Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(5); 2017 > Article
-
Case Study
A Rare Case of Aggressive Melanotic Schwannoma Occurred in Spinal Nerve of a 59-Year-Old Male - Sung-eun Choi,*, Yoon Jin Cha,*, Jisup Kim, Hyunseo Cha, Jayeong Seo, Sung-Uk Kuh1, Sung-Jun Kim2, Se Hoon Kim,3
-
Journal of Pathology and Translational Medicine 2017;51(5):505-508.
DOI: https://doi.org/10.4132/jptm.2017.01.04
Published online: April 4, 2017
Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
1Department of Neurosurgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
2Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
3Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author Se Hoon Kim, MD Department of Pathology, Severance Hospital, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea Tel: +82-2-2228-1764 Fax: +82-2-362-0860 E-mail: paxco@yuhs.ac
- *Sung-eun Choi and Yoon Jin Cha contributed equally to this work.
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Melanotic schwannoma (MS) is a rare variant of nerve sheath neoplasm that shows ultrastructural and immunophenotypical features of Schwann cells but also has cytoplasmic melanosomes and is reactive for melanocytic markers as well. Unlike conventional schwannoma, which is totally benign, MS has an unpredictable prognosis and is thought to have low-malignant potential. Herein, we present a rare case of recurrent MS in lumbar spine of a 59-year-old male.
- This study was approved by the Institutional Review Board of Gangnam Severance Hospital with a waiver of informed consent (IRB No. 3-2016-0255).
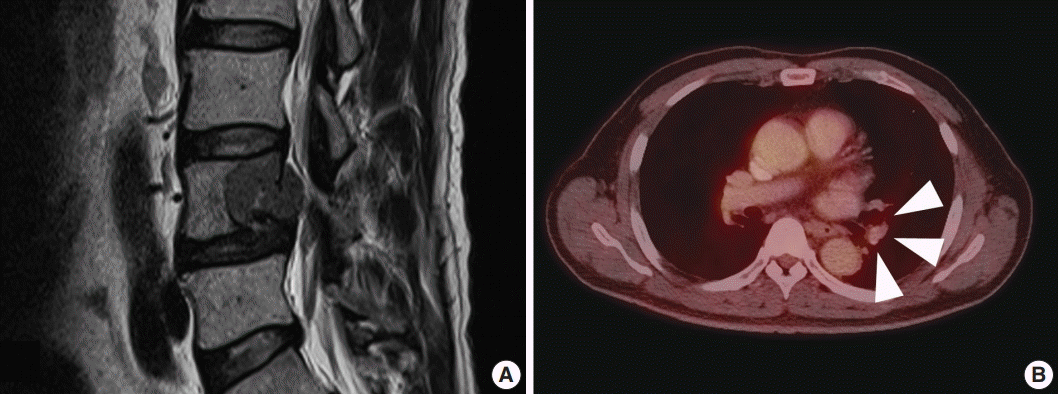
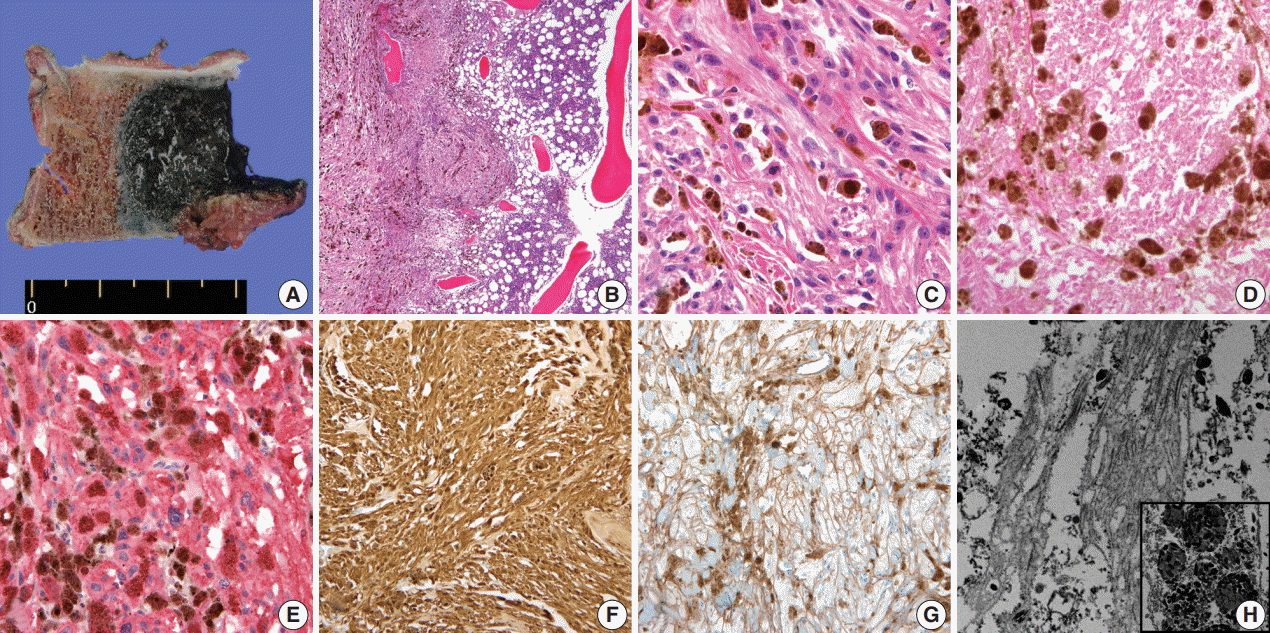
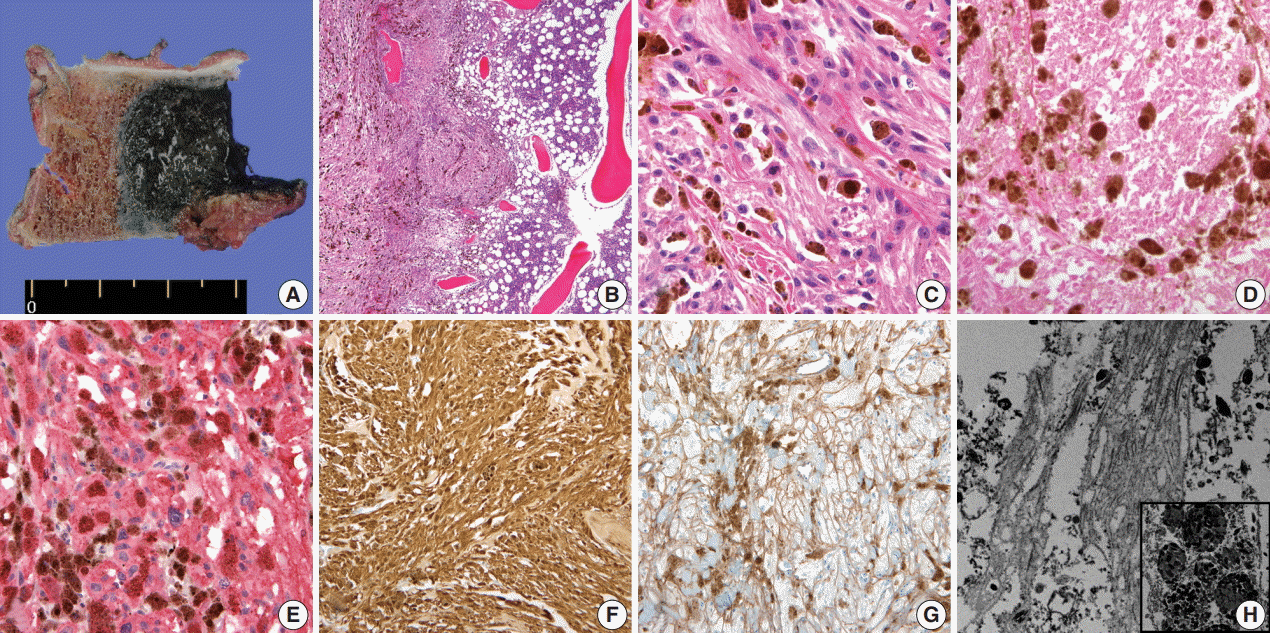
- A 59-year-old male presented with right buttock pain and radiating leg pain. He had a medical history of hypertension on medication for 10 years. Five years ago, he had a hemi-laminectomy with removal of a spinal cord mass of left L4 level, at the outside hospital, and the diagnosis was MS. On spinal magnetic resonance imaging, tumor in previous operative site, L4 level of spinal cord, was recognized with involvement of L4 vertebral body (Fig. 1A). Tumor recurrence was considered based on the patient’s history. Subsequent positron emission tomography–computed tomography revealed strong fluorodeoxyglucose (FDG) uptake in the L4/5 level. Multiple lung nodules with increased FDG uptake were found in the left upper lobe, right middle lobe, and right lower lobe, which appeared to be consistent with metastatic nodules (Fig. 1B). Patient received total laminectomy of L4 and subtotal laminectomy of L3 lower and L5 upper bodies. The resected specimen submitted in fresh state consisted of a product of L4 corpectomy, including a body of L4 spinal bone and separately sent soft tissue. A 3.3×2.2-cm-sized black, soft mass was located in the posterior side of the L4 body. On cut section, the black mass was infiltrating into the bone (Fig. 2A).
- On histological examination, a cellular pigmented mass was infiltrating the bone marrow (Fig. 2B). Discohesive tumor cells were arranged in solid sheets or nests. Tumor cells had eosinophilic ample cytoplasm, long cytoplasmic process with fuzzy cell borders, and variable amounts of cytoplasmic melanin pigments. Nuclear pleomorphism and prominent macronucleoli were observed with a few mitoses up to 2/10 high power fields (HPFs) (Fig. 2C). Foci of tumor necrosis were observed (Fig. 2D). Immunohistochemical staining for human melanoma black 45 (HMB45; 1:100, clone HMB45, Dako, Carpinteria, CA, USA), S-100 protein (1:2,000, clone bBS/NC/VI-H14, Dako), Ki-67 (1:150, clone MIB-1, Dako), and collagen type IV (1:100, clone CIV 22, Dako) was performed. Tumor cells showed diffuse and strong expression of HMB45 and S-100 protein (Fig. 2E, F). Collagen type IV was expressed along the pericellular membrane (Fig. 2G). Ki-67 was positive in approximately 1% of the tumor cells. Under electron microscopy, abundant basal lamina (Fig. 2H) was observed along with the cytoplasmic melanosomes (Fig. 2H, inset).
CASE REPORT
- We reported a rare case of spinal MS that showed local recurrence and pulmonary metastasis. MS is a rare variant of nerve sheath neoplasm of which less than 200 cases have been reported to date with three cases in Korean reports [2-5]. MS can be divided into psammomatous and nonpsammomatous type [2], and approximately half of the psammomatous MS are related to Carney complex, an autosomal dominant disease with cardiac myxomas and Cushing syndrome [6]. Nonpsammomatous MS is considered to be a sporadic type and commonly affects spinal nerves and paraspinal ganglia, whereas psammomatous type often involves autonomic nerves of viscera. In contrast to the typical encapsulation of conventional schwannoma, MS is a circumscribed but unencapsulated tumor, which may reflect the potential of more aggressive nature of MS such as an invasive growth pattern. In present study, the tumor showed infiltrative border that permeated the bone marrow space. Unlike the conventional schwannoma, which is a totally benign neoplasm, MS follows an unpredictable clinical course. Even devoid of overt histologic atypia, approximately 10% of MS follow a malignant course [2,7]. Although MS could demonstrate nuclear pleomorphism and macronucleoli with expression of melanocytic markers, findings mimicking malignant melanoma, these histologic features are poorly correlated with the clinical outcome. However, unlike malignant melanoma which usually has frequent mitosis, MS has rare mitosis. In addition, histologic features of ample cytoplasm, cytoplasmic process, and indiscernable cell border as well as low proliferative index contribute to the diagnosis of MS rather than malignant melanoma. Presence of mitosis itself, particularly over one mitosis/10HPFs, is the only known risk factor of metastasis in MS [8]. In present case, the tumor had histologic atypia—nuclear pleomorphism, prominent macronucleoli—and foci of necrosis which are worrisome histologic features in routine pathologic diagnosis. Moreover, more importantly, the mitotic count was up to 2 /10HPFs, which may have been a factor attributing to the lung metastasis.
- Recently, Torres-Mora et al. [8] carried out gene microarray study covering over 1,700 genes, showed different gene expression profile of MS from conventional schwannoma or malignant melanoma, and suggested that MS is a distinctive neoplasm, belonging neither to the conventional schwannoma nor malignant melanoma. Among pigmented lesions of central nervous system, MS lacks GNAQ codon 209 mutations, which is one of mutational descriptors found in leptomeningeal melanocytic lesions [9]. Although MS is a genetically interesting and ambiguous tumor, a genetic study of the present case was not available. Instead, immunohistochemical staining and electron microscopy helped to identify the abundant basal lamina and cytoplasmic melanosomes, which elucidated the features of both Schwann cell and melanocyte. Previous study described different basement membrane staining patterns of MS from conventional schwannoma and leptomeningeal melanocytic lesion [9]. MS demonstrated pericellular staining of basement membrane on collagen type IV, similar to that of conventional schwannoma with or without a nesting pattern, whereas other melanocytic lesions had predominant nesting pattern [9].
- So far, only about 20 cases of metastatic MS have been reported [7,8,10-16], which are shown in Table 1. This is the first metastatic and recurrent MS case in a Korean patient. The sporadic, spinal MS showed an aggressive biologic behavior—local recurrence and pulmonary metastasis—and the ancillary examination delineated the pericellular basal lamina and cytoplasmic melanosomes.
DISCUSSION
Acknowledgments

| Case No. | Sex | Age (yr) | Primary site | Metastasis site | References |
|---|---|---|---|---|---|
| 1 | M | 27 | Bronchus | Brain | Rowlands et al. [15] (1987) |
| 2 | F | 48 | T9–T10 | Lung, skin | Killeen et al. [16] (1988) |
| 3 | M | 27 | L5 | Lung, pleura | Vallat-Decouvelaere et al. [7] (1999) |
| 4 | F | 35 | L3–L5 | Bone, lymph node | Vallat-Decouvelaere et al. [7] (1999) |
| 5 | F | 45 | T6 | Lung, bone, liver | Vallat-Decouvelaere et al. [7] (1999) |
| 6 | M | 35 | C4–C5 | Leptomeninges | Santaguida et al. [10] (2004) |
| 7 | M | 61 | T7 | Leptomeninges | Tawk et al. [11] (2005) |
| 8 | M | 33 | L5–S1 | Lung | Shields et al. [12] (2011) |
| 9 | F | 32 | C4–C5 | Lung | Faria et al. [13] (2013) |
| 10 | F | 15 | Cervical paraspinal | Leptomeninges, parascapular, and neck soft tissues | Torres-Mora et al. [8] (2014) |
| 11 | F | 23 | L4 | Liver | Torres-Mora et al. [8] (2014) |
| 12 | F | 25 | Sacrum | Lung, pleura, lymph nodes | Torres-Mora et al. [8] (2014) |
| 13 | M | 27 | L2–L3 | Lung, lymph nodes, abdomen | Torres-Mora et al. [8] (2014) |
| 14 | M | 32 | C2 nerve root | Lung, skeleton | Torres-Mora et al. [8] (2014) |
| 15 | M | 40 | Paraspinal L3–L4 | Spine (T12) | Torres-Mora et al. [8] (2014) |
| 16 | F | 44 | T5–6 | Lung, posterior chest wall | Torres-Mora et al. [8] (2014) |
| 17 | M | 47 | L3–L4 | Lung, liver, pleura, leptomeninges, bone | Torres-Mora et al. [8] (2014) |
| 18 | M | 47 | C5 | Lumbar/thoracic, brain | Torres-Mora et al. [8] (2014) |
| 19 | M | 61 | T6–T8 | Spinal cord | Torres-Mora et al. [8] (2014) |
| 20 | F | 67 | T10 | Liver | Torres-Mora et al. [8] (2014) |
| 21 | M | 46 | L3 | Brain, leptomeninges | Khoo et al. [14] (2016) |
- 1. Carney JA. Carney complex: the complex of myxomas, spotty pigmentation, endocrine overactivity, and schwannomas. Semin Dermatol 1995; 14: 90-8. ArticlePubMed
- 2. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. Lyon: IARC Press, 2007.
- 3. Yim H, Go JH, Ahn CS, Hong SW, Jung WH. Pigmented (melanotic) schwannoma of the cervical spinal canal: a case report. Korean J Pathol 1995; 29: 256-62.
- 4. Yi S, Chin DK, Jin BH, Cho YE, Kim YS. Melanotic schwannoma in cervical spine: a case report. J Korean Neurosurg Soc 2001; 30: 916-20.
- 5. You SH, Suh YL, Kim JH. Melanotic acoustic schwannoma. J Korean Neurosurg Soc 2002; 31: 485-7.
- 6. Carney JA. Psammomatous melanotic schwannoma: a distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol 1990; 14: 206-22. PubMed
- 7. Vallat-Decouvelaere AV, Wassef M, Lot G, et al. Spinal melanotic schwannoma: a tumour with poor prognosis. Histopathology 1999; 35: 558-66. ArticlePubMedPDF
- 8. Torres-Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL. Malignant melanotic schwannian tumor: a clinicopathologic, immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of “melanotic schwannoma”. Am J Surg Pathol 2014; 38: 94-105. PubMed
- 9. Kusters-Vandevelde HV, van Engen-van Grunsven IA, Kusters B, et al. Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol 2010; 120: 755-64. ArticlePubMedPMC
- 10. Santaguida C, Sabbagh AJ, Guiot MC, Del Maestro RF. Aggressive intramedullary melanotic schwannoma: case report. Neurosurgery 2004; 55: 1430.ArticlePubMedPDF
- 11. Tawk RG, Tan D, Mechtler L, Fenstermaker RA. Melanotic schwannoma with drop metastases to the caudal spine and high expression of CD117 (c-kit). J Neurooncol 2005; 71: 151-6. ArticlePubMedPDF
- 12. Shields LB, Glassman SD, Raque GH, Shields CB. Malignant psammomatous melanotic schwannoma of the spine: a component of Carney complex. Surg Neurol Int 2011; 2: 136.ArticlePubMedPMC
- 13. Faria MH, Doria-Netto RH, Osugue GJ, Queiroz Lde S, Chaddad-Neto FE. Melanotic schwannoma of the cervical spine progressing with pulmonary metastasis: case report. Neurol Med Chir (Tokyo) 2013; 53: 712-6. ArticlePubMedPMC
- 14. Khoo M, Pressney I, Hargunani R, Tirabosco R. Melanotic schwannoma: an 11-year case series. Skeletal Radiol 2016; 45: 29-34. ArticlePubMedPDF
- 15. Rowlands D, Edwards C, Collins F. Malignant melanotic schwannoma of the bronchus. J Clin Pathol 1987; 40: 1449-55. ArticlePubMedPMC
- 16. Killeen RM, Davy CL, Bauserman SC. Melanocytic schwannoma. Cancer 1988; 62: 174-83. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- The “Pigmented Side” of Nerve Sheaths: Malignant Melanotic Nerve Sheath Tumor
Raduan Ahmed Franca, Rosa Maria Di Crescenzo, Lorenzo Ugga, Rosa Della Monica, Elena D'Avella
International Journal of Surgical Pathology.2025; 33(4): 1068. CrossRef - Case Report: Cutaneous melanocytic schwannoma with concomitant melanocytoma in a canine
Olwam H. Monakali, Nicolize O'Dell, Louise van der Weyden
Wellcome Open Research.2024; 8: 364. CrossRef - Intradural Melanotic Schwannoma of the Sacral Spine: An Illustrated Case Report of Diagnostic Conundrum
Jiunn-Kai Chong, Navneet Kumar Dubey, Wen-Cheng Lo
Reports.2024; 7(3): 56. CrossRef - Rare giant retroperitoneal melanotic schwannoma: a case report and literature review
Pan Chen, Junfeng Cheng, Lin Zhang
Frontiers in Oncology.2024;[Epub] CrossRef - A Rare Case of Melanotic Schwannoma Occurred Intraosseous of Sacrum: A Literature Review
Xiaobo Yan, Keyi Wang, Nong Lin, Xin Huang, YanBiao Fu, Zhaoming Ye
Orthopaedic Surgery.2023; 15(2): 655. CrossRef - Sporadic spinal psammomatous malignant melanotic nerve sheath tumor: A case report and literature review
Giulio Bonomo, Alessandro Gans, Elio Mazzapicchi, Emanuele Rubiu, Paolo Alimonti, Marica Eoli, Rosina Paterra, Bianca Pollo, Guglielmo Iess, Francesco Restelli, Jacopo Falco, Francesco Acerbi, Marco Paolo Schiariti, Paolo Ferroli, Morgan Broggi
Frontiers in Oncology.2023;[Epub] CrossRef - Case Report: Cutaneous melanocytic schwannoma with concomitant melanocytoma in a canine
Olwam H. Monakali, Nicolize O'Dell, Louise van der Weyden
Wellcome Open Research.2023; 8: 364. CrossRef - Fine‐needle aspiration cytology of melanotic schwannoma in the submandibular gland
Yu‐Hua Huang, Ying‐Chou Lu, Hsuan‐Ying Huang, Chien‐Chin Chen
Diagnostic Cytopathology.2021; 49(1): 142. CrossRef - Checkpoint inhibitors and radiotherapy in refractory malignant melanocytic schwannoma with Carney complex: first evidence of efficacy
Jyoti Bajpai, Akhil Kapoor, Rakesh Jalali, Mrinal M Gounder
BMJ Case Reports.2021; 14(5): e240296. CrossRef - 18F-FDG PET/CT imaging for aggressive melanotic schwannoma of the L3 spinal root
Xun-Ze Shen, Wei Wang, Zhou-Ye Luo
Medicine.2021; 100(8): e24803. CrossRef - Hemorrhagic spinal melanotic schwannoma presenting as acute chest pain: A case report and literature review
Dallas J. Soyland, Dylan R. Goehner, Kayla M. Hoerschgen, Troy D. Gust, Shawn M. Vuong
Surgical Neurology International.2021; 12: 164. CrossRef - Retroperitoneal Recurrence of Melanotic Schwannoma on 18F-FDG PET/CT
Xiangliu OuYang, Lichun Zheng, Xiaoming Zhang
Clinical Nuclear Medicine.2021; 46(12): 991. CrossRef - Schwannoma originating from the common iliac artery: a case report
Seung-Myoung Son, Chang Gok Woo
Journal of International Medical Research.2020;[Epub] CrossRef - Intraosseous Melanotic Schwannoma in the Sacrum Mimicking Primary Bone Tumor
Yoshitaka Nagashima, Yusuke Nishimura, Kaoru Eguchi, Takayuki Awaya, Satoshi Yoshikawa, Shoichi Haimoto, Toshihiko Wakabayashi, Masahito Hara
NMC Case Report Journal.2020; 7(3): 107. CrossRef - Extramedullary melanotic schwannoma recurrence in the cervical vertebral arch: a case report and review of the literature
Zongbin Hou, Teng Shi, Guangrun Li, Lin Tian, Xinna Li, Xiaoyang Liu
Journal of International Medical Research.2020;[Epub] CrossRef - Extramedullary malignant melanotic schwannoma of the spine: Case report and an up to date systematic review of the literature
Georgios Solomou, Adikarige Haritha Dulanka Silva, Adrianna Wong, Ute Pohl, Nikolaos Tzerakis
Annals of Medicine and Surgery.2020; 59: 217. CrossRef - Melanotic Schwannoma of the Vagina: A Report of a Very Rare Tumor and Review of the Literature
Kofi Effah, Stefan Seidl, Edith Gorges, Patrick Kafui Akakpo
Case Reports in Obstetrics and Gynecology.2019; 2019: 1. CrossRef - Melanotic Schwannomas Are Rarely Seen Pigmented Tumors with Unpredictable Prognosis and Challenging Diagnosis
Elif Keskin, Sumeyye Ekmekci, Ozgur Oztekin, Gulden Diniz
Case Reports in Pathology.2017; 2017: 1. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Fig. 2.
| Case No. | Sex | Age (yr) | Primary site | Metastasis site | References |
|---|---|---|---|---|---|
| 1 | M | 27 | Bronchus | Brain | Rowlands et al. [15] (1987) |
| 2 | F | 48 | T9–T10 | Lung, skin | Killeen et al. [16] (1988) |
| 3 | M | 27 | L5 | Lung, pleura | Vallat-Decouvelaere et al. [7] (1999) |
| 4 | F | 35 | L3–L5 | Bone, lymph node | Vallat-Decouvelaere et al. [7] (1999) |
| 5 | F | 45 | T6 | Lung, bone, liver | Vallat-Decouvelaere et al. [7] (1999) |
| 6 | M | 35 | C4–C5 | Leptomeninges | Santaguida et al. [10] (2004) |
| 7 | M | 61 | T7 | Leptomeninges | Tawk et al. [11] (2005) |
| 8 | M | 33 | L5–S1 | Lung | Shields et al. [12] (2011) |
| 9 | F | 32 | C4–C5 | Lung | Faria et al. [13] (2013) |
| 10 | F | 15 | Cervical paraspinal | Leptomeninges, parascapular, and neck soft tissues | Torres-Mora et al. [8] (2014) |
| 11 | F | 23 | L4 | Liver | Torres-Mora et al. [8] (2014) |
| 12 | F | 25 | Sacrum | Lung, pleura, lymph nodes | Torres-Mora et al. [8] (2014) |
| 13 | M | 27 | L2–L3 | Lung, lymph nodes, abdomen | Torres-Mora et al. [8] (2014) |
| 14 | M | 32 | C2 nerve root | Lung, skeleton | Torres-Mora et al. [8] (2014) |
| 15 | M | 40 | Paraspinal L3–L4 | Spine (T12) | Torres-Mora et al. [8] (2014) |
| 16 | F | 44 | T5–6 | Lung, posterior chest wall | Torres-Mora et al. [8] (2014) |
| 17 | M | 47 | L3–L4 | Lung, liver, pleura, leptomeninges, bone | Torres-Mora et al. [8] (2014) |
| 18 | M | 47 | C5 | Lumbar/thoracic, brain | Torres-Mora et al. [8] (2014) |
| 19 | M | 61 | T6–T8 | Spinal cord | Torres-Mora et al. [8] (2014) |
| 20 | F | 67 | T10 | Liver | Torres-Mora et al. [8] (2014) |
| 21 | M | 46 | L3 | Brain, leptomeninges | Khoo et al. [14] (2016) |
M, male; F, female; T, thoracic spine; L lumbar spine; C, cervical spine.

 E-submission
E-submission