Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(3); 2018 > Article
-
Original Article
Molecular Screening of Small Biopsy Samples Using Next-Generation Sequencing in Korean Patients with Advanced Non-small Cell Lung Cancer: Korean Lung Cancer Consortium (KLCC-13-01) - Bo Mi Ku,*, Mi Hwa Heo1,*, Joo-Hang Kim2, Byoung Chul Cho3, Eun Kyung Cho4, Young Joo Min5, Ki Hyeong Lee6, Jong-Mu Sun1, Se-Hoon Lee1, Jin Seok Ahn1, Keunchil Park1, Tae Jung Kim7, Ho Yun Lee7, Hojoong Kim8, Kyung-Jong Lee8, Myung-Ju Ahn1
-
Journal of Pathology and Translational Medicine 2018;52(3):148-156.
DOI: https://doi.org/10.4132/jptm.2018.03.12
Published online: March 26, 2018
Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul, Korea
1Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
2CHA Bundang Medical Center, CHA University, Seongnam, Korea
3Division of Medical Oncology, Yonsei Cancer Center, Seoul, Korea
4Division of Hematology and Medical Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
5Division of Oncology, Department of Hematology and Oncology, Ulsan University Hospital, Ulsan, Korea
6Division of Medical Oncology, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
7Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
8Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
-
Corresponding Author Kyung-Jong Lee, MD Division of Pulmonary and Clinical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-0777 Fax: +82-2-3410-6956 E-mail: kj2011.lee@samsung.com
Myung-Ju Ahn, MD Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 06351, Korea Tel: +82-2-3410-3438 Fax: +82-2-3410-1754 E-mail: silk.ahn@samsung.com - *Bo Mi Ku and Mi Hwa Heo contributed equally to this work.
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Non-small cell lung cancer (NSCLC) is a common type of cancer with poor prognosis. As individual cancers exhibit unique mutation patterns, identifying and characterizing gene mutations in NSCLC might help predict patient outcomes and guide treatment. The aim of this study was to evaluate the clinical adequacy of molecular testing using next-generation sequencing (NGS) for small biopsy samples and characterize the mutational landscape of Korean patients with advanced NSCLC.
-
Methods
- DNA was extracted from small biopsy samples of 162 patients with advanced NSCLC. Targeted NGS of genomic alterations was conducted using Ion AmpliSeq Cancer Hotspot Panel v2.
-
Results
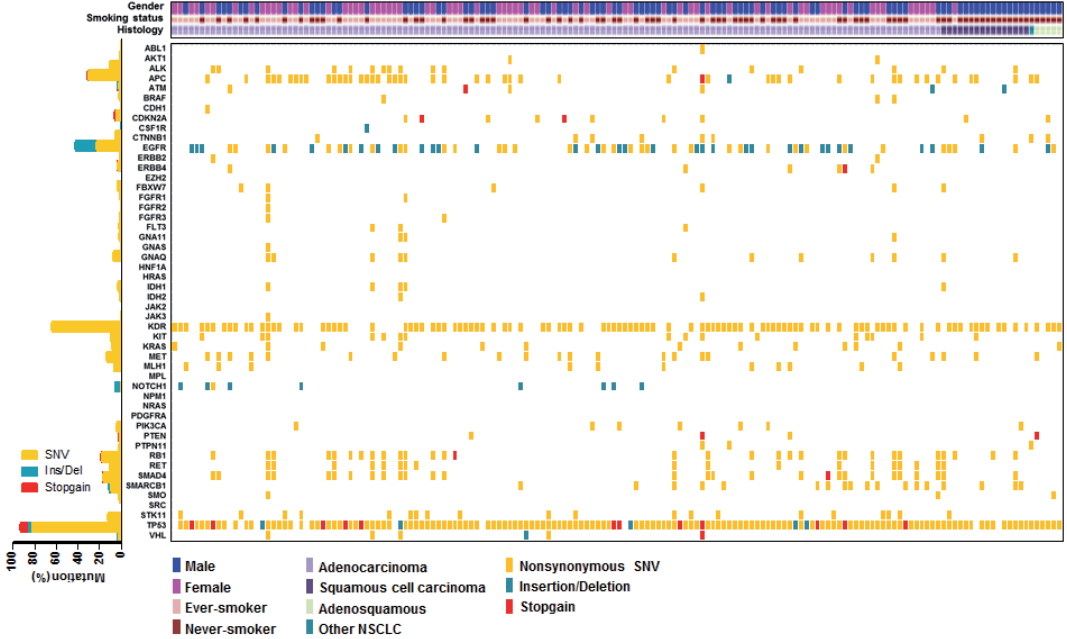
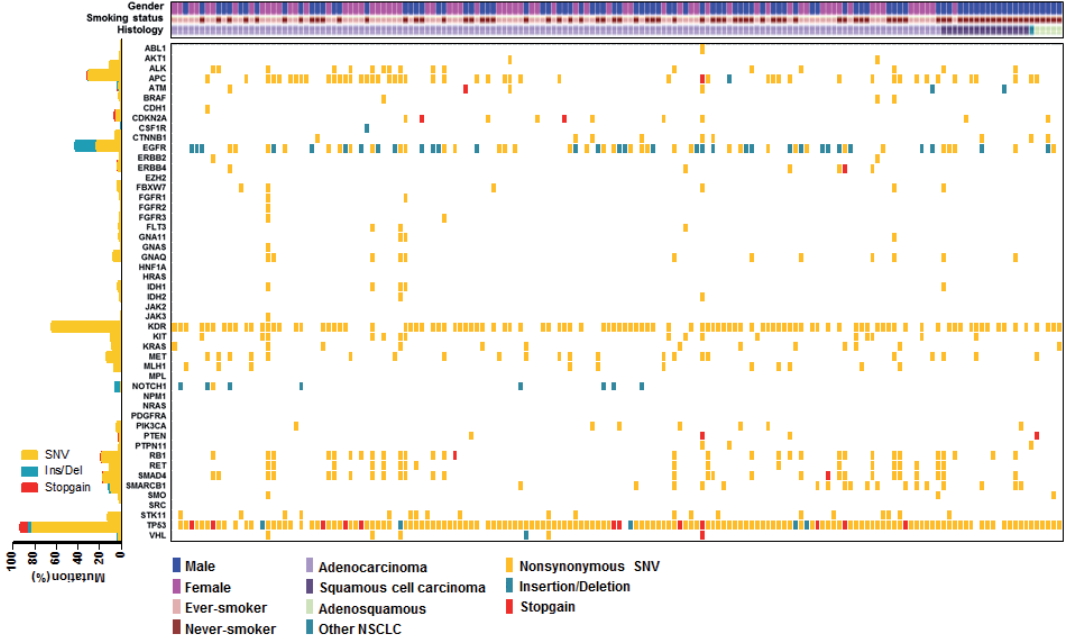
- The median age of patients was 64 years (range, 32 to 83 years) and the majority had stage IV NSCLC at the time of cancer diagnosis (90%). Among the 162 patients, 161 patients (99.4%) had novel or hotspot mutations (range, 1 to 21 mutated genes). Mutations were found in 41 genes. Three of the most frequently mutated genes were TP53 (151, 93.2%), KDR (104, 64.2%), and epidermal growth factor receptor (EGFR; 69, 42.6%). We also observed coexistence of EGFR and other oncogene (such as KRAS, PIC3CA, PTEN, and STK11) mutations. Given that 69.6% (48/69) of EGFR mutant patients were treated with EGFR tyrosine kinase inhibitors, EGFR mutant status had higher prognostic ability in this study.
-
Conclusions
- These results suggest that targeted NGS using small biopsy samples is feasible and allows for the detection of both common and rare mutations in NSCLC.
- Patients and tumor samples
- We analyzed 162 FFPE or frozen tumor tissue specimens from advanced NSCLC patients between January 2014 and December 2015 at Samsung Medical Center (SMC). All samples were collected before any treatments were initiated. Procedures used for tumor tissue sampling varied, including video-assisted thoracoscopic surgery, core-needle biopsy, bronchoscopy, and endobronchial ultrasonography. Clinical data were obtained retrospectively from electronic medical records. The clinical variables assessed were sex, age at diagnosis, smoking history, tumor subtype, cancer stage, EGFR mutation, ALK rearrangement, chemotherapy regimen, TKIs, and tumor response. Separately, EGFR mutation status was tested by real-time PCR using the peptide nucleic acid (PNA)–clamping EGFR Mutation Detection Kit (Panagene, Inc., Daejeon, Korea). Real-time PCR was performed using a CFX96 (Bio-Rad, Hercules, CA, USA) and all reagents were included in the kit. PCR cycling and mutation detection were done as previously described [12]. ALK rearrangement status was tested by immunohistochemistry and confirmed by fluorescence in situ hybridization (FISH). All procedures involving tumor specimens were reviewed and approved by the Institutional Review Board (IRB) of SMC and all data were fully anonymized (SMC 2013-08-113-020). Written informed consent was provided by all patients.
- DNA extraction
- All tissue sections were reviewed by pathologists, and only those with tumor content more than 10% were included in the study. Genomic DNA was extracted from FFPE samples using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) or FF tumor specimens using QIAamp DNA mini kit. Purified DNA was quantitated by NanoDrop (Invitrogen Life Technologies, Carlsbad, CA, USA) and Qubit Fluorometer (Invitrogen Life Technologies).
- Next-generation sequencing and data analysis
- The Ion Torrent Ion AmpliSeq Cancer Hotspot Panel v2 (Life Technologies) was used. This panel detects hotspot regions, including ~2,800 COSMIC mutations of 50 oncogenes and tumor suppressor genes. A total of 162 cases of NSCLC specimens were subjected to NGS on the AmpliSeq platform. Sequencings were done according to previously described methods [13]. Variants calls were further processed to reduce potential false-positives. Coverage (> 500 ×) was considered as filtering criteria and the minimal variant allele frequency was 2% for confirming variants as real. After filtering using these criteria, variants causing amino acid change and frameshift were finally used for statistical analysis.
- Statistical analysis
- Clinical and radiological response to treatment was assessed according to Response Evaluation Criteria In Solid Tumor ver. 1.1. Kaplan-Meier estimates were used for the analysis of all time-to-event variables. Progression-free survival (PFS) was calculated from the date of chemotherapy to the date of disease progression or death from any cause or the date of last follow-up. The overall survival (OS) was measured from the date of chemotherapy to the date of death from any cause and was censored at the date of the last follow-up visit. Variables with p < .05 were considered significant. All statistical analyses were performed using PASW Statistics ver. 23.0 (SPSS Inc., Chicago, IL, USA).
MATERIALS AND METHODS
- Patient characteristics
- The clinical characteristics of advanced NSCLC patients included in the present study are summarized in Table 1. The median age was 64 years (range, 32 to 83 years) and gender proportions were roughly equal (male 57% vs female 43%). Seventy-nine patients (59%) were smokers or former smokers, 83 patients (51%) were never smokers. The NSCLC subtype distribution was as follows: adenocarcinoma (139/162, 85.8%), squamous cell carcinoma (17/162, 10.5%), adenosquamous cell carcinoma (1/162, 0.6%), and other (5/162, 3.1%). The majority of patients had stage IV NSCLC at the time of cancer diagnosis (145/162; 90%). In stage IV NSCLC patients, the median PFS was 6.2 months (95% CI, 4.2 to 8.1) and OS was 19.6 months (95% CI, 15.4 to 23.7). EGFR mutation test was done in 145 patients, and the mutation was detected in 64 patients (44.1%) by realtime PCR using PNA-clamping. Positive results for ALK rearrangement by FISH were detected in 14 patients (8.6%). In this cohort, 81 patients were treated with cytotoxic chemotherapy (81/162, 50.0%), 51 with EGFR TKIs (51/162, 31.5%), and three with ALK TKIs (3/162, 1.8%) as first-line therapy.
- Molecular profiling of advanced NSCLC
- We employed targeted NGS technology to evaluate somatic mutations occurring in advanced NSCLC, using the Ion Torrent Ion AmpliSeq Cancer Hotspot Panel. Among the detected mutations, only those annotated in the Catalogue of Somatic Mutations in Cancer (COSMIC) database were considered. Mutations were found in 41 genes and commonly detected in the following genes: TP53 (151, 93.2%), KDR (104, 64.2%), EGFR (69, 42.6%), APC (51, 31.5%), RB1 (30, 18.5%), SMAD4 (28, 17.3%), MET (22, 13.6%), STK11 (20, 12.3%), RET (18, 11.1%), ALK (17, 10.5%), and KRAS (13, 8.0%), as shown in Fig. 1. Only one patient had no mutation while 161 (99.4%) patients possessed more than one mutation (range, 1 to 21; median, 4). The vast majority of identified mutations were single nucleotide variant (SNV) followed by deletion (Del) and insertion (Ins). In accordance with the frequency described in previous studies [1,3], EGFR mutations were found in 42.6% patients and most of them (54/69, 78.3%) were typical mutations (30 Del exon 19 and 24 L858R). One of these was a triple EGFR mutant (L858R/G873R/Q787L) and ten were double EGFR mutant (3 Del exon 19/G873R, 2 Del exon 19/A750P, 1 Del exon 19/S752Y, 1 Del exon 19/K754N, 2 L858R/G873R, 1 L858R/T790M). Besides TP53 and EGFR, the most frequently mutated gene was KDR, and KDR mutations appeared in codon 472 (103 Q472H) and codon 875 (1 T875A). One patient with KDR Q472H had concurrent KDR S1148C. MET mutations were found in codon 375 (17 N375S) and codon 179 (5 A179T). Although one MET R970C in exon 14 was simultaneously found with N375S, this has not been reported as cause of MET exon 14 skipping [14]. STK11 mutations were found in codon 354 (19 F354L) and codon 176 (1 D176G). Two samples detecting STK11 F354L had other STK11 mutation in codon 281 (P281L). Seventeen ALK mutations were all in codon 1184 (G1184E). In concordance with the known frequency of KRAS mutations in Asian population (5%–10%) [15], they were found in 8% of this cohort. Most KRAS mutations appeared in codon 12 (1 G12A, 4 G12C, 3 G12D, 1 G12R, and 2 G12V) with two mutations in codon 50 (T50P). PIK3CA mutations (E81K, R401Q, E542G, E545A, E545K, Q546K, and H1047R) were detected in seven samples and one patient had double PIK3CA mutants (E542G/E545A). PTEN mutations (K66E, R130X, Q171X, and P246L) were identified in four patients.
- We also observed co-occurrence of some of the most frequently mutated and clinically significant genes. Five patients simultaneously had mutations in both EGFR and KRAS. EGFR mutations also harbored PIK3CA and PTEN mutations, which were detected in three and two patients, respectively. In addition, although STK11 mutations were most commonly seen in association with KRAS mutations, we found seven cases with co-occurrence of EGFR and STK11 mutations in this study.
- Comparison of mutational profiles obtained with the AmpliSeq assay
- Based on mutation results considering the location of mutation sites, EGFR mutations were consistently detected by targeted NGS using AmpliSeq Cancer Panel and conventional PNA-clamping PCR (42.6% vs 44.1%). In 145 patients tested for EGFR mutation, the comparison results of EGFR mutations detected by targeted NGS and conventional PNA-clamping PCR are summarized in Table 2. When comparing mutation detection of EGFR in FFPE and FF samples, a high concordance rate (92.4%) was seen between NGS and PNA-clamping PCR. However, targeted NGS method identified additional EGFR mutations in 14 concordant cases and seven discordant cases that were not identified by PNA-clamping PCR. The most frequently found additional EGFR mutation was G873R which was found in 12 patients. We observed three discordant cases that showed positive results (all Del exon 19) in PNA-clamping, but negative in NGS. Considering the high sensitivity of NGS, these results may be due to tumor heterogeneity.
- Although most of the patients had a single biopsy, four patients had repeated biopsies and had double tumors tested. Except for one consistent case, the other three cases showed slightly different mutation profiles (Table 3). These differences may be due to tumor heterogeneity or tumor evolution.
- Impact of mutation status on survival
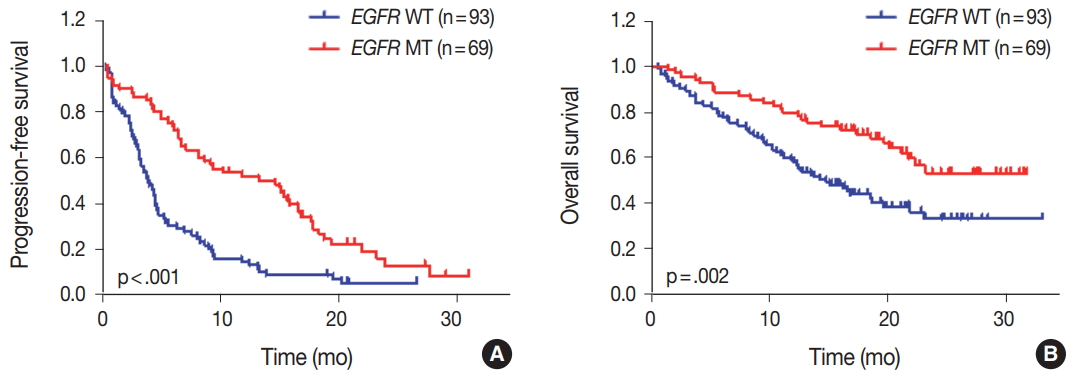
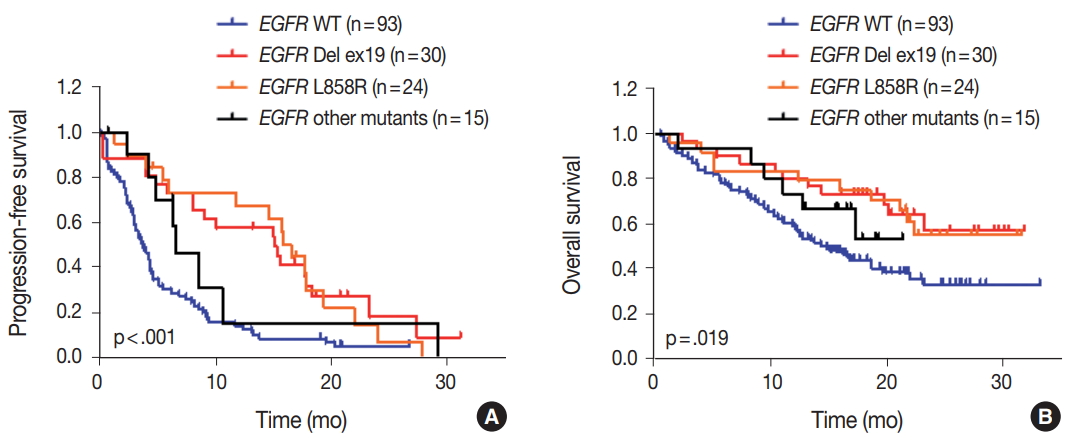
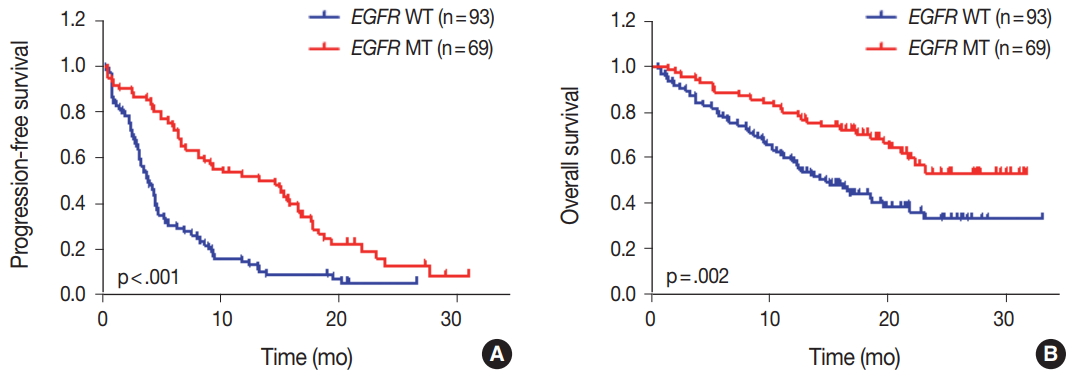
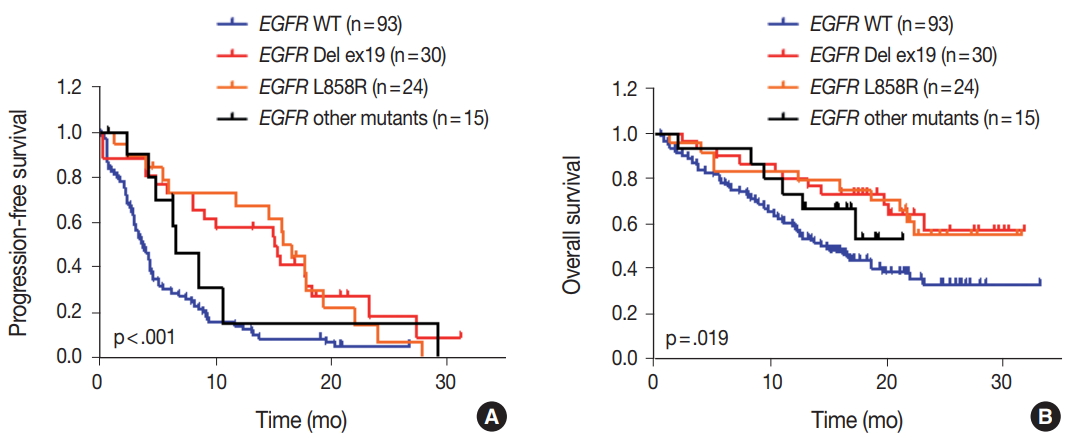
- We evaluated the relationships between EGFR somatic mutations and survival. Activating EGFR mutations have been reported as prognostic factors in other studies [16,17]. In this study cohort, 69.6% (48/69) of patients with EGFR mutations were treated with EGFR TKIs. The presence of EGFR mutations were definitive predictive markers of both PFS (hazard ratio [HR], 2.59; 95% confidence interval [CI], 1.75 to 3.85) (Fig. 2A) and OS (HR, 2.00; 95% CI, 1.30 to 3.09) (Fig. 2B). Median PFSs were 3.8 months for EGFR wild-type group and 14.6 months for EGFR mutant group. The median OS for EGFR wild-type group was 14.8 months, but the median OS for EGFR mutant group was not reached. When PFS was analyzed after grouping the patients according to the three types of EGFR mutations (Del ex19, L858R, and others), the median PFSs were different between activating mutations (15.1 months for Del ex19 and 17.7 months for L858R) and others (6.6 months) (Fig. 3A). However, the median OS was not reached in the three types of EGFR mutations (Fig. 3B).
RESULTS
- Currently, molecular testing for EGFR mutations and ALK rearrangements is essential for targeted therapy in patients with NSCLC. However, the heterogeneous nature of NSCLC can lead to inaccurate molecular classification and therapeutic resistance. Other genetic alterations that have been found and have potential therapeutics include ROS1, RET, and NTRX gene rearrangement, MET exon 14 skipping, and MET amplification [4,18,19]. However, there are no standard molecular diagnostic tests for these genetic alterations. In addition, an important limitation in current routine diagnosis is that the quantity of DNA extracted from small biopsy samples (FFPE or FF) is not adequate for multiple molecular tests in most cases. To cope with this limitation, comprehensive multiplex testing using NGS is necessary to improve the efficacy of targeted therapy for NSCLC patients. With advances in NGS technology, several target regions of interest can be sequenced concurrently and thereby improve the chances of identifying rare mutations. In this study, 162 Korean advanced NSCLC samples were assessed for mutations in oncogenes and tumor suppressor genes using an NGS platform (AmpliSeq Cancer Hotspot Panel). This targeted sequencing method shows high accuracy and requires only small quantities of sample (10 ng DNA), enabling researchers to sequence challenging small biopsy samples such as FFPE. Genetic alterations were confirmed in 99.4% of samples and 14 additional EGFR mutations (L707F, G719A, G719C, L747S, A750P, S752Y, K754N, S768I, V769L, V774M, T783A, S784P, Q787L, and G873R) were identified that were not detected with PNA-clamping PCR. In addition, we found some of the most frequently altered and clinically significant genes such as KRAS, MET, STK11, PIK3CA, and PTEN mutations. Moreover, the higher sensitivity of NGS platform should increase the identification of concomitant mutations. These results suggest the feasibility and usefulness of targeted sequencing to identify low frequency mutations and detect additional mutations that are helpful to understand the clinical outcomes of the patients in each group.
- For patients with EGFR-mutant NSCLC, EGFR TKIs are found to increase response rates and survival time [16,17]. In concordance with these data, EGFR mutations were associated with significant improvements of PFS and OS compared to EGFR wild-type patients, because most patients were treated with EGFR TKIs. Despite these benefits of EGFR TKIs, not all patients respond to treatment and most EGFR-mutant NSCLC patients develop acquired resistance.
- Tumor suppressor TP53 mutations are frequently detected in most human cancers. TP53 was also the most commonly altered gene in this study, and this result is consistent with those of previous studies [20,21]. TP53 was concurrently mutated with many other genes such as EGFR and KRAS in this study, perhaps due to the high frequency of TP53 mutations found in our samples. Whereas the frequency of TP53 mutation is well known, therapeutic options based on this alteration are scarce and controversial in patients with lung cancer. A previous study on advanced NSCLC found an association between TP53 mutation and shorter median OS, but another study, on the other hand, reported no association between TP53 mutation and survival [22,23].
- Our data also identified KDR Q472H polymorphism in 103 patients (31 homozygotes and 72 heterozygotes). KDR Q472H has been reported to increase tumor microvasculature and shown to mediate vascular endothelial growth factor receptor 2 phosphorylation in NSCLC [24]. Furthermore, KDR Q472H had a higher proliferative and invasive capacity in melanoma [25]. Although we did not find a significant correlation between KDR Q472H and survival in EGFR TKI- or chemotherapytreated NSCLC patients, the prognostic value of KDR Q472H should be different after treatment with vascular endothelial growth factor pathway inhibitors.
- Although KRAS mutations are the most common oncogenic driver, there are some ethnic differences. The frequency of KRAS mutations in Asian is 5%–15%. In addition, KRAS mutations usually occur in EGFR wild-type tumors [18,26]. In this study, we detected 13 KRAS mutations (8%) and five concurrent KRAS/EGFR mutations (3.0%) via NGS. In these five patients, three patients, who were clinically confirmed to have EGFR L858R mutations, received EGFR TKI (gefitinib) treatment with partial response or progression of the disease. Other two patients were treated with chemotherapy and showed 0.8 and 9.3 months of PFS. Although the prognostic effect of KRAS mutations was not clear due to small sample size, these results suggest that KRAS mutation test using NGS platform may help determine the appropriate therapy for NSCLC patients.
- Recent studies have demonstrated that mutations in EGFR-downstream genes such as PIK3CA, PTEN, and STK11 are associated with de novo resistance to EGFR TKI [27]. Furthermore, PIK3CA and PTEN mutations may result in resistance to EGFR TKI [4]. In this study, we found PIK3CA and PTEN mutations in seven (4.3%) and four (2.5%) patients, respectively. Concurrent EGFR/PIK3CA mutations were detected in three patients. All of them received EGFR TKI (2 gefitinib and 1 erlotinib) treatment and showed partial response with different range of PFS (6.6–21.3 months). Concurrent EGFR/PTEN mutations were found in two patients and one received EGFR TKI (afatinib) treatment with partial response (PFS, 8.1 months). However, neither PIK3CA nor PTEN mutation status alone had significant effects on PFS and OS in the EGFR-mutant group. In STK11, we identified mutations in 20 patients (19 F354L and 1 D176G). STK11 encodes the serine/threonine protein kinase and is part of the STK11/AMPK/mammalian target of rapamycin signaling pathway. STK11 mutations were commonly found, and inactivation of STK11 is known to promote tumorigenesis and is associated with worse survival outcome [20,28]. The overall rate of STK11 mutations (12.3%) was slightly lower than that indicated by The Cancer Genome Atlas (17%) [18]. This discrepancy can be explained by the origin of the population; STK11 mutations have been reported to be associated with European ancestry [19,21]. STK11 mutations often coexist with KRAS mutations and have confounding prognostic significance [29,30]. However, in this study, we found only one concurrent KRAS/STK11 (G12A/D176G) mutation. This patient received chemotherapy (AP: doxorubicin, cisplatin) and showed partial response with 4.4 months of PFS. A recent study reported that pathogenic STK11 F354L mutations had been recurrently identified in three EGFR TKI non-responders, while these mutations had not been found in EGFR TKI responders [20]. In our study, seven STK11 F354L mutations were recurrently found in EGRK TKI–treated patients. Among them, six (treated with gefitinib and erlotinib) showed partial response (PFS, 4.1 to 17.8 months) and one (treated with afatinib) showed stable disease (PFS, 19.3 months). This discrepancy may be due to the small sample size of STK11 mutant patients. Thus, more research is required to identify the clinical implications of STK11 mutations.
- Our study has a few limitations. Our analysis relied on targeted sequencing to investigate genetic alterations and thus the genes selected in this study may only explain a portion of the total genetic alterations. The NGS platform used in this study (AmpliSeq Cancer Hotspot Panel) detected only SNVs, and thus it was impossible to detect copy number variations (CNVs) and translocations. Furthermore, other novel genetic or epigenetic alterations may have been missed. Most tumor samples were acquired from small biopsy samples, and thus there were not enough tissue available for more comprehensive analysis. Therefore, there is a need for new NGS platforms to simultaneously detect SNVs, CNVs, and translocations, even with small amounts of tissue samples. In addition, functional effects of the detected mutations were not evaluated in vitro.
- Our results demonstrate that targeted sequencing using NGS is feasible for mutation profiling of small biopsy samples in NSCLC. We also demonstrated previously unappreciated mutations, enabling further refinements of subclassification for the prediction of therapeutic effects. In conclusion, we suggest that more comprehensive genomic characterizations of NSCLC with small biopsy samples would reveal coexisting alterations that might influence the efficacy of therapy.
DISCUSSION
Acknowledgments
| Sample type | No. of cases compared |
Concordant (NGS/PNA) |
Discordant (NGS/PNA) |
Concordance (%) | |||
|---|---|---|---|---|---|---|---|
| –/– | +/+ | –/+ | +/– | +/+ | |||
| FFPE | 131 | 69 | 53 | 2 | 6 | 1 | 93.1 |
| FF | 14 | 5 | 7 | 1 | 1 | 0 | 85.7 |
| Total | 145 | 74 | 60 | 3 | 7 | 1 | 92.4 |
- 1. Jung KW, Won YJ, Oh CM, et al. Prediction of cancer incidence and mortality in Korea, 2016. Cancer Res Treat 2016; 48: 451-7. ArticlePubMedPMCPDF
- 2. Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014; 14: 535-46. ArticlePubMedPMCPDF
- 3. Richer AL, Friel JM, Carson VM, Inge LJ, Whitsett TG. Genomic profiling toward precision medicine in non-small cell lung cancer: getting beyond EGFR. Pharmgenomics Pers Med 2015; 8: 63-79. PubMedPMC
- 4. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014; 11: 473-81. ArticlePubMedPDF
- 5. Han JY, Kim SH, Lee YS, et al. Comparison of targeted next-generation sequencing with conventional sequencing for predicting the responsiveness to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in never-smokers with lung adenocarcinoma. Lung Cancer 2014; 85: 161-7. ArticlePubMed
- 6. Rathi V, Wright G, Constantin D, et al. Clinical validation of the 50 gene AmpliSeq Cancer Panel V2 for use on a next generation sequencing platform using formalin fixed, paraffin embedded and fine needle aspiration tumour specimens. Pathology 2017; 49: 75-82. ArticlePubMed
- 7. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014; 311: 1998-2006. ArticlePubMedPMC
- 8. Ballester LY, Luthra R, Kanagal-Shamanna R, Singh RR. Advances in clinical next-generation sequencing: target enrichment and sequencing technologies. Expert Rev Mol Diagn 2016; 16: 357-72. ArticlePubMed
- 9. Padmanabhan V, Steinmetz HB, Rizzo EJ, et al. Improving adequacy of small biopsy and fine-needle aspiration specimens for molecular testing by next-generation sequencing in patients with lung cancer: a quality improvement study at Dartmouth-Hitchcock Medical Center. Arch Pathol Lab Med 2017; 141: 402-9. ArticlePubMedPDF
- 10. Zheng G, Tsai H, Tseng LH, et al. Test feasibility of next-generation sequencing assays in clinical mutation detection of small biopsy and fine needle aspiration specimens. Am J Clin Pathol 2016; 145: 696-702. ArticlePubMedPMCPDF
- 11. Kim ST, Lee J, Hong M, et al. The NEXT-1 (Next generation pErsonalized tX with mulTi-omics and preclinical model) trial: prospective molecular screening trial of metastatic solid cancer patients, a feasibility analysis. Oncotarget 2015; 6: 33358-68. ArticlePubMedPMC
- 12. Kim HJ, Lee KY, Kim YC, et al. Detection and comparison of peptide nucleic acid-mediated real-time polymerase chain reaction clamping and direct gene sequencing for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Lung Cancer 2012; 75: 321-5. ArticlePubMed
- 13. Ku BM, Jung HA, Sun JM, et al. High-throughput profiling identifies clinically actionable mutations in salivary duct carcinoma. J Transl Med 2014; 12: 299.ArticlePubMedPMCPDF
- 14. Lee GD, Lee SE, Oh DY, et al. MET exon 14 skipping mutations in lung adenocarcinoma: clinicopathologic implications and prognostic Values. J Thorac Oncol 2017; 12: 1233-46. ArticlePubMed
- 15. Karachaliou N, Mayo C, Costa C, et al. KRAS mutations in lung cancer. Clin Lung Cancer 2013; 14: 205-14. ArticlePubMed
- 16. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239-46. PubMed
- 17. Kuan FC, Kuo LT, Chen MC, et al. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer 2015; 113: 1519-28. ArticlePubMedPMCPDF
- 18. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014; 511: 543-50. ArticlePubMedPMCPDF
- 19. Dearden S, Stevens J, Wu YL, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol 2013; 24: 2371-6. ArticlePubMedPMCPDF
- 20. Lim SM, Kim HR, Cho EK, et al. Targeted sequencing identifies genetic alterations that confer primary resistance to EGFR tyrosine kinase inhibitor (Korean Lung Cancer Consortium). Oncotarget 2016; 7: 36311-20. ArticlePubMedPMC
- 21. Mäki-Nevala S, Sarhadi VK, Rönty M, et al. Hot spot mutations in Finnish non-small cell lung cancers. Lung Cancer 2016; 99: 102-10. ArticlePubMed
- 22. Murakami I, Hiyama K, Ishioka S, Yamakido M, Kasagi F, Yokosaki Y. p53 gene mutations are associated with shortened survival in patients with advanced non-small cell lung cancer: an analysis of medically managed patients. Clin Cancer Res 2000; 6: 526-30. PubMed
- 23. Lim EH, Zhang SL, Li JL, et al. Using whole genome amplification (WGA) of low-volume biopsies to assess the prognostic role of EGFR, KRAS, p53, and CMET mutations in advanced-stage nonsmall cell lung cancer (NSCLC). J Thorac Oncol 2009; 4: 12-21. PubMed
- 24. Glubb DM, Cerri E, Giese A, et al. Novel functional germline variants in the VEGF receptor 2 gene and their effect on gene expression and microvessel density in lung cancer. Clin Cancer Res 2011; 17: 5257-67. ArticlePubMedPMCPDF
- 25. Silva IP, Salhi A, Giles KM, et al. Identification of a novel pathogenic germline KDR variant in melanoma. Clin Cancer Res 2016; 22: 2377-85. ArticlePubMedPDF
- 26. Roberts PJ, Stinchcombe TE. KRAS mutation: should we test for it, and does it matter? J Clin Oncol 2013; 31: 1112-21. ArticlePubMed
- 27. Kim HR, Cho BC, Shim HS, et al. Prediction for response duration to epidermal growth factor receptor-tyrosine kinase inhibitors in EGFR mutated never smoker lung adenocarcinoma. Lung Cancer 2014; 83: 374-82. ArticlePubMed
- 28. Pécuchet N, Laurent-Puig P, Mansuet-Lupo A, et al. Different prognostic impact of STK11 mutations in non-squamous non-small-cell lung cancer. Oncotarget 2017; 8: 23831-40. ArticlePubMed
- 29. Gleeson FC, Kipp BR, Levy MJ, et al. Somatic STK11 and concomitant STK11/KRAS mutational frequency in stage IV lung adenocarcinoma adrenal metastases. J Thorac Oncol 2015; 10: 531-4. ArticlePubMed
- 30. Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012; 150: 1107-20. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- The clinical relevance of surgical specimens for RNA sequencing in lung cancer: a cohort study
Jung Seop Eom, Soo Han Kim, Kyungbin Kim, Ahrong Kim, Hyo Yeong Ahn, Jeongha Mok, Jeong Su Cho, Min Ki Lee, Ju Sun Song, Mi-Hyun Kim
Frontiers in Oncology.2024;[Epub] CrossRef - PTEN, PTENP1, microRNAs, and ceRNA Networks: Precision Targeting in Cancer Therapeutics
Glena Travis, Eileen M. McGowan, Ann M. Simpson, Deborah J. Marsh, Najah T. Nassif
Cancers.2023; 15(20): 4954. CrossRef - Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis
Barbara Melosky, Kato Kambartel, Maik Häntschel, Margherita Bennetts, Dana J. Nickens, Julia Brinkmann, Antonin Kayser, Michael Moran, Federico Cappuzzo
Molecular Diagnosis & Therapy.2022; 26(1): 7. CrossRef - Landscape of EGFR mutations in lung adenocarcinoma: a single institute experience with comparison of PANAMutyper testing and targeted next-generation sequencing
Jeonghyo Lee, Yeon Bi Han, Hyun Jung Kwon, Song Kook Lee, Hyojin Kim, Jin-Haeng Chung
Journal of Pathology and Translational Medicine.2022; 56(5): 249. CrossRef - Suitability of transbronchial brushing cytology specimens for next‐generation sequencing in peripheral lung cancer
Naoki Furuya, Shingo Matsumoto, Kazutaka Kakinuma, Kei Morikawa, Takeo Inoue, Hisashi Saji, Koichi Goto, Masamichi Mineshita
Cancer Science.2021; 112(1): 380. CrossRef - KLHL38 involvement in non-small cell lung cancer progression via activation of the Akt signaling pathway
Yitong Xu, Chenglong Wang, Xizi Jiang, Yao Zhang, Hongbo Su, Jun Jiang, Hongjiu Ren, Xueshan Qiu
Cell Death & Disease.2021;[Epub] CrossRef - Molecular biomarker testing for non–small cell lung cancer: consensus statement of the Korean Cardiopulmonary Pathology Study Group
Sunhee Chang, Hyo Sup Shim, Tae Jung Kim, Yoon-La Choi, Wan Seop Kim, Dong Hoon Shin, Lucia Kim, Heae Surng Park, Geon Kook Lee, Chang Hun Lee
Journal of Pathology and Translational Medicine.2021; 55(3): 181. CrossRef - Targeting non-small cell lung cancer: driver mutation beyond epidermal growth factor mutation and anaplastic lymphoma kinase fusion
Quincy S. Chu
Therapeutic Advances in Medical Oncology.2020;[Epub] CrossRef Concomitant Mutations in EGFR 19Del/L858R Mutation and Their Association with Response to EGFR-TKIs in NSCLC Patients
Hengrui Liang, Caichen Li, Yi Zhao, Shen Zhao, Jun Huang, Xiuyu Cai, Bo Cheng, Shan Xiong, Jianfu Li, Wei Wang, Changbin Zhu, Weiwei Li, Jianxing He, Wenhua Liang
Cancer Management and Research.2020; Volume 12: 8653. CrossRef- Prognostic role of Rab27A and Rab27B expression in patients with non‐small cell lung carcinoma
Hyun Min Koh, Dae Hyun Song
Thoracic Cancer.2019; 10(2): 143. CrossRef - PD‐L1 expression in ROS1‐rearranged non‐small cell lung cancer: A study using simultaneous genotypic screening of EGFR, ALK, and ROS1
Jongmin Lee, Chan Kwon Park, Hyoung‐Kyu Yoon, Young Jo Sa, In Sook Woo, Hyo Rim Kim, Sue Youn Kim, Tae‐Jung Kim
Thoracic Cancer.2019; 10(1): 103. CrossRef - Targeted Next-Generation Sequencing Validates the Use of Diagnostic Biopsies as a Suitable Alternative to Resection Material for Mutation Screening in Colorectal Cancer
Hersh A. Ham-Karim, Henry Okuchukwu Ebili, Kirsty Manger, Wakkas Fadhil, Narmeen S. Ahmad, Susan D. Richman, Mohammad Ilyas
Molecular Diagnosis & Therapy.2019; 23(3): 383. CrossRef - Small lung tumor biopsy samples are feasible for high quality targeted next generation sequencing
Hidenori Kage, Shinji Kohsaka, Aya Shinozaki‐Ushiku, Yoshihisa Hiraishi, Jiro Sato, Kazuhiro Nagayama, Tetsuo Ushiku, Daiya Takai, Jun Nakajima, Kiyoshi Miyagawa, Hiroyuki Aburatani, Hiroyuki Mano, Takahide Nagase
Cancer Science.2019; 110(8): 2652. CrossRef - PTEN in Lung Cancer: Dealing with the Problem, Building on New Knowledge and Turning the Game Around
Anastasios Gkountakos, Giulia Sartori, Italia Falcone, Geny Piro, Ludovica Ciuffreda, Carmine Carbone, Giampaolo Tortora, Aldo Scarpa, Emilio Bria, Michele Milella, Rafael Rosell, Vincenzo Corbo, Sara Pilotto
Cancers.2019; 11(8): 1141. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Characteristic | No. (%) (n = 162) |
|---|---|
| Age (yr) | |
| Median | 64 |
| Range | 32–83 |
| Sex | |
| Male | 92 (57) |
| Female | 70 (43) |
| Smoking history | |
| Never-smoker | 83 (51) |
| Current | 33 (20) |
| Ex-smoker | 46 (28) |
| Histology | |
| Adenocarcinoma | 139 (86) |
| Squamous cell carcinoma | 17 (10) |
| Adenosquamous | 1 (1) |
| NSCLC, other | 5 (3) |
| Clinical stage | |
| I–II | 7 (4) |
| IIIA | 5 (3) |
| IIIB | 5 (3) |
| IV | 145 (90) |
| Brain metastasis | |
| Present | 49 (30) |
| Absent | 113 (70) |
| Biopsy type | |
| VATS | 45 (28) |
| CNB_lung | 23 (14) |
| CNB_others | 22 (14) |
| Bronchoscopy | 31 (19) |
| EBUS | 41 (25) |
| First treatment | |
| Chemotherapy | 81 (50) |
| EGFR TKI | 51 (32) |
| ALK TKI | 3 (2) |
| No treatment | 27 (16) |
| Sample type | No. of cases compared | Concordant (NGS/PNA) |
Discordant (NGS/PNA) |
Concordance (%) | |||
|---|---|---|---|---|---|---|---|
| –/– | +/+ | –/+ | +/– | +/+ | |||
| FFPE | 131 | 69 | 53 | 2 | 6 | 1 | 93.1 |
| FF | 14 | 5 | 7 | 1 | 1 | 0 | 85.7 |
| Total | 145 | 74 | 60 | 3 | 7 | 1 | 92.4 |
| Patient No. | Mutations identified in first sample | Mutations identified in second sample |
|---|---|---|
| 1 | KDR Q472H, APC A1582P, MET N375S, TP53 R248W, TP53 P72R | KDR Q472H, APC A1582P, TP53 R248W, TP53 P72R |
| 2 | ERBB4 T926M, KIT M541L, TP53 H179R, TP53 P72R | ERBB4 T926M, KIT M541L, FLT F590L, TP53 H179R, TP53 P72R |
| 3 | PIK3CA E542K, PTPN11 G503V, TP53 E285K, TP53 P72R, TP53 P72A, SRC Q529X | KIT M541L, KDR Q472H, TP53 P72R |
| 4 | CTNNB1 D32A, KDR Q472H, EGFR ex19 del, EGFR A750P, CDKN2A H66R, TP53 P72R | CTNNB1 D32A, KDR Q472H, EGFR ex19 del, EGFR A750P, CDKN2A H66R, TP53 P72R |
NSCLC, non-small cell lung carcinoma; VATS, video-assisted thoracoscopic surgery; CNB, core-needle biopsy; EBUS, endobronchial ultrasonography; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; ALK, anaplastic lymphoma kinase.
EGFR, epidermal growth factor receptor; NGS, next-generation sequencing; PNA, peptide nucleic acid; PCR, polymerase chain reaction; FFPE, formalin-fixed paraffin-embedded; FF, fresh frozen.

 E-submission
E-submission