Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 51(4); 2017 > Article
-
Original Article
Implication of PHF2 Expression in Clear Cell Renal Cell Carcinoma - Cheol Lee1, Bohyun Kim1, Boram Song1, Kyung Chul Moon1,2
-
Journal of Pathology and Translational Medicine 2017;51(4):359-364.
DOI: https://doi.org/10.4132/jptm.2017.03.16
Published online: June 13, 2017
1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea
2Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, Seoul, Korea
- Corresponding Author Kyung Chul Moon, MD Department of Pathology, Kidney Research Institute, Medical Research Center, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-1767 Fax: +82-2-743-5530 E-mail: blue7270@snu.ac.kr
• Received: February 9, 2017 • Revised: March 8, 2017 • Accepted: March 14, 2017
© 2017 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Clear cell renal cell carcinoma (CCRCC) is presumed to be associated with adipogenic differentiation. Histone modification is known to be important for adipogenesis, and the function of histone demethylase plant homeodomain finger 2 (PHF2) has been noted. In addition, PHF2 may act as a tumor suppressor via epigenetic regulation of p53 and is reported to be reduced in colon cancer and stomach cancer tissues. In this study, we examined PHF2 expression in CCRCC specimens by immunohistochemistry.
-
Methods
- We studied 254 CCRCCs and 56 non-neoplastic renal tissues from patients who underwent radical or partial nephrectomy between 2000 and 2003 at the Seoul National University Hospital. Tissue microarray blocks were prepared, and immunohistochemical staining for PHF2 was performed.
-
Results
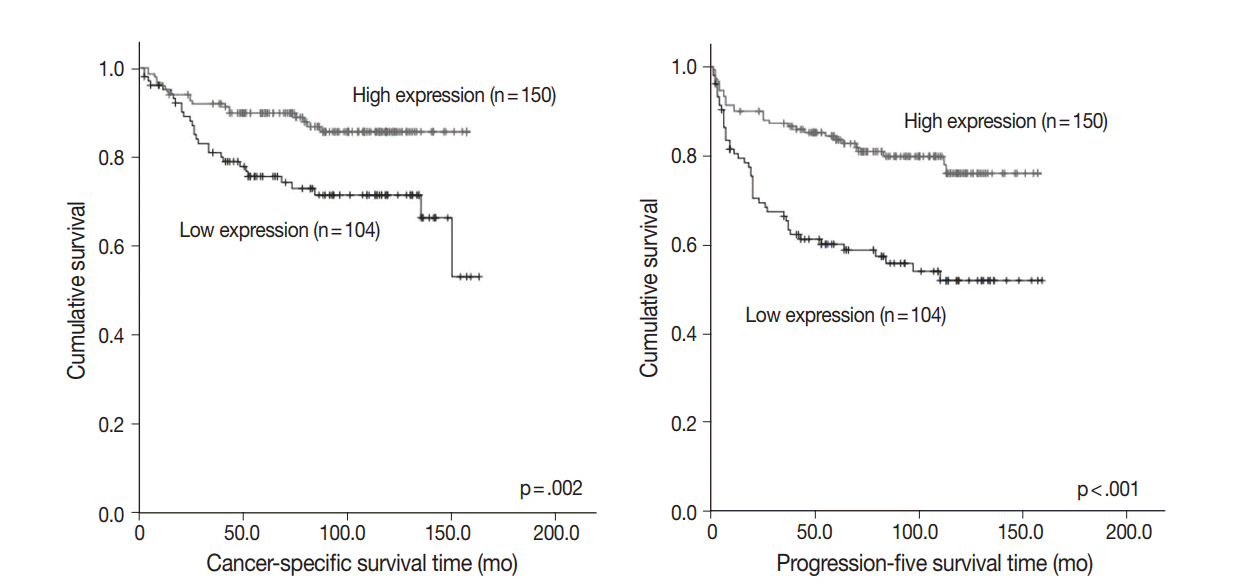
- Among 254 CCRCC cases, 150 cases (59.1%) showed high expression and 104 cases (40.1%) showed low expression. High expression of PHF2 was significantly correlated with a low Fuhrman nuclear grade (p < .001), smaller tumor size (p < .001), low overall stage (p = .003), longer cancer-specific survival (p = .002), and progression-free survival (p < .001) of the patients. However, it was not an independent prognostic factor in multivariate analysis adjusted for Fuhrman nuclear grade and overall stage.
-
Conclusions
- Our study showed that low expression of PHF2 is associated with aggressiveness and poor prognosis of CCRCC.
- Patients and tissue microarray
- We examined 254 CCRCCs and 56 non-neoplastic renal tissues including the cortex and medulla from patients who underwent radical or partial nephrectomy between 2000 and 2003 in Seoul National University Hospital. Tissue microarray (TMA) blocks containing representative tumor and non-neoplastic tissue core sections (2 mm in diameter) were prepared from each formalin-fixed paraffin block (SuperBioChips Laboratories, Seoul, Korea). All available hematoxylin and eosin–stained slides were reviewed to assess the validity of the diagnosis, and tumors were graded from 1 to 4 according to the Fuhrman nuclear grade system. Clinical and pathological information was collected from electronic medical records and pathological reports. The follow-up period ranged from 2 to 163 months, and the median follow-up period was 84.5 months. This study was approved by Seoul National University Hospital’s Institutional Review Board (IRB).
- Immunohistochemistry
- Immunohistochemical staining for PHF2 was performed on 4 μm-thick sections taken from TMA. Immunohistochemistry (IHC) was performed using the Ventana Benchmark XT automated dyeing system (Ventana Medical Systems, Tucson, AZ, USA). Polyclonal rabbit anti-PHF2 antibody (Novus Biologicals, Littleton, CO, USA) was diluted 1:200. Immunohistochemically stained TMA slides were individually reviewed by two pathologists without clinicopathological information.
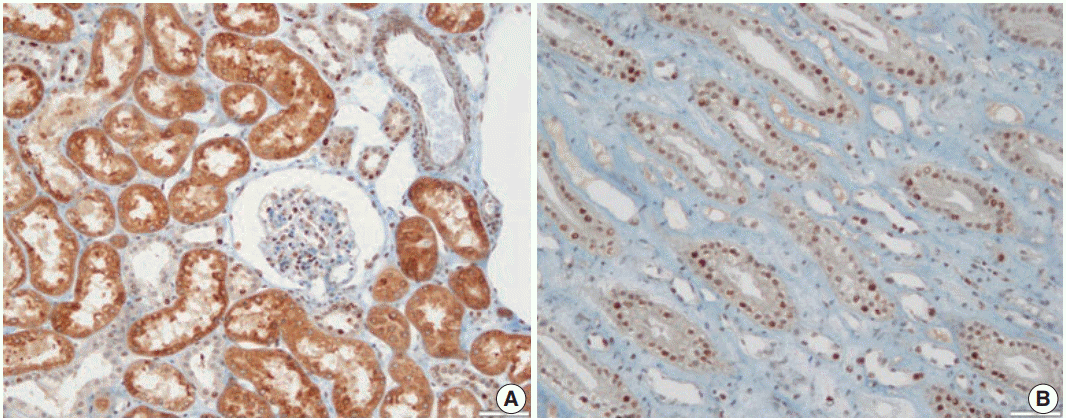
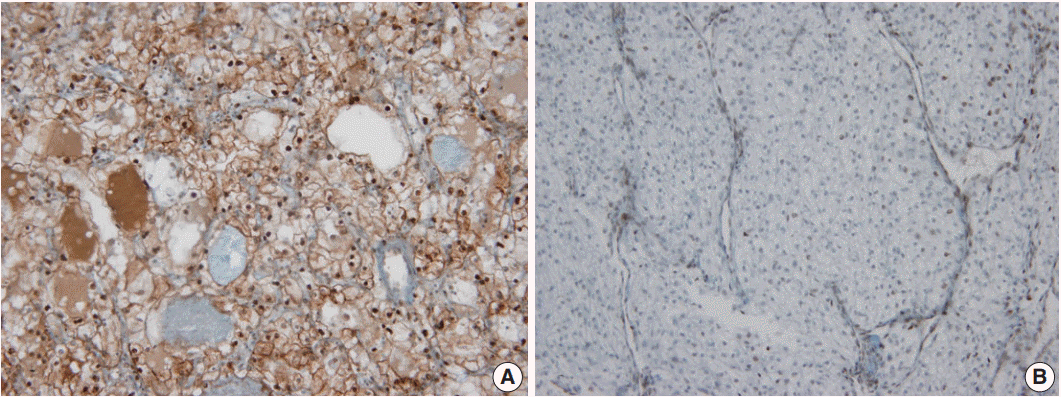
- In non-neoplastic renal tissue, PHF2 was commonly expressed in the nucleus and cytoplasm of the proximal tubular epithelium, but only in the nucleus of the distal tubular epithelium and podocytes of the glomerulus (Fig. 1). The expression of PHF2 was assessed in both the nucleus and cytoplasm of CCRCCs and classified as high when nuclear expression was observed in more than 10% of the tumor cells, regardless of cytoplasmic expression (Fig. 2).
- Statistical analysis
- Statistical analysis was performed using the statistical program SPSS ver. 21.0 (IBM Corp., Armonk, NY, USA). The chi-squared test was used to analyze the relationship between PHF2 expression and clinicopathological characteristics. Survival curves were generated using the Kaplan-Meier method, and survival rate differences were compared using the log-rank test. The multivariate Cox proportional hazard model was used to analyze the importance of various variables for survival. p-values less than .05 were considered statistically significant.
MATERIALS AND METHODS
- Basic clinicopathological characteristics
- Of the 254 patients in this study, 187 (73.6%) were males and 67 (26.4%) were females. The age at diagnosis was between 28 and 82 years old. The mean age±standard deviation (SD) and median age were 56.2±11.3 years and 57 years, respectively. We have classified them into two groups: older than 57 years or not. The Fuhrman nuclear grade distribution was 16 cases in grade I (6.3%), 113 cases in grade II (44.5%), 93 cases in grade III (36.6%), and 32 cases in grade IV (12.6%). The tumor size ranged from 1.0 to 22.0 cm and the mean±SD size was 5.6±3.5 cm. At the time of diagnosis, 23 patients (9.1%) had metastatic cancers and 231 (90.9%) did not. According to the prognostic classification of the American Joint Committee on Cancer 7th edition [17], the T stage was as follows: T1 was 164 cases (64.6%), T2 was 38 cases (15.0%), T3 was 50 cases (19.7%), and T4 was two cases (0.8%). Additionally, the overall stage was 162 cases in stage I (63.8%), 31 cases in stage II (12.2%), 37 cases in stage III (14.6%), and 24 cases in stage IV (9.4%) (Table 1).
- Immunohistochemical result of PHF2 and relationship with clinicopathological characteristics
- Of the 254 cases, 150 cases (59.1%) showed high expression, and 104 cases (40.1%) showed low expression. The high expression of PHF2 was associated with a younger age group (p=.006) and significantly correlated with low Fuhrman nuclear grade, small tumor size and low overall stage (p<.001, p<.001, and p=.003, respectively) (Table 1). However, there was no association with the patients’ sex, T stage, or metastasis at diagnosis.
- The association between PHF2 expression and survival time of the patients
- To assess prognostic value, we evaluated the association between PHF2 expression and patient survival by a log-rank test using Kaplan-Meier analysis. We observed that high expression of PHF2 was significantly associated with longer cancer-specific survival (p=.002) and progression-free survival (p<.001) in CCRCC patients (Fig. 3).
- Univariate and multivariate Cox regression analysis of cancer-specific survival and progression-free survival
- Univariate cox regression analysis showed significant correlation with age (less than 57 or not), Fuhrman nuclear grade, tumor size (less than 5 cm or not), T stage, metastasis at diagnosis, overall stage, PHF2 expression status, cancer-specific survival (p=.009 for age, p=.003 for PHF2 expression status, and p<.001 for the others), and progression-free survival (p=.001 for age and p<.001 for the others) (Table 2). However, multivariate analysis adjusted for age, Fuhrman nuclear grade and overall stage showed that high expression of PHF2 was not an independent prognostic factor in CCRCC patients with cancer-specific survival (p=.566) and progression-free survival (p=.099) (Table 2).
RESULTS
- In this study, we analyzed the association of PHF2 expression with other clinicopathological parameters and survival time of the CCRCC patients. High expression of PHF2 was associated with a young age group and correlated significantly with low Fuhrman nuclear grade and overall stage. We also observed that high expression of PHF2 was significantly correlated with longer cancer-specific survival and progression-free survival in patients. Multivariate analysis adjusted for age, Fuhrman nuclear grade and overall stage showed that high expression of PHF2 was not an independent prognostic factor for cancer-specific survival and progression-free survival in CCRCC patients.
- PHF2 is a type of histone demethylase, and histone modification is known to be important in adipogenesis [9,10,18]. PHF2 has been shown to play a role in gluconeogenesis in hepatocytes of fasted mice [11] and proinflammatory gene regulation in macrophages in vitro [12]. In a recent study, PHF2 was shown to play an important role in adipogenesis in transgenic mice [9]. PHF2 demethylates the AT-rich interactive domain-containing protein 5B (ARID5B) and binds the promoter regions of target genes by forming a complex with demethylated ARID5B [10,11]. PHF2 interacts with CCAAT/enhancer-binding protein alpha (CEBPA), one of the major regulators of adipogenesis, and promotes adipogenesis by demethylating H3K9me2 in the promoter region of CEBPA target gene. These results indicate that PHF2 promotes adipogenesis by coactivation of CEBPA and that PHF2 is a novel histone methylation-modifying enzyme that modulates adipogenesis [9,10].
- As mentioned earlier, some studies have shown that adipogenic differentiation is associated with CCRCC [5-7]. One study showed that gene expression patterns and IHC results in CCRCC tissues were associated with adipogenesis [7]. They also found that CCRCC cells undergo adipogenic transdifferentiation, based on the observation that the clear cell morphology of CCRCC cells disappeared in standard cell culture media and was redeveloped in adipogenic media [7]. This study concluded that adipogenesis was a type of epithelial mesenchymal transition [7]. Another study has shown that adipose differentiation-related protein (ADFP) expression is increased at both mRNA and protein levels of CCRCC compared to non-neoplastic renal tissues and other types of renal cell carcinoma [5]. ADFP was originally known as a protein associated with lipid metabolism, highly expressed in adipocytes and variably expressed in other cells [19,20]. Higher ADFP levels in CCRCC were associated with a lower grade, lower stage and better prognosis, while higher ADFP levels were an independent good prognostic factor in multivariate analysis [5,6].
- In previous studies, CCRCC with low nuclear grade showed a typical clear cell morphology, but CCRCC with a higher nuclear grade showed reduced clear cell morphology and other morphological features that are relatively frequently observed in metastatic renal cell carcinoma, such as eosinophilic cytoplasm and rhabdoid feature [2,21]. These results suggest that clear cell morphology due to adipogenesis in CCRCC is associated with low nuclear grade and good prognosis. In this regard, our findings suggest that adipogenic differentiation by histone modification is a new tumorigenic mechanism that reflects the clear cell morphology in CCRCC. In addition, the loss of PHF2 expression seems to be correlated with tumor progression and a poor prognosis of CCRCC due to the loss of adipogenic differentiation.
- The role of PHF2 in tumorigenesis is not yet well understood, and only a few studies have examined the association between human cancer and PHF2 expression. The expression of PHF2 in breast cancer was not correlated with patient prognosis [13]. In esophageal squamous cell carcinoma, PHF2 was overexpressed in cancer cells compared to the non-neoplastic epithelium, and high cytoplasmic expression of PHF2 tended to be associated with decreased overall survival of the patients, but there was no statistical significance [14]. In head and neck squamous cell carcinoma, loss of heterozygosity was observed in the chromosomal 9q22 locus located in the PHF2 gene in relation to dysplastic lesions, but it was not related to the clinicopathological index or patient survival rate [15,22].
- A recent study showed that PHF2 can modulate p53 by demethylating H3K9me2, and down-regulation of PHF2 expression occurs in colon and stomach cancer [16]. In the study, they suggested that PHF2 acts as a tumor suppressor by regulating the function of p53 [16]. Even p53-positive colon cancer cells have a malignant phenotype associated with the suppression of p21, a downstream molecule of p53 [16]. They also suggested that PHF2 expression levels could help predict patient outcomes in tumors expressing functional p53 [16]. These results provide another perspective on the role of PHF2 in CCRCC, although the role of p53 in the progression of CCRCC remains controversial [23-25].
- Our study showed the clinicopathological significance of PHF2 expression in CCRCC. Low expression of PHF2 is associated with aggressiveness and poor prognosis of CCRCC, and this effect of PHF2 may be related to the role of PHF2 in adipogenesis or the regulation of p53. More functional studies will help clarify the role of PHF2 in the development and progression of CCRCC.
DISCUSSION
Fig. 1.Plant homeodomain finger 2 (PHF2) expression in non-neoplastic renal tissue. PHF2 is expressed in the nucleus and cytoplasm of the proximal tubular epithelium and in the nucleus of the distal tubular epithelium and podocytes in the glomerulus. (A) Cortex. (B) Medulla.
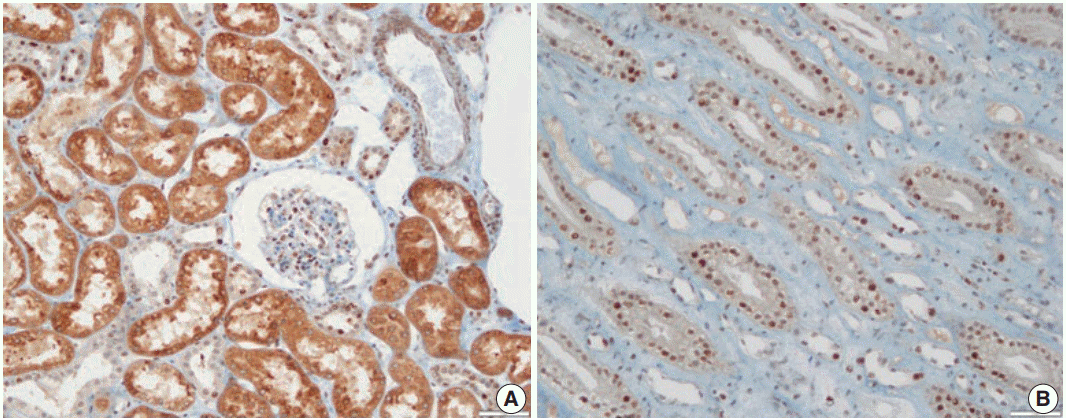

Fig. 2.Plant homeodomain finger 2 expression in clear cell renal cell carcinoma. (A) High expression (B) Low expression.
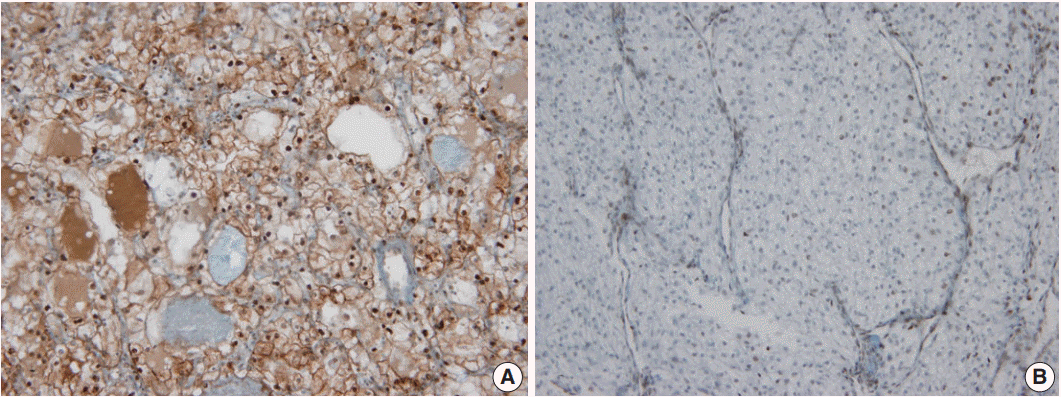

Fig. 3.Kaplan-Meier curves. Cancer-specific and progression-free survival according to plant homeodomain finger 2 (PHF2) expression.
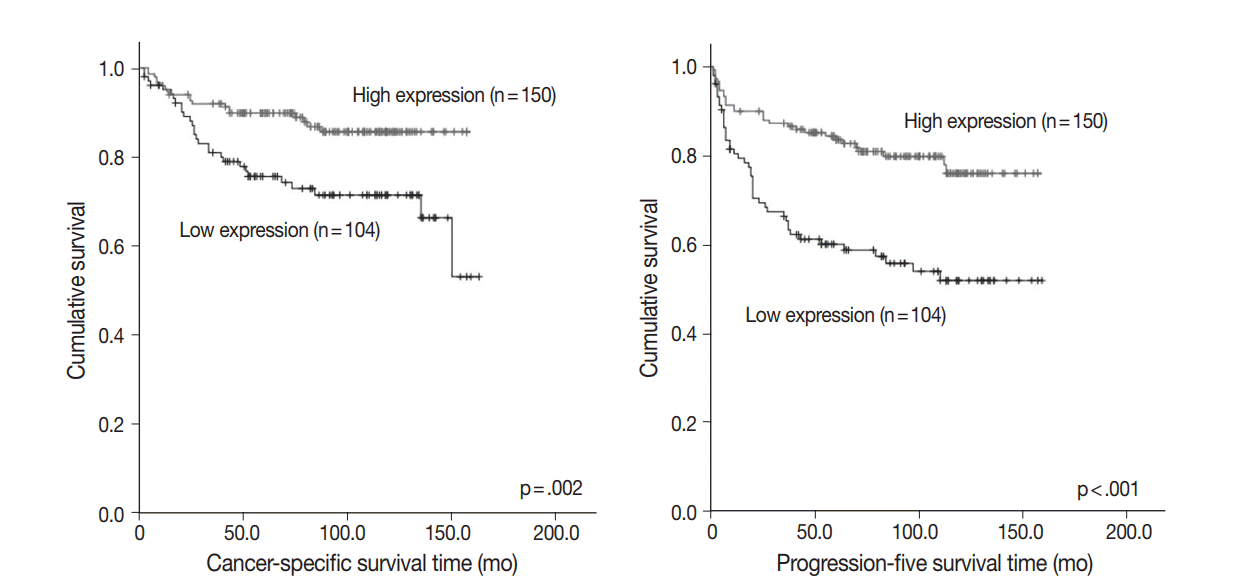

Table 1.Relationship between PHF2 expression and clinicopathological characteristics
Table 2.Univariate and multivariate Cox regression analysis of cancer-specific and progression-free survival
- 1. Jung KW, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat 2013; 45: 1-14. ArticlePubMedPMCPDF
- 2. Lee C, Park JW, Suh JH, Nam KH, Moon KC. Histologic variations and immunohistochemical features of metastatic clear cell renal cell carcinoma. Korean J Pathol 2013; 47: 426-32. ArticlePubMedPMC
- 3. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press, 2004; 12-39.
- 4. Rezende RB, Drachenberg CB, Kumar D, et al. Differential diagnosis between monomorphic clear cell adenocarcinoma of salivary glands and renal (clear) cell carcinoma. Am J Surg Pathol 1999; 23: 1532-8. ArticlePubMed
- 5. Yao M, Tabuchi H, Nagashima Y, et al. Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J Pathol 2005; 205: 377-87. ArticlePubMed
- 6. Yao M, Shioi K, et al. Expression of adipose differentiation-related protein: a predictor of cancer-specific survival in clear cell renal carcinoma. Clin Cancer Res 2007; 13: 152-60. ArticlePubMedPDF
- 7. Tun HW, Marlow LA, von Roemeling CA, et al. Pathway signature and cellular differentiation in clear cell renal cell carcinoma. PLoS One 2010; 5: e10696. Article
- 8. Hasenpusch-Theil K, Chadwick BP, Theil T, Heath SK, Wilkinson DG. PHF2, a novel PHD finger gene located on human chromosome 9q22. Mamm Genome 1999; 10: 294-8. ArticlePubMedPDF
- 9. Okuno Y, Ohtake F, Igarashi K, et al. Epigenetic regulation of adipogenesis by PHF2 histone demethylase. Diabetes 2013; 62: 1426-34. ArticlePubMedPMCPDF
- 10. Okuno Y, Inoue K, Imai Y. Novel insights into histone modifiers in adipogenesis. Adipocyte 2013; 2: 285-8. ArticlePubMedPMC
- 11. Baba A, Ohtake F, Okuno Y, et al. PKA-dependent regulation of the histone lysine demethylase complex PHF2-ARID5B. Nat Cell Biol 2011; 13: 668-75. ArticlePubMedPDF
- 12. Pascual G, Liu W, et al. Control of proinflammatory gene programs by regulated trimethylation and demethylation of histone H4K20. Mol Cell 2012; 48: 28-38. ArticlePubMedPMC
- 13. Sinha S, Singh RK, Alam N, Roy A, Roychoudhury S, Panda CK. Alterations in candidate genes PHF2, FANCC, PTCH1 and XPA at chromosomal 9q22.3 region: pathological significance in early- and late-onset breast carcinoma. Mol Cancer 2008; 7: 84.ArticlePubMedPMCPDF
- 14. Sun LL, Sun XX, Xu XE, et al. Overexpression of JumonjiAT-rich interactive domain 1B and PHD finger protein 2 is involved in the progression of esophageal squamous cell carcinoma. Acta Histochem 2013; 115: 56-62. ArticlePubMed
- 15. Ghosh A, Maiti GP, Bandopadhyay MN, et al. Inactivation of 9q22.3 tumor suppressor genes predict outcome for patients with head and neck squamous cell carcinoma. Anticancer Res 2013; 33: 1215-20. PubMed
- 16. Lee KH, Park JW, Sung HS, et al. PHF2 histone demethylase acts as a tumor suppressor in association with p53 in cancer. Oncogene 2015; 34: 2897-909. ArticlePubMedPDF
- 17. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A 3rd. AJCC cancer staging manual. 7th ed. New York: Springer, 2010; 479-90.
- 18. Musri MM, Carmona MC, Hanzu FA, Kaliman P, Gomis R, Párrizas M. Histone demethylase LSD1 regulates adipogenesis. J Biol Chem 2010; 285: 30034-41. ArticlePubMedPMC
- 19. Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 1997; 38: 2249-63. ArticlePubMed
- 20. Gao J, Serrero G. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J Biol Chem 1999; 274: 16825-30. ArticlePubMed
- 21. Thoenes W, Störkel S, Rumpelt HJ. Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas): the basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract 1986; 181: 125-43. PubMed
- 22. Tripathi A, Dasgupta S, Roy A, et al. Sequential deletions in both arms of chromosome 9 are associated with the development of head and neck squamous cell carcinoma in Indian patients. J Exp Clin Cancer Res 2003; 22: 289-97. PubMed
- 23. Baytekin F, Tuna B, Mungan U, Aslan G, Yorukoglu K. Significance of P-glycoprotein, p53, and survivin expression in renal cell carcinoma. Urol Oncol 2011; 29: 502-7. ArticlePubMed
- 24. Noon AP, Polanski R, El-Fert AY, et al. Combined p53 and MDM2 biomarker analysis shows a unique pattern of expression associated with poor prognosis in patients with renal cell carcinoma undergoing radical nephrectomy. BJU Int 2012; 109: 1250-7. ArticlePubMed
- 25. Noon AP, Vlatkovic N, Polanski R, et al. p53 and MDM2 in renal cell carcinoma: biomarkers for disease progression and future therapeutic targets? Cancer 2010; 116: 780-90. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- The role of histone demethylase PHF2 as a tumour suppressor in hepatocellular carcinoma by regulating SRXN1
Dexter Kai Hao Thng, Lissa Hooi, Wai Khang Yong, Dennis Kappei, Tan Boon Toh, Edward Kai-Hua Chow
Oncogenesis.2026;[Epub] CrossRef - Phosphoproteomics identifies determinants of PAK inhibitor sensitivity in leukaemia cells
Pedro Casado, Santiago Marfa, Marym M. Hadi, Henry Gerdes, Sandra M. Martin-Guerrero, Farideh Miraki-Moud, Vinothini Rajeeve, Pedro R. Cutillas
Cell Communication and Signaling.2025;[Epub] CrossRef - The role of histone methylation in renal cell cancer: an update
Yanguang Hou, Yan Yuan, Yanze Li, Lei Wang, Juncheng Hu, Xiuheng Liu
Molecular Biology Reports.2023; 50(3): 2735. CrossRef - Phosphorylation of PHF2 by AMPK releases the repressive H3K9me2 and inhibits cancer metastasis
Ying Dong, Hao Hu, Xuan Zhang, Yunkai Zhang, Xin Sun, Hanlin Wang, Weijuan Kan, Min-jia Tan, Hong Shi, Yi Zang, Jia Li
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - HIF-1α-mediated augmentation of miRNA-18b-5p facilitates proliferation and metastasis in osteosarcoma through attenuation PHF2
Peng Luo, Yan-dong Zhang, Feng He, Chang-jun Tong, Kai Liu, He Liu, Shi-zhuang Zhu, Jian-zhou Luo, Bing Yuan
Scientific Reports.2022;[Epub] CrossRef - Integration of meta-analysis and supervised machine learning for pattern recognition in breast cancer using epigenetic data
Reza Panahi, Esmaeil Ebrahimie, Ali Niazi, Alireza Afsharifar
Informatics in Medicine Unlocked.2021; 24: 100629. CrossRef - PHF2 regulates homology-directed DNA repair by controlling the resection of DNA double strand breaks
Ignacio Alonso-de Vega, Maria Cristina Paz-Cabrera, Magdalena B Rother, Wouter W Wiegant, Cintia Checa-Rodríguez, Juan Ramón Hernández-Fernaud, Pablo Huertas, Raimundo Freire, Haico van Attikum, Veronique A J Smits
Nucleic Acids Research.2020; 48(9): 4915. CrossRef - Emerging of lysine demethylases (KDMs): From pathophysiological insights to novel therapeutic opportunities
Sarder Arifuzzaman, Mst Reshma Khatun, Rabeya Khatun
Biomedicine & Pharmacotherapy.2020; 129: 110392. CrossRef - Biology and targeting of the Jumonji-domain histone demethylase family in childhood neoplasia: a preclinical overview
Tyler S. McCann, Lays M. Sobral, Chelsea Self, Joseph Hsieh, Marybeth Sechler, Paul Jedlicka
Expert Opinion on Therapeutic Targets.2019; 23(4): 267. CrossRef - MiR-221 Promotes Hepatocellular Carcinoma Cells Migration via Targeting PHF2
Yi Fu, Mingyan Liu, Fengxia Li, Li Qian, Ping Zhang, Fengwei Lv, Wenting Cheng, Ruixing Hou
BioMed Research International.2019; 2019: 1. CrossRef - PHF2 histone demethylase prevents DNA damage and genome instability by controlling cell cycle progression of neural progenitors
Stella Pappa, Natalia Padilla, Simona Iacobucci, Marta Vicioso, Elena Álvarez de la Campa, Claudia Navarro, Elia Marcos, Xavier de la Cruz, Marian A. Martínez-Balbás
Proceedings of the National Academy of Sciences.2019; 116(39): 19464. CrossRef - Plant homeodomain finger protein 2 as a novel IKAROS target in acute lymphoblastic leukemia
Zheng Ge, Yan Gu, Qi Han, Justin Sloane, Qinyu Ge, Goufeng Gao, Jinlong Ma, Huihui Song, Jiaojiao Hu, Baoan Chen, Sinisa Dovat, Chunhua Song
Epigenomics.2018; 10(1): 59. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Implication of PHF2 Expression in Clear Cell Renal Cell Carcinoma



Fig. 1. Plant homeodomain finger 2 (PHF2) expression in non-neoplastic renal tissue. PHF2 is expressed in the nucleus and cytoplasm of the proximal tubular epithelium and in the nucleus of the distal tubular epithelium and podocytes in the glomerulus. (A) Cortex. (B) Medulla.
Fig. 2. Plant homeodomain finger 2 expression in clear cell renal cell carcinoma. (A) High expression (B) Low expression.
Fig. 3. Kaplan-Meier curves. Cancer-specific and progression-free survival according to plant homeodomain finger 2 (PHF2) expression.
Fig. 1.
Fig. 2.
Fig. 3.
Implication of PHF2 Expression in Clear Cell Renal Cell Carcinoma
| Characteristic | No. of cases (n = 254) | PHF2 expression |
p-value | |
|---|---|---|---|---|
| Low (n = 104, 40.1%) | High (n = 150, 59.1%) | |||
| Age (yr) | .006 | |||
| < 57 | 124 (48.8) | 40 | 84 | |
| ≥ 57 | 130 (51.2) | 64 | 66 | |
| Sex | .320 | |||
| Male | 187 (73.6) | 80 | 107 | |
| Female | 67 (26.4) | 24 | 43 | |
| Fuhrman grade | < .001 | |||
| 1, 2 | 129 (50.8) | 38 | 91 | |
| 3, 4 | 125 (49.2) | 66 | 59 | |
| Tumor size (cm) | < .001 | |||
| < 5 | 145 (57.1) | 44 | 101 | |
| ≥ 5 | 109 (42.9) | 60 | 49 | |
| T stage | .071 | |||
| 1, 2 | 202 (79.5) | 77 | 125 | |
| 3, 4 | 52 (20.5) | 27 | 25 | |
| Metastasis at diagnosis | .111 | |||
| No | 231 (90.9) | 91 | 140 | |
| Yes | 23 (9.1) | 13 | 10 | |
| Overall stage | .003 | |||
| I, II | 193 (76.0) | 69 | 124 | |
| III, IV | 61 (24.0) | 35 | 26 | |
| Parameter | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| p-value | HR | 95% CI | p-value | HR | 95% CI | |
| Cancer-specific survival | ||||||
| Age (< 57 yr vs ≥ 57 yr) | .009 | 2.239 | 1.227–4.088 | .393 | 1.312 | 0.704–2.4480 |
| Sex (male vs female) | .870 | 0.947 | 0.492–1.821 | |||
| Fuhrman grade (1, 2 vs 3, 4) | < .001 | 7.754 | 3.463–17.363 | < .001 | 4.391 | 1.911–10.089 |
| Tumor size (< 5 cm vs ≥ 5 cm) | < .001 | 8.130 | 3.801–17.391 | |||
| T stage (1, 2 vs 3, 4) | < .001 | 9.021 | 5.021–16.207 | |||
| Metastasis at diagnosis (no vs yes) | < .001 | 26.511 | 14.102–49.840 | |||
| Overall stage (I, II vs III, IV) | < .001 | 15.961 | 8.233–30.945 | < .001 | 10.616 | 5.313–21.214 |
| PHF2 expression (low vs high) | .003 | 0.419 | 0.235–0.749 | .566 | 0.840 | 0.463–1.524 |
| Progression-free survival | ||||||
| Age (< 57 yr vs ≥ 57 yr) | .001 | 2.324 | 1.436–3.762 | .121 | 1.483 | 0.902–2.440 |
| Sex (male vs female) | .959 | 0.987 | 0.586–1.660 | |||
| Fuhrman grade (1, 2 vs 3, 4) | < .001 | 3.972 | 2.357–6.692 | .001 | 2.491 | 1.447–4.287 |
| Tumor size (< 5 cm vs ≥ 5 cm) | < .001 | 8.416 | 4.692–15.096 | |||
| T stage (1, 2 vs 3, 4) | < .001 | 6.573 | 4.121–10.484 | |||
| Metastasis at diagnosis (no vs yes) | < .001 | 19.269 | 11.058–33.578 | |||
| Overall stage (I, II vs III, IV) | < .001 | 9.230 | 5.744–14.832 | < .001 | 6.470 | 3.933–10.643 |
| PHF2 expression (low vs high) | < .001 | 0.385 | 0.242–0.611 | .099 | 0.666 | 0.411–1.079 |
Table 1. Relationship between PHF2 expression and clinicopathological characteristics
Values are presented as number (%). PHF2, plant homeodomain finger 2.
Table 2. Univariate and multivariate Cox regression analysis of cancer-specific and progression-free survival
HR, hazard ratio; CI, confidence interval.

 E-submission
E-submission