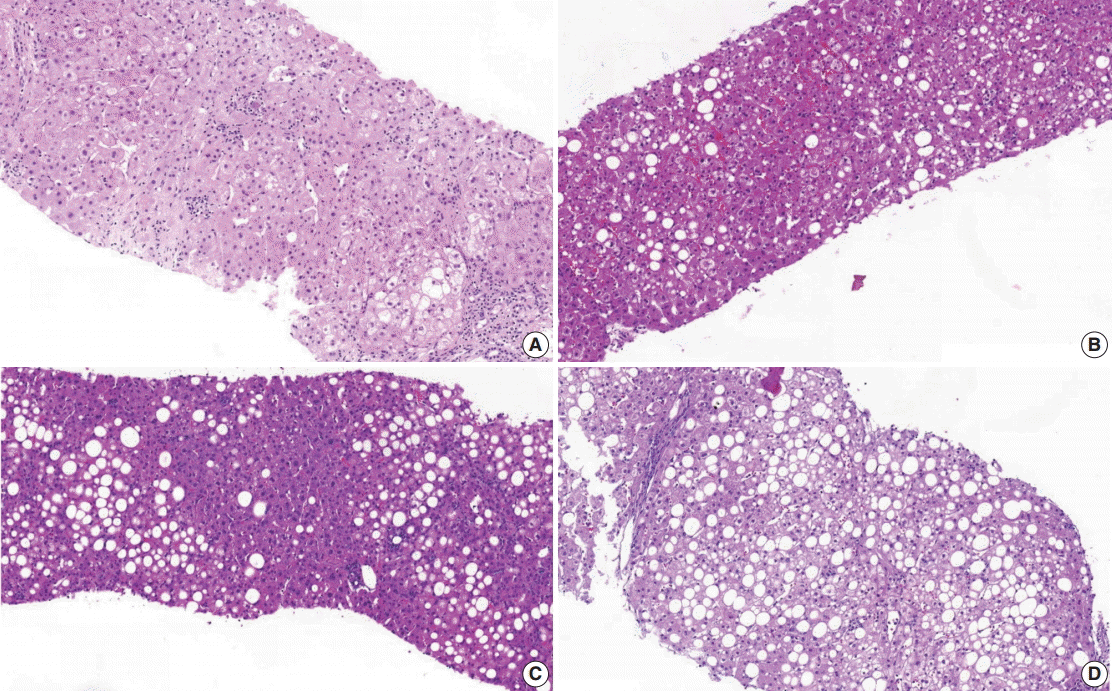
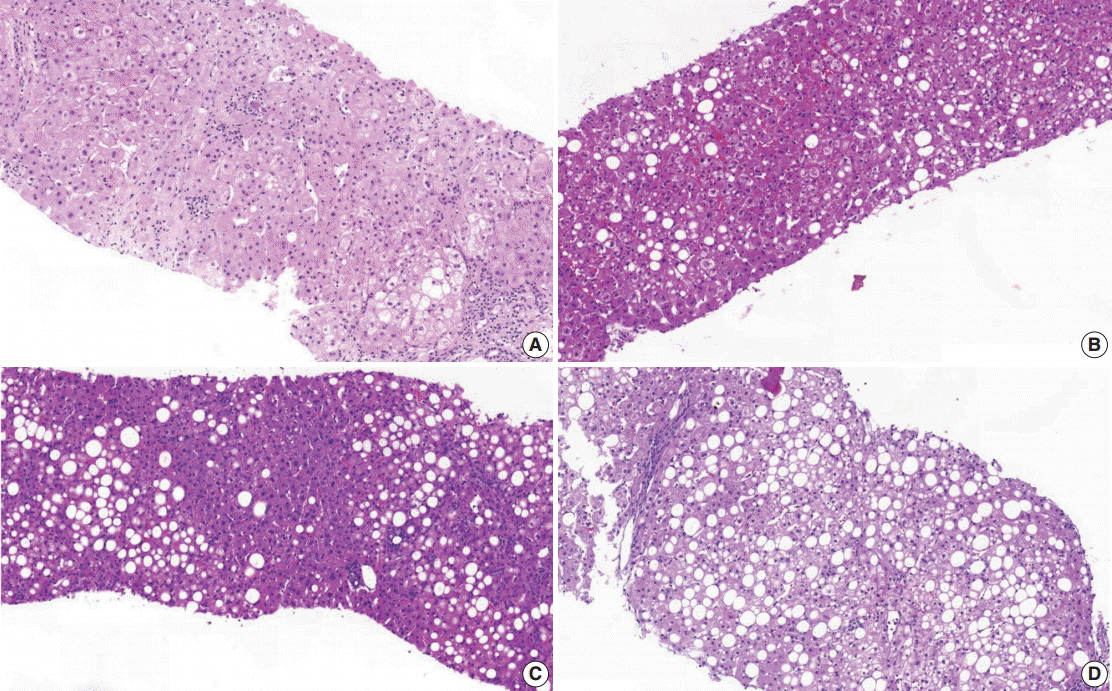
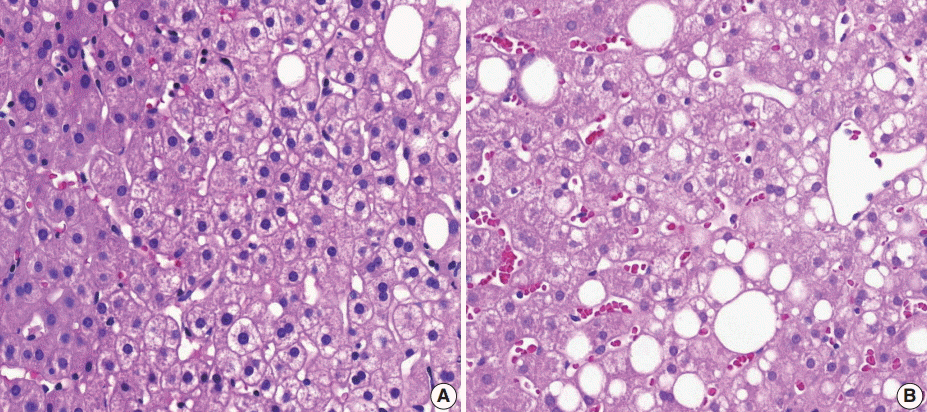
- (1) Steatosis grade: steatosis grade should be determined by fat volume rather than the number of fatty hepatocytes at 100×optical magnification (Fig. 1).
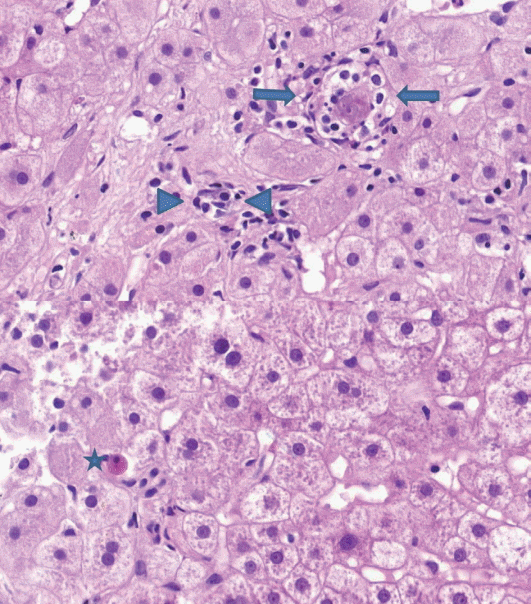
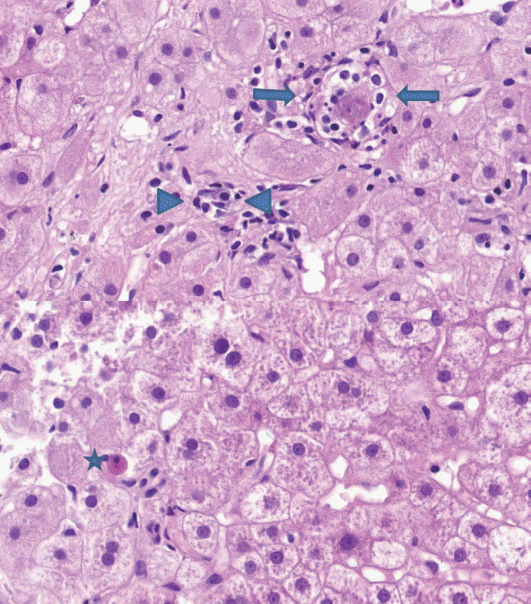
- (2) Lobular inflammation: lobular inflammation should be graded under 200× magnification throughout the entire biopsy field, and the mean, not the maximum number in the most active field, should be determined. Spotty necrosis of hepatocytes, lymphocyte aggregations, and acidophilic bodes should be included, whereas lipogranuloma resulting from fat phagocytosis should be excluded as in the histomorphologic criteria for chronic hepatitis grading (Fig. 2).
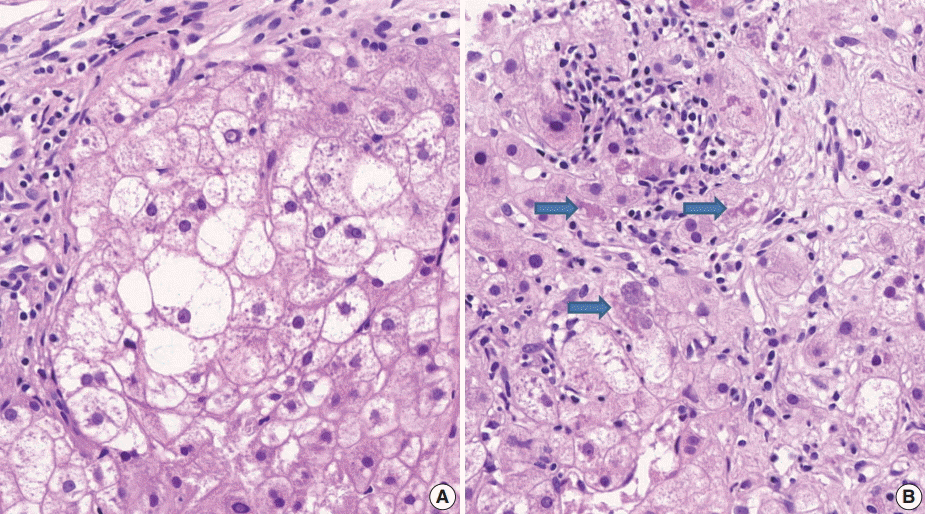
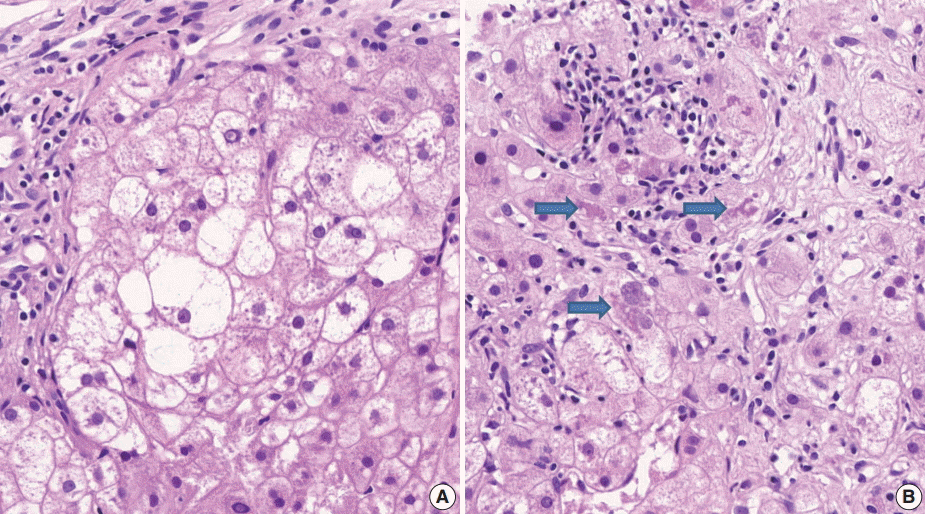
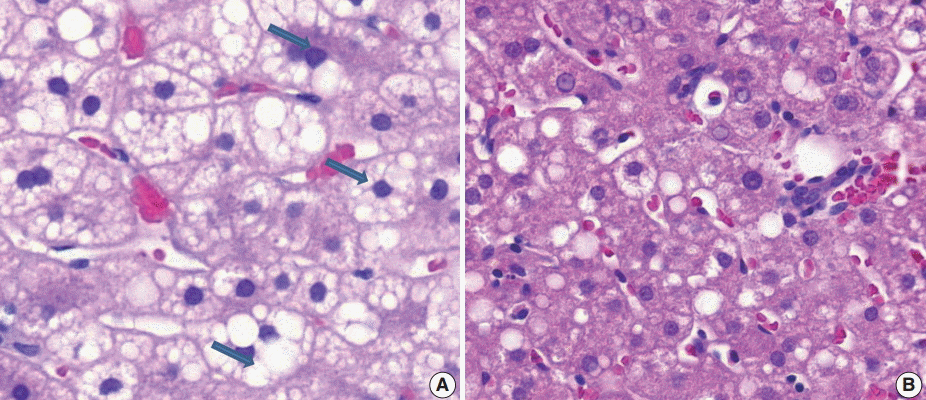
- (3) Ballooning degeneration: the histomorphological criteria of ballooning degeneration are enlarged round cells with loss of polygonal features and cytoplasm showing heterogeneous granular features (Fig. 3). Hydropic swelling and microvesicular fatty changes should be carefully distinguished from ballooning degeneration. In cases of hydropic swelling, the hepatocyte has large, swollen, and homogenously granular cytoplasm with well-preserved polygonal features. In particular, microvesicular fatty changes can also be enlarged and can be confused with ballooning degeneration. Microvesicular fatty changes show centrally located nuclei indented by a small fat droplet with a lipoblast-like feature (Fig. 4). When only one or two ballooned cells can be seen throughout the entire field, the term “few” can be applied.
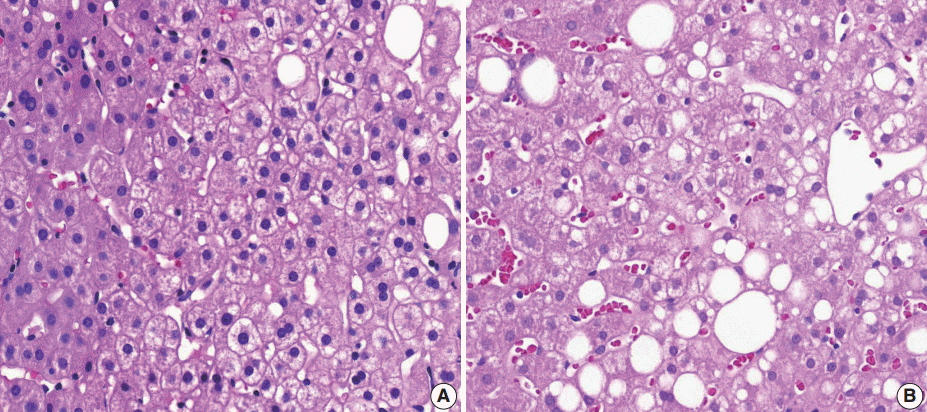
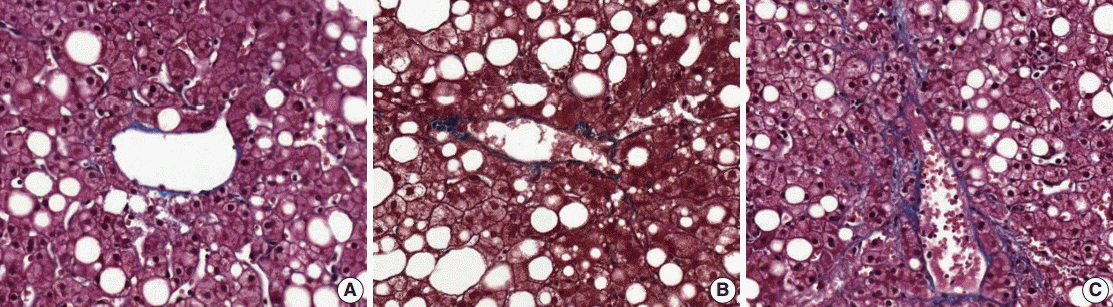
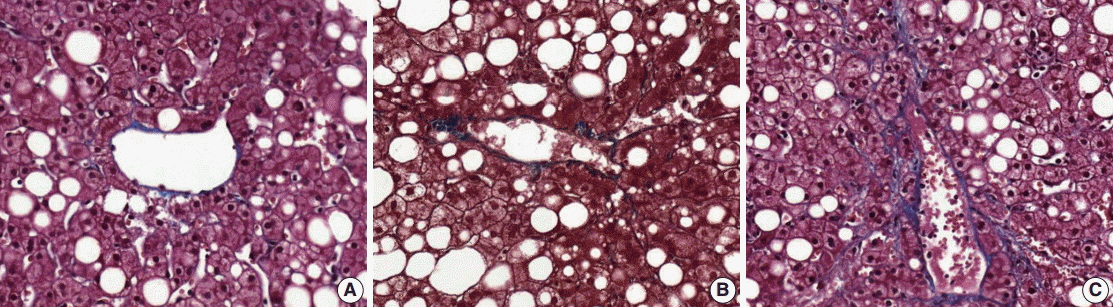
- (4) Fibrosis: mild fibrosis should be carefully distinguished from the normal framework around the central area. Only obvious fibrosis with pericellular collagen deposition should be considered as existence of fibrosis (Fig. 5).
- (5) Mallory’s body: Mallory’s body should be defined as a definite eosinophilic lump in the cytoplasm (Fig. 3B).
- (6) Microvesicular fatty changes: these are defined as lipoblast-like features showing centrally located nucleus and numerous intracytoplasmic micro-fat vacuoles inducing nuclear indentation. Microvesicular fatty changes should be carefully differentiated from cells having small- or medium-sized fat vacuoles without cytoplasmic enlargement and nuclear indentation, as mentioned by Yeh and Brunt (Fig. 6) [21].
- All kappa values increased in the second review based on the above criteria. In particular, kappa values for the four major parameters increased from 0.3830 to 0.5638 for steatosis grade, from 0.1398 to 0.2815 for lobular inflammation, from 0.1923 to 0.3362 for ballooning degeneration, and from 0.3303 to 0.4664 for fibrosis.
- This increased agreement likely resulted from the consensus meeting and the determination of more detailed histomorphologic criteria. However, the method used in the second review is more familiar to most pathologists and may have also contributed to increased agreement. Despite the differences in review method (virtual vs glass), there is no doubt that the exact histomorphologic criteria of NAFLD remain ambiguous and contribute to low interobserver agreement between pathologists.
- Therefore, our more detailed suggestions for NAFLD histomorphologic criteria—including steatosis grade, lobular inflammation, ballooning degeneration, and fibrosis, as mentioned above—will increase the accuracy of diagnosis and grading of NAFLD and improve interobserver agreement. Through this work and recommendations, we expect that a more exact basis for research of NAFLD and development of a new grading and scoring system will follow.
DISCUSSION
Acknowledgments
- 1. Brunt EM, Tiniakos DG. Pathology of steatohepatitis. Best Pract Res Clin Gastroenterol 2002; 16: 691-707. ArticlePubMed
- 2. Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol 1989; 20: 594-8. ArticlePubMed
- 3. Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a followup study of forty-two patients for up to 21 years. Hepatology 1990; 11: 74-80. ArticlePubMed
- 4. Teli MR, James OF, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology 1995; 22: 1714-9. ArticlePubMed
- 5. Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 2007; 11: 1-16. ArticlePubMed
- 6. Dunn W, Xu R, Wingard DL, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008; 103: 2263-71. ArticlePubMedPMC
- 7. Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liverrelated mortality in non-alcoholic fatty liver disease. J Hepatol 2008; 49: 608-12. ArticlePubMed
- 8. Carter-Kent C, Yerian LM, Brunt EM, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology 2009; 50: 1113-20. PubMedPMC
- 9. Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010; 52: 79-104. ArticlePubMed
- 10. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010; 51: 1820-32. ArticlePubMed
- 11. Abrams GA, Kunde SS, Lazenby AJ, Clements RH. Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of nonalcoholic fatty liver disease. Hepatology 2004; 40: 475-83. ArticlePubMed
- 12. Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005; 42: 132-8. ArticlePubMed
- 13. Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol 2006; 4: 226-32. ArticlePubMed
- 14. Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2010; 7: 195-203. ArticlePubMedPDF
- 15. Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012; 32: 3-13. ArticlePubMed
- 16. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999; 94: 2467-74. ArticlePubMed
- 17. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313-21. ArticlePubMed
- 18. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980; 55: 434-8. ArticlePubMed
- 19. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116: 1413-9. ArticlePubMed
- 20. Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology 2004; 39: 188-96. ArticlePubMed
- 21. Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol 2007; 128: 837-47. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Chronic polypharmacy, monotherapy, and deprescribing: Understanding complex effects on the hepatic proteome of aging mice
Kevin Winardi, John Mach, Matthew J. McKay, Mark P. Molloy, Sarah J. Mitchell, Michael R. MacArthur, Catriona McKenzie, David G. Le Couteur, Sarah N. Hilmer
Aging Cell.2025;[Epub] CrossRef - Utility of AI digital pathology as an aid for pathologists scoring fibrosis in MASH
Desiree Abdurrachim, Serene Lek, Charlene Zhi Lin Ong, Chun Kit Wong, Yongqi Zhou, Aileen Wee, Gwyneth Soon, Timothy J. Kendall, Michael O. Idowu, Christopher Hendra, Ashmita Saigal, Radha Krishnan, Elaine Chng, Dean Tai, Gideon Ho, Thomas Forest, Annaswa
Journal of Hepatology.2025; 82(5): 898. CrossRef - Artificial intelligence scoring of liver biopsies in a phase II trial of semaglutide in nonalcoholic steatohepatitis
Vlad Ratziu, Sven Francque, Cynthia A. Behling, Vanja Cejvanovic, Helena Cortez-Pinto, Janani S. Iyer, Niels Krarup, Quang Le, Anne-Sophie Sejling, Dina Tiniakos, Stephen A. Harrison
Hepatology.2024; 80(1): 173. CrossRef - Classification of the Stages of Nonalcoholic Steatohepatitis via Federated General Visual Representation Learning
Mehmet Nergiz
International Journal of Imaging Systems and Technology.2024;[Epub] CrossRef - Outcome prediction in metabolic dysfunction‐associated steatotic liver disease using stain‐free digital pathological assessment
Timothy J. Kendall, Elaine Chng, Yayun Ren, Dean Tai, Gideon Ho, Jonathan A. Fallowfield
Liver International.2024; 44(10): 2511. CrossRef - Non-alcoholic fatty liver disease: the pathologist’s perspective
Wei-Qiang Leow, Anthony Wing-Hung Chan, Paulo Giovanni L. Mendoza, Regina Lo, Kihan Yap, Haeryoung Kim
Clinical and Molecular Hepatology.2023; 29(Suppl): S302. CrossRef - CT-based Hounsfield unit values reflect the degree of steatohepatitis in patients with low-grade fatty liver disease
Ha Neul Kim, Hong Jae Jeon, Hei Gwon Choi, In Sun Kwon, Woo Sun Rou, Jeong Eun Lee, Tae Hee Lee, Seok Hyun Kim, Byung Seok Lee, Kyung Sook Shin, Hyun Jung Lee, Hyuk Soo Eun
BMC Gastroenterology.2023;[Epub] CrossRef - Artificial intelligence and deep learning: New tools for histopathological diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis
Yoshihisa Takahashi, Erdenetsogt Dungubat, Hiroyuki Kusano, Toshio Fukusato
Computational and Structural Biotechnology Journal.2023; 21: 2495. CrossRef - An integrated gene-to-outcome multimodal database for metabolic dysfunction-associated steatotic liver disease
Timothy J. Kendall, Maria Jimenez-Ramos, Frances Turner, Prakash Ramachandran, Jessica Minnier, Michael D. McColgan, Masood Alam, Harriet Ellis, Donald R. Dunbar, Gabriele Kohnen, Prakash Konanahalli, Karin A. Oien, Lucia Bandiera, Filippo Menolascina, An
Nature Medicine.2023; 29(11): 2939. CrossRef - Improved pathology reporting in NAFLD/NASH for clinical trials
Caitlin Rose Langford, Marc H Goldinger, Darren Treanor, Clare McGenity, Jonathan R Dillman, Daniela S Allende, Robert Goldin, Elizabeth M Brunt, Kurt Zatloukal, Helmut Denk, Kenneth A Fleming
Journal of Clinical Pathology.2022; 75(2): 73. CrossRef - Standardizing the histological assessment of late posttransplantation biopsies from pediatric liver allograft recipients
Stefan G. Hübscher, Sandy Feng, Annette S. H. Gouw, Hironori Haga, Hyo Jeong Kang, Deirdre A. Kelly, Mina Komuta, Andrew Lesniak, Benjamin A. Popp, Henkjan J. Verkade, Eunsil Yu, Anthony J. Demetris
Liver Transplantation.2022; 28(9): 1475. CrossRef - Discordant pathological diagnosis of non‐alcoholic fatty liver disease: A prospective multicenter study
Takuya Kuwashiro, Hirokazu Takahashi, Hideyuki Hyogo, Yuji Ogawa, Kento Imajo, Masato Yoneda, Takashi Nakahara, Satoshi Oeda, Kenichi Tanaka, Yuichiro Amano, Shinji Ogawa, Atsushi Kawaguchi, Shinichi Aishima, Masayoshi Kage, Kazuaki Chayama, Atsushi Nakaj
JGH Open.2020; 4(3): 497. CrossRef - Obeticholic acid for the treatment of nonalcoholic steatohepatitis: Expectations and concerns
Stergios A. Polyzos, Jannis Kountouras, Christos S. Mantzoros
Metabolism.2020; 104: 154144. CrossRef - A scoring system for the diagnosis of non-alcoholic steatohepatitis from liver biopsy
Kyoungbun Lee, Eun Sun Jung, Eunsil Yu, Yun Kyung Kang, Mee-Yon Cho, Joon Mee Kim, Woo Sung Moon, Jin Sook Jeong, Cheol Keun Park, Jae-Bok Park, Dae Young Kang, Jin Hee Sohn, So-Young Jin
Journal of Pathology and Translational Medicine.2020; 54(3): 228. CrossRef - An Improved qFibrosis Algorithm for Precise Screening and Enrollment into Non-Alcoholic Steatohepatitis (NASH) Clinical Trials
Wei-Qiang Leow, Pierre Bedossa, Feng Liu, Lai Wei, Kiat-Hon Lim, Wei-Keat Wan, Yayun Ren, Jason Pik-Eu Chang, Chee-Kiat Tan, Aileen Wee, George Boon-Bee Goh
Diagnostics.2020; 10(9): 643. CrossRef - Deep learning quantification of percent steatosis in donor liver biopsy frozen sections
Lulu Sun, Jon N. Marsh, Matthew K. Matlock, Ling Chen, Joseph P. Gaut, Elizabeth M. Brunt, S. Joshua Swamidass, Ta-Chiang Liu
EBioMedicine.2020; 60: 103029. CrossRef - Magnetic resonance elastography SE-EPI vs GRE sequences at 3T in a pediatric population with liver disease
Juan S. Calle-Toro, Suraj D. Serai, Erum A. Hartung, David J. Goldberg, Bradley D. Bolster, Kassa Darge, Sudha A. Anupindi
Abdominal Radiology.2019; 44(3): 894. CrossRef - R2 relaxometry based MR imaging for estimation of liver iron content: A comparison between two methods
Juan S. Calle-Toro, Christian A. Barrera, Dmitry Khrichenko, Hansel J. Otero, Suraj D. Serai
Abdominal Radiology.2019; 44(9): 3058. CrossRef - Inhibition of mitochondrial fatty acid oxidation in drug-induced hepatic steatosis
Bernard Fromenty
Liver Research.2019; 3(3-4): 157. CrossRef - Standardising the interpretation of liver biopsies in non‐alcoholic fatty liver disease clinical trials
Rish K. Pai, David E. Kleiner, John Hart, Oyedele A. Adeyi, Andrew D. Clouston, Cynthia A. Behling, Dhanpat Jain, Sanjay Kakar, Mayur Brahmania, Lawrence Burgart, Kenneth P. Batts, Mark A. Valasek, Michael S. Torbenson, Maha Guindi, Hanlin L. Wang, Veeral
Alimentary Pharmacology & Therapeutics.2019; 50(10): 1100. CrossRef - NAFLD Histology: a Critical Review and Comparison of Scoring Systems
Rish K. Pai
Current Hepatology Reports.2019; 18(4): 473. CrossRef - Hepatic sonic hedgehog protein expression measured by computer assisted morphometry significantly correlates with features of non-alcoholic steatohepatitis
Michael Estep, Rohini Mehta, Gary Bratthauer, Lakshmi Alaparthi, Fanny Monge, Simon Ali, Dinan Abdelatif, Zahra Younoszai, Maria Stepanova, Zachary D. Goodman, Zobair M. Younossi
BMC Gastroenterology.2019;[Epub] CrossRef - Validation of intimate correlation between visceral fat and hepatic steatosis: Quantitative measurement techniques using CT for area of fat and MR for hepatic steatosis
Moon Hyung Choi, Joon-Il Choi, Michael Yong Park, Sung Eun Rha, Soon Nam Oh, Seung Eun Jung, Jae Young Byun, Stephan Kannengiesser, Yohan Son
Clinical Nutrition.2018; 37(1): 214. CrossRef - Ultrasound or MR elastography of liver: which one shall I use?
Meng Yin, Sudhakar K. Venkatesh
Abdominal Radiology.2018; 43(7): 1546. CrossRef - Feasibility and agreement of stiffness measurements using gradient-echo and spin-echo MR elastography sequences in unselected patients undergoing liver MRI
Guilherme Moura Cunha, Kevin J Glaser, Anke Bergman, Rodrigo P Luz, Eduardo H de Figueiredo, Flavia Paiva Proença Lobo Lopes
The British Journal of Radiology.2018; : 20180126. CrossRef - Second harmonic generation microscopy provides accurate automated staging of liver fibrosis in patients with non-alcoholic fatty liver disease
Pik Eu Chang, George Boon Bee Goh, Wei Qiang Leow, Liang Shen, Kiat Hon Lim, Chee Kiat Tan, Manlio Vinciguerra
PLOS ONE.2018; 13(6): e0199166. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure






Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
| Pathologic parameter | Criteria for scoring | |
|---|---|---|
| 1 | Steatosis grade | 1: < 5%, 2: 5–33%, 3: 34–66%, 4: > 66% |
| 2 | Steatosis location | 1: zone 1, 2: zone 3, 3: azonal, 4: panacinar |
| 3 | Microvesicular steatosis | 0: absent, 1: present |
| 4 | Fibrosis | 0: none |
| 1A: mild, zone 3, perisinusoidal | ||
| 1B: moderate, zone 3, perisinusoidal | ||
| 1C: portal/periportal | ||
| 2: perisinusoidal and portal/periportal | ||
| 3: bridging fibrosis | ||
| 4: cirrhosis | ||
| 5 | Lobular inflammation | 0: no foci |
| 1: < 2 foci per 200 × field | ||
| 2: 2–4 foci per 200 × field | ||
| 3: > 4 foci per 200 × field | ||
| 6 | Microgranuloma | 0: absent, 1: present |
| 7 | Large lipogranuloma | 0: absent, 1: present |
| 8 | Portal inflammation | 0: none to minimal, 1: greater than minimal |
| 9 | Ballooning | 0: absent, 1: few, 2: many/prominent |
| 10 | Acidophilic bodies | 0: none to rare, 1: many |
| 11 | Mallory’s hyaline | 0: none to rare, 1: many |
| 12 | Glycogenated nuclei | 0: none to rare, 1: many |
| Pathologic parameters | First review | Second review | |
|---|---|---|---|
| 1 | Steatosis grade | 0.3830 | 0.5638 |
| 2 | Steatosis location | 0.3388 | 0.4502 |
| 3 | Microvesicular steatosis | 0.0091 | 0.2916 |
| 4 | Fibrosis | 0.3303 | 0.4664 |
| 5 | Lobular inflammation | 0.1398 | 0.2815 |
| 6 | Microgranuloma | 0.0984 | 0.1199 |
| 7 | Large lipogranuloma | 0.4822 | 0.5004 |
| 8 | Portal inflammation | 0.5914 | 0.7386 |
| 9 | Ballooning | 0.1923 | 0.3362 |
| 10 | Acidophilic bodies | 0.7618 | 0.6493 |
| 11 | Mallory's hyaline | 0.4603 | 0.5236 |
| 12 | Glycogenated nuclei | 0.3218 | 0.3846 |

 E-submission
E-submission