Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(1); 2017 > Article
-
Original Article
Increased Expression of Thymosin β4 Is Independently Correlated with Hypoxia Inducible Factor-1α (HIF-1α) and Worse Clinical Outcome in Human Colorectal Cancer - Seung Yun Lee, Mee Ja Park, Hye Kyung Lee, Hyun Jin Son, Chang Nam Kim1, Joo Heon Kim,, Dong Wook Kang
-
Journal of Pathology and Translational Medicine 2017;51(1):9-16.
DOI: https://doi.org/10.4132/jptm.2016.08.23
Published online: October 16, 2016
Department of Pathology, Eulji University Hospital, Daejeon, Korea
1Department of Surgery, Eulji University Hospital, Daejeon, Korea
-
Corresponding Author: Joo Heon Kim, MD, PhD Department of Pathology, Eulji University Hospital, 95 Dunsanseo-ro, Seo-gu, Daejeon 35233, Korea Tel: +82-42-611-3454 Fax: +82-42-611-3459 E-mail: kjh2000@eulji.ac.kr
Dong Wook Kang, MD, PhD Department of Pathology, Eulji University Hospital, 95 Dunsanseo-ro, Seo-gu, Daejeon 35233, Korea Tel: +82-42-611-3454 Fax: +82-42-611-3459 E-mail: astrias@eulji.ac.kr
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Thymosin β4 Is an Endogenous Iron Chelator and Molecular Switcher of Ferroptosis
Joanna I. Lachowicz, Giusi Pichiri, Marco Piludu, Sara Fais, Germano Orrù, Terenzio Congiu, Monica Piras, Gavino Faa, Daniela Fanni, Gabriele Dalla Torre, Xabier Lopez, Kousik Chandra, Kacper Szczepski, Lukasz Jaremko, Mitra Ghosh, Abdul-Hamid Emwas, Mass
International Journal of Molecular Sciences.2022; 23(1): 551. CrossRef - Metal coordination of thymosin β4: Chemistry and possible implications
Joanna Izabela Lachowicz, Mariusz Jaremko, Lukasz Jaremko, Giuseppina Pichiri, Pierpaolo Coni, Marco Piludu
Coordination Chemistry Reviews.2019; 396: 117. CrossRef - Adipose-Derived Mesenchymal Stem Cells Enhance Ovarian Cancer Growth and Metastasis by Increasing Thymosin Beta 4X-Linked Expression
Yijing Chu, Min You, Jingjing Zhang, Guoqiang Gao, Rendong Han, Wenqiang Luo, Tingting Liu, Jianxin Zuo, Fuling Wang
Stem Cells International.2019; 2019: 1. CrossRef - An Investigation on the Therapeutic Effect of Thymosinβ4 and Its Expression Levels in Streptozotocin-Induced Diabetic Mice
Kyung Sook Cho, Dong-Jin Kim, Bomee Shim, Jung Yeon Kim, Jun Mo Kang, Seon Hwa Park, Sang-Ho Lee, Hyung-In Yang, Kyoung Soo Kim
BioMed Research International.2018; 2018: 1. CrossRef - Hypoxia-inducible factor-1α expression in colorectal carcinoma
Ahmed M. Abd ElAziz, Hanan S. Abd ElHamid, Rasha R. Mostafa, Yousra R.A. Shalaby
Egyptian Journal of Pathology.2018; 38(1): 18. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


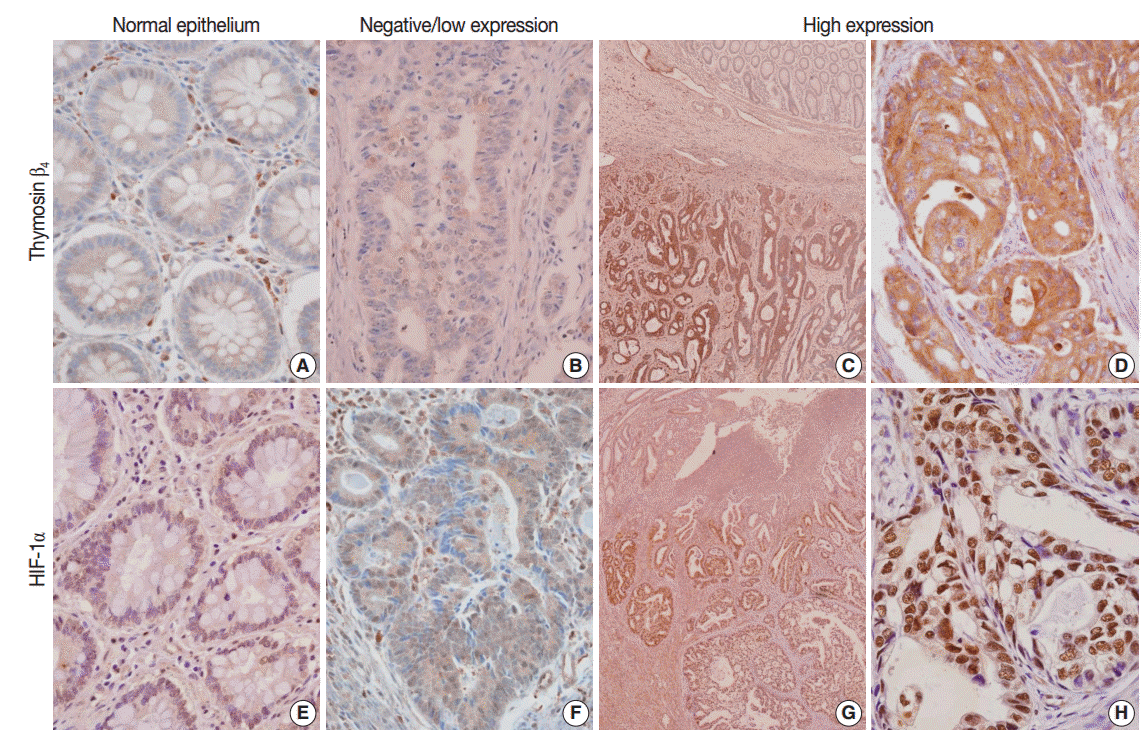
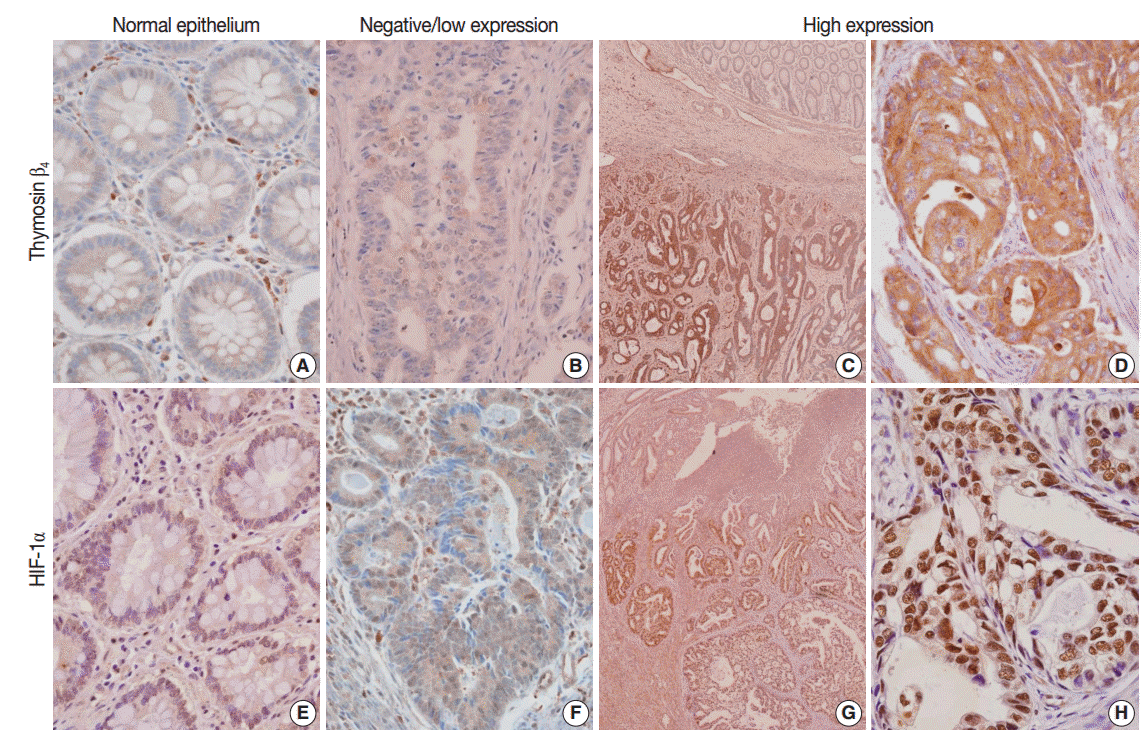
Fig. 1.
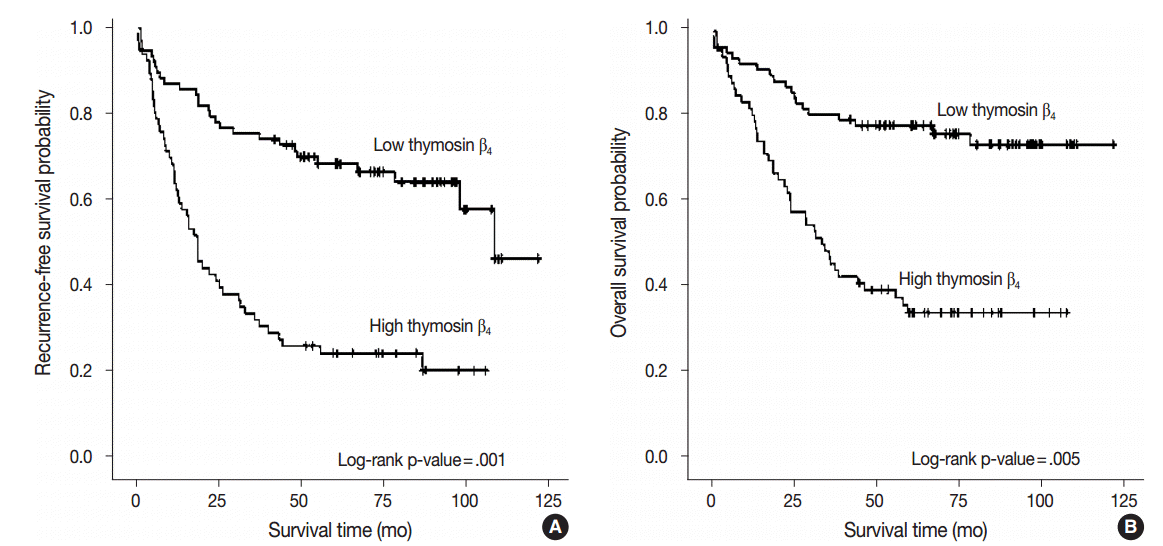
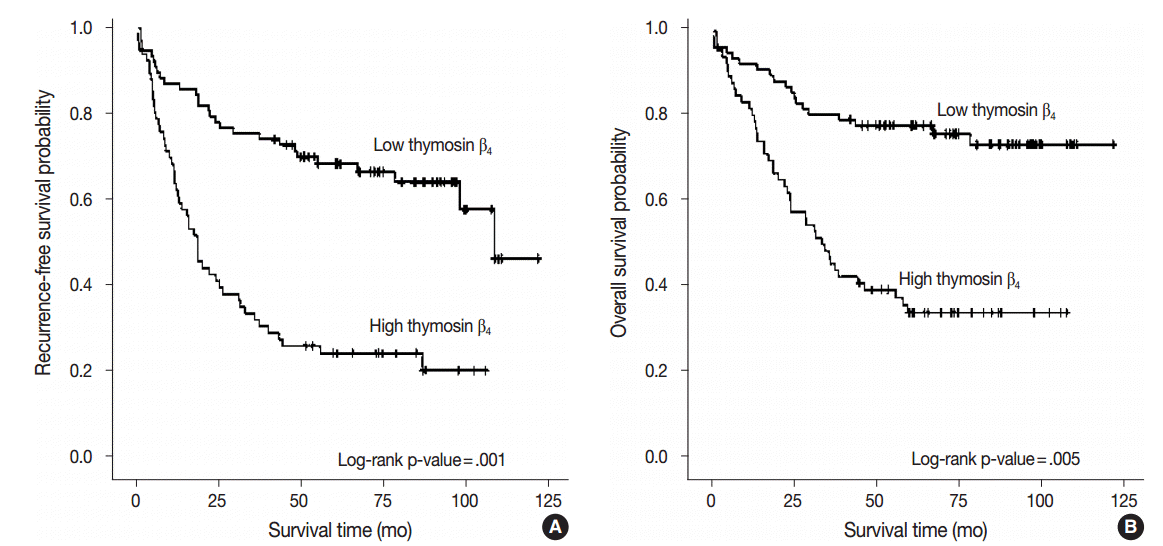
Fig. 2.
| Characteristic | Total | Thymosin β4 expression level |
p-value | |
|---|---|---|---|---|
| Negative/Low | High | |||
| Age (yr) | ||||
| < 50 | 26 | 10 (13.0) | 16 (24.2) | < .082 |
| ≥ 50 | 117 | 67 (87.0) | 50 (75.8) | |
| Gender | ||||
| Female | 68 | 35 (45.5) | 33 (50.0) | < .587 |
| Male | 75 | 42 (54.5) | 33 (50.0) | |
| Site | ||||
| Right/Transverse colon | 34 | 20 (26.0) | 14 (21.2) | < .505 |
| Left colon and rectum | 109 | 57 (74.0) | 52 (78.8) | |
| Size | < .814 | |||
| < 5 cm in diameter | 60 | 33 (42.9) | 27 (40.9) | |
| ≥ 5 cm in diameter | 83 | 44 (57.1) | 39 (59.1) | |
| Grade | < .926 | |||
| Low | 111 | 60 (54.1) | 51 (45.9) | |
| High | 32 | 17 (53.1) | 15 (46.9) | |
| LV invasion | < .001 |
|||
| Not identified | 39 | 31 (40.3) | 8 (12.1) | |
| Present | 104 | 46 (59.7) | 58 (87.9) | |
| Tumor border | .560 | |||
| Pushing | 13 | 8 (10.4) | 5 (7.6) | |
| Infiltrating | 130 | 69 (90.6) | 61 (92.4) | |
| Tumor budding | < .118 | |||
| Low | 37 | 24 (31.2) | 13 (19.7) | |
| High | 106 | 53 (68.6) | 53 (80.3) | |
| Invasion depth | < .001 |
|||
| pT1 | 5 | 5 (6.5) | 0 | |
| pT2 | 21 | 18 (23.4) | 3 (4.5) | |
| pT3 | 105 | 50 (64.9) | 55 (83.3) | |
| pT4 | 12 | 4 (5.2) | 8 (12.1) | |
| LN metastasis | < .001 |
|||
| pN0 | 67 | 52 (67.5) | 15 (22.7) | |
| pN1 | 23 | 8 (10.4) | 15 (22.7) | |
| pN2 | 53 | 17 (22.1) | 36 (54.5) | |
| Distant metastasis | < .001 |
|||
| M0 | 121 | 74 (96.1) | 47 (71.2) | |
| M1 | 22 | 3 (3.9) | 19 (28.8) | |
| TNM stage | < .001 |
|||
| I | 21 | 20 (26.0) | 1 (1.5) | |
| II | 45 | 32 (41.6) | 13 (19.7) | |
| III | 55 | 22 (28.6) | 33 (50.0) | |
| IV | 22 | 3 (3.9) | 19 (28.8) | |
| Characteristic | No. | Recurrence-free survival |
Overall survival |
||
|---|---|---|---|---|---|
| Relative risk (95% CI) | p-value | Relative risk (95% CI) | p-value | ||
| Thymosin β4 | .001 |
.005 |
|||
| Low/negative | 77 | 1.000 | 1.000 | ||
| High | 66 | 2.540 (1.479–4.362) | 2.457 (1.315–4.592) | ||
| Size (diameter, cm) | .230 | .094 | |||
| < 5 | 60 | 1.000 | 1.000 | ||
| ≥ 5 | 83 | 1.349 (0.828–2.197) | 1.636 (0.919–2.913) | ||
| Grade | .048 |
.013 |
|||
| Low | 111 | 1.000 | 1.000 | ||
| High | 32 | 1.785 (1.005–3.171) | 2.241 (1.186–4.236) | ||
| LV invasion | .938 | .974 | |||
| Not identified | 39 | 1.000 | 1.000 | ||
| Present | 104 | 1.025 (0.547–1.922) | 1.012 (0.485–2.111) | ||
| Budding | .022 |
.047 |
|||
| Low | 37 | 1.000 | 1.000 | ||
| High | 106 | 2.094 (1.112–3.942) | 2.068 (1.010–4.235) | ||
| Invasion depth | .022 |
.074 | |||
| pT1 + pT2 | 26 | 1.000 | 1.000 | ||
| pT3 + pT4 | 117 | 3.491 (1.194–10.206) | 3.849 (0.878–16.868) | ||
| LN metastasis | .833 | .437 | |||
| Not identified | 67 | 1.000 | 1.000 | ||
| Present | 76 | 1.060 (0.617–1.821) | 1.290 (0.678–2.455) | ||
| Distant metastasis | .005 |
.001 |
|||
| M0 | 121 | 1.000 | 1.000 | ||
| M1 | 22 | 2.368 (1.301–4.311) | 2.997 (1.586–5.664) | ||
| Frequency | Total | HIF-1α |
p-value | ||
|---|---|---|---|---|---|
| High | Low/negative | ||||
| Thymosin β4 | High | 66 | 49 (74.2) | 17 (25.8) | < .001 |
| Low/negative | 77 | 18 (23.4) | 59 (76.6) | ||
Values are presented as number (%). LV, lympho-vascular invasion; LN, lymph node. p < .05. Fisher exact test.
p-values were obtained by Cox proportional hazards analysis modeled. CI, confidence interval; LV, lympho-vascular invasion; LN, lymph node. p < .05.
Values are presented as number (%). HIF-1α, hypoxia inducible factor-1α.

 E-submission
E-submission