Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 52(6); 2018 > Article
-
Original Article
High Cytoplasmic CXCR4 Expression Predicts Prolonged Survival in Triple-Negative Breast Cancer Patients Treated with Adjuvant Chemotherapy - Bobae Shim, Min-Sun Jin1, Ji Hye Moon, In Ae Park, Han Suk Ryu
-
Journal of Pathology and Translational Medicine 2018;52(6):369-377.
DOI: https://doi.org/10.4132/jptm.2018.09.19
Published online: October 1, 2018
Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
1Department of Pathology, Bucheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Bucheon, Korea
- Corresponding Author Han Suk Ryu, MD, PhD Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-3361 Fax: +82-2-743-5530 E-mail: karlnash@naver.com
© 2018 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Bisphenol A-induced cancer-associated adipocytes promotes breast carcinogenesis via CXCL12/AKT signaling
Zhiyuan Dong, Liping He, Jinyi Wu, Chunfeng Xie, Shanshan Geng, Jieshu Wu, Caiyun Zhong, Xiaoting Li
Molecular and Cellular Endocrinology.2025; 599: 112473. CrossRef - Dissecting the tumor microenvironment in primary breast angiosarcoma: insights from single-cell RNA sequencing
Peikai Ding, Shengbin Pei, Yi Zhai, Zheng Qu, Yazhe Yang, Xiaolong Feng, Qiang Liu, Xiangyu Wang, Wenxiang Zhang, Zhongzhao Wang, Xiangyi Kong, Jing Wang, Yi Fang
Breast Cancer Research.2025;[Epub] CrossRef - CXCR4 expression in colorectal cancer and tumor stage correlation
Fang Cao, Yang Zhang, Jianhao Xu, Jiarui Min, Jihao Su, Zhe Chen, Jingfeng Gu, Zijie Xu, Song Xu
Gene Reports.2025; 40: 102281. CrossRef - Distinct profiles of proliferating CD8+/TCF1+ T cells and CD163+/PD-L1+ macrophages predict risk of relapse differently among treatment-naïve breast cancer subtypes
Konstantinos Ntostoglou, Sofia D. P. Theodorou, Tanja Proctor, Ilias P. Nikas, Sinclair Awounvo, Athanasia Sepsa, Vassilis Georgoulias, Han Suk Ryu, Ioannis S. Pateras, Christos Kittas
Cancer Immunology, Immunotherapy.2024;[Epub] CrossRef - Unravelling the CXCL12/CXCR4 Axis in breast cancer: Insights into metastasis, microenvironment interactions, and therapeutic opportunities
Priyanka Garg, Venkateswara Rao Jallepalli, Sonali Verma
Human Gene.2024; 40: 201272. CrossRef - New Emerging Chemokine Receptors: CCR5 or CXCR5 on Tumor Is Associated with Poor Response to Chemotherapy and Poor Prognosis in Locally Advanced Triple-Negative Breast Cancer
Neslihan Cabioglu, Semen Onder, Hüseyin Karatay, Aysel Bayram, Gizem Oner, Mustafa Tukenmez, Mahmut Muslumanoglu, Abdullah Igci, Ahmet Dinccag, Vahit Ozmen, Adnan Aydiner, Pınar Saip, Ekrem Yavuz
Cancers.2024; 16(13): 2388. CrossRef - Cancer-Associated-Fibroblast-Mediated Paracrine and Autocrine SDF-1/CXCR4 Signaling Promotes Stemness and Aggressiveness of Colorectal Cancers
Chao-Yang Chen, Shih-Hsien Yang, Ping-Ying Chang, Su-Feng Chen, Shin Nieh, Wen-Yen Huang, Yu-Chun Lin, Oscar Kuang-Sheng Lee
Cells.2024; 13(16): 1334. CrossRef - Associations of CXCL12 polymorphisms with clinicopathological features in breast cancer: a case-control study
Shuai Lin, Yi Zheng, Meng Wang, Linghui Zhou, Yuyao Zhu, Yujiao Deng, Ying Wu, Dai Zhang, Na Li, Huafeng Kang, Zhijun Dai
Molecular Biology Reports.2022; 49(3): 2255. CrossRef - The clinicopathological and prognostic value of CXCR4 expression in patients with lung cancer: a meta-analysis
Liping Qiu, Yuanyuan Xu, Hui Xu, Biyun Yu
BMC Cancer.2022;[Epub] CrossRef - Demystifying the CXCR4 conundrum in cancer biology: Beyond the surface signaling paradigm
Mushtaq Ahmad Nengroo, Muqtada Ali Khan, Ayushi Verma, Dipak Datta
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2022; 1877(5): 188790. CrossRef - Targeted dendrimers for antagonizing the migration and viability of NALM-6 lymphoblastic leukemia cells
Chuda Chittasupho, Chaiyawat Aonsri, Witcha Imaram
Bioorganic Chemistry.2021; 107: 104601. CrossRef - CXCR4 and RANK Combination as a Predictor of Breast Cancer Bone Metastasis in Indonesia
Yulian Erwin D
Journal of Surgery and Surgical Research.2021; : 020. CrossRef - CXCL12/CXCR4 axis in the microenvironment of solid tumors: A critical mediator of metastasis
Keywan Mortezaee
Life Sciences.2020; 249: 117534. CrossRef - Impact of the Chemokine Receptors CXCR4 and CXCR7 on Clinical Outcome in Adrenocortical Carcinoma
Irina Chifu, Britta Heinze, Carmina T. Fuss, Katharina Lang, Matthias Kroiss, Stefan Kircher, Cristina L. Ronchi, Barbara Altieri, Andreas Schirbel, Martin Fassnacht, Stefanie Hahner
Frontiers in Endocrinology.2020;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



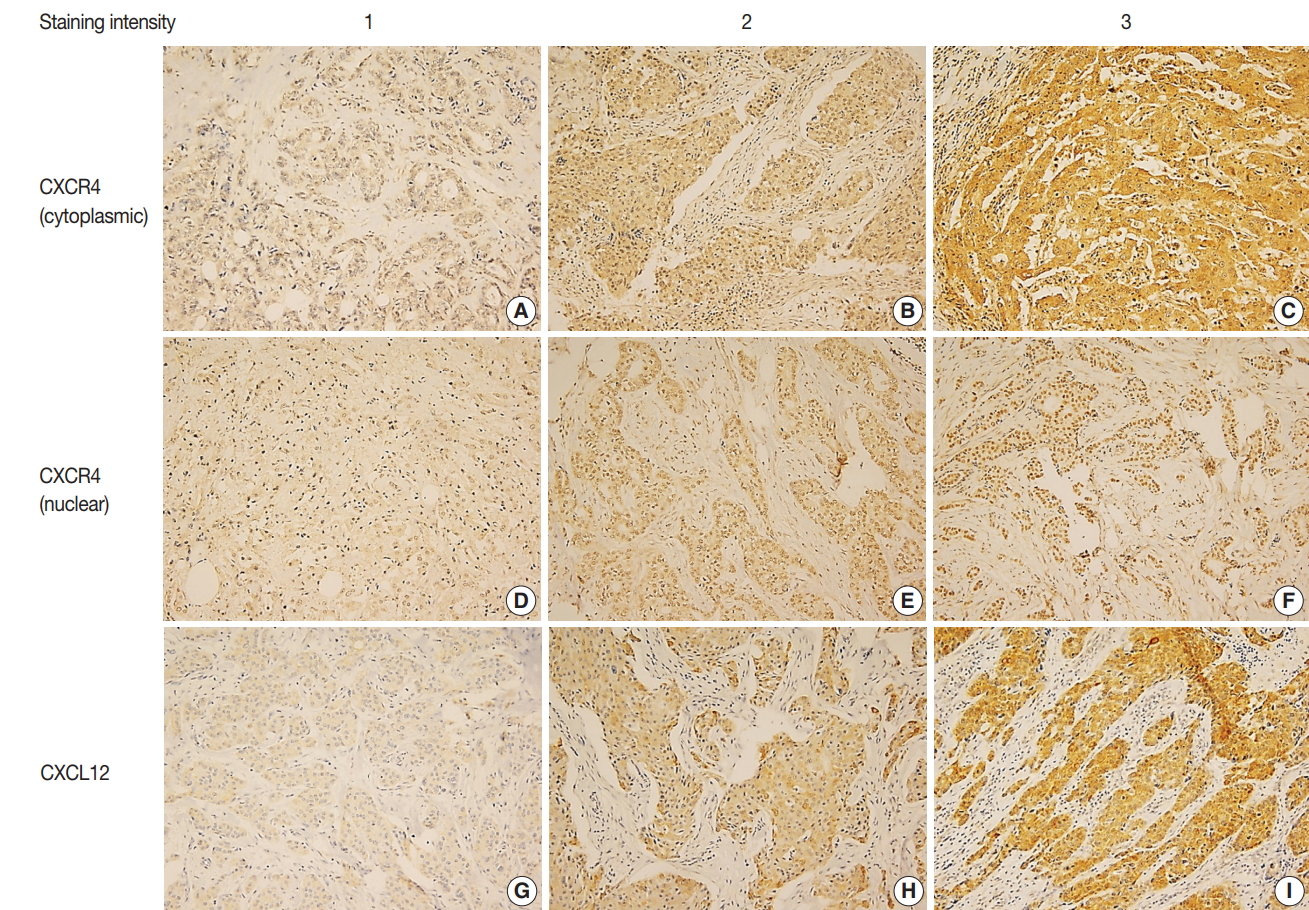
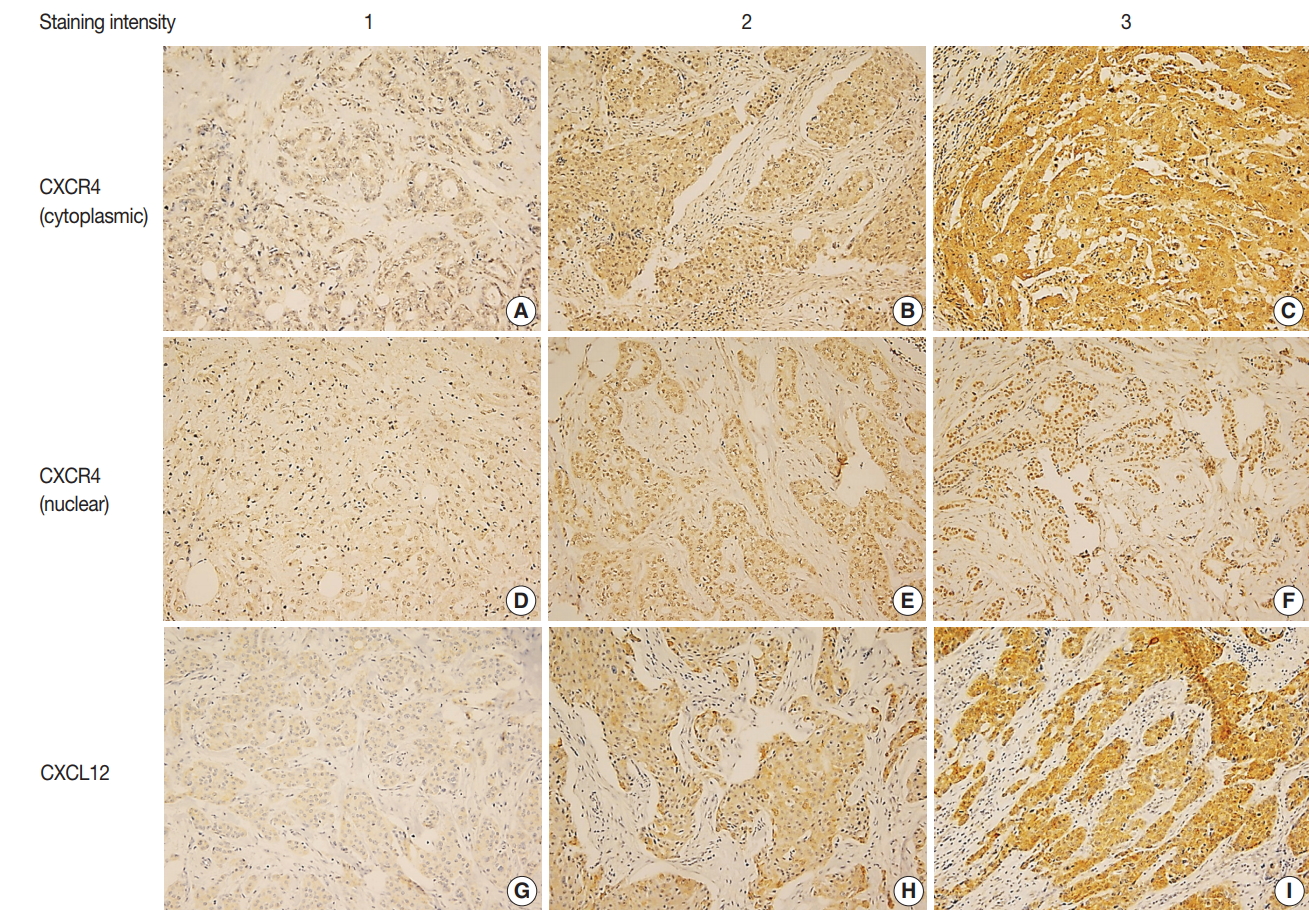
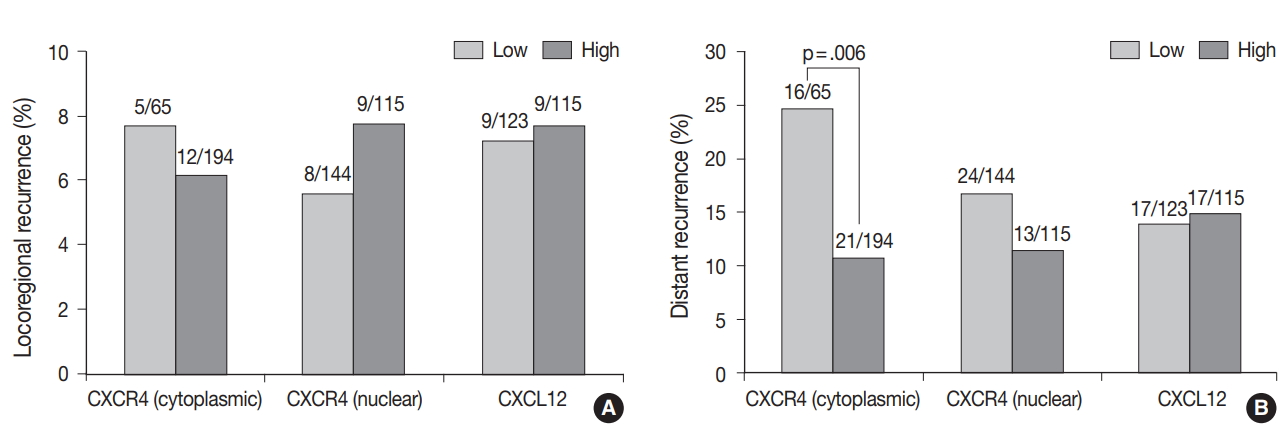
Fig. 1.
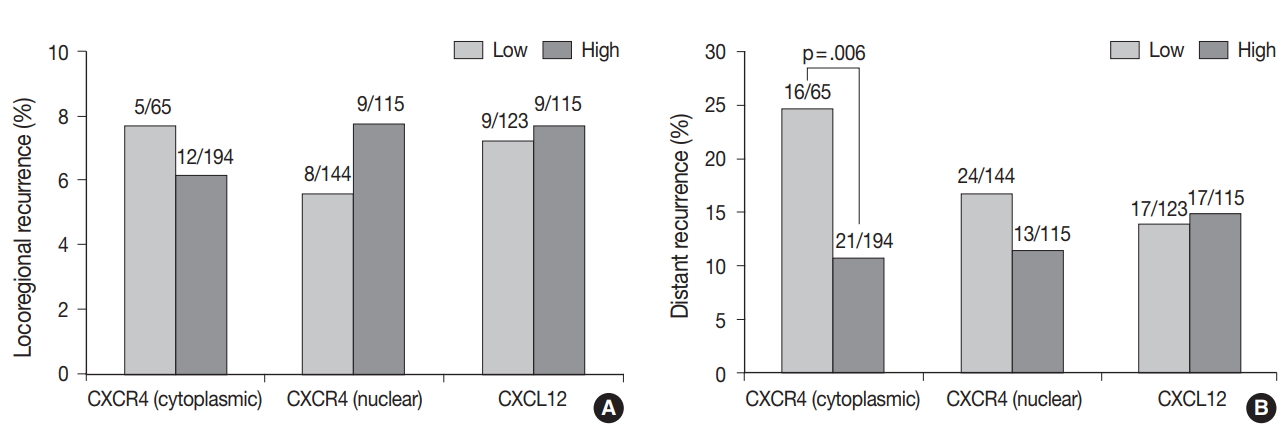
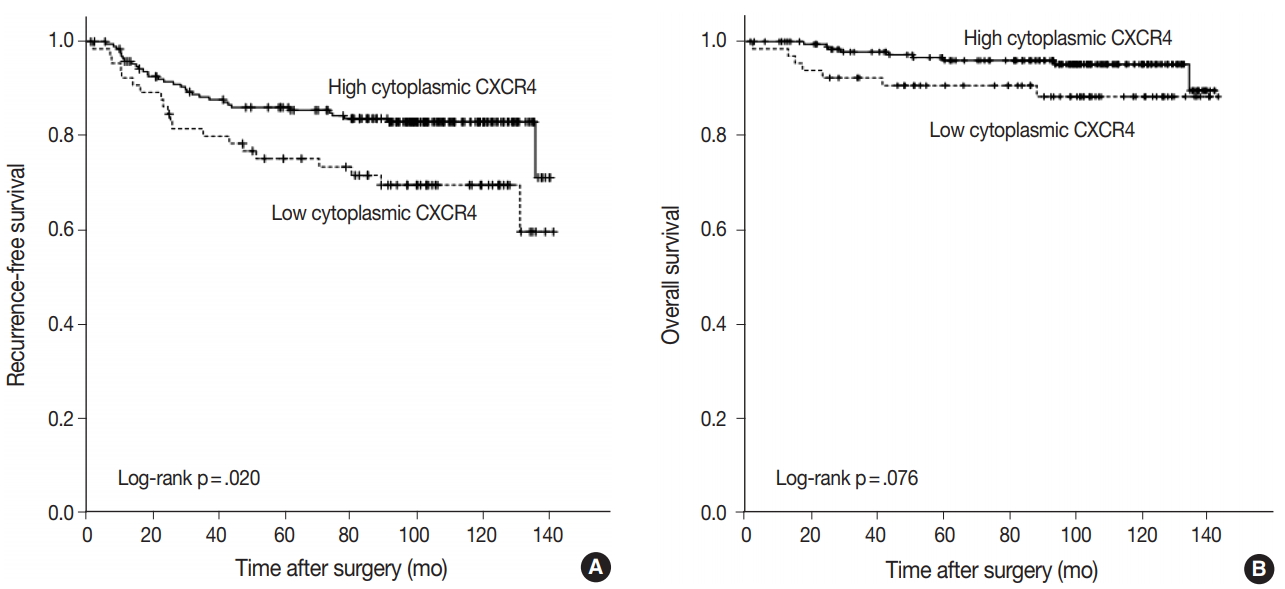
Fig. 2.
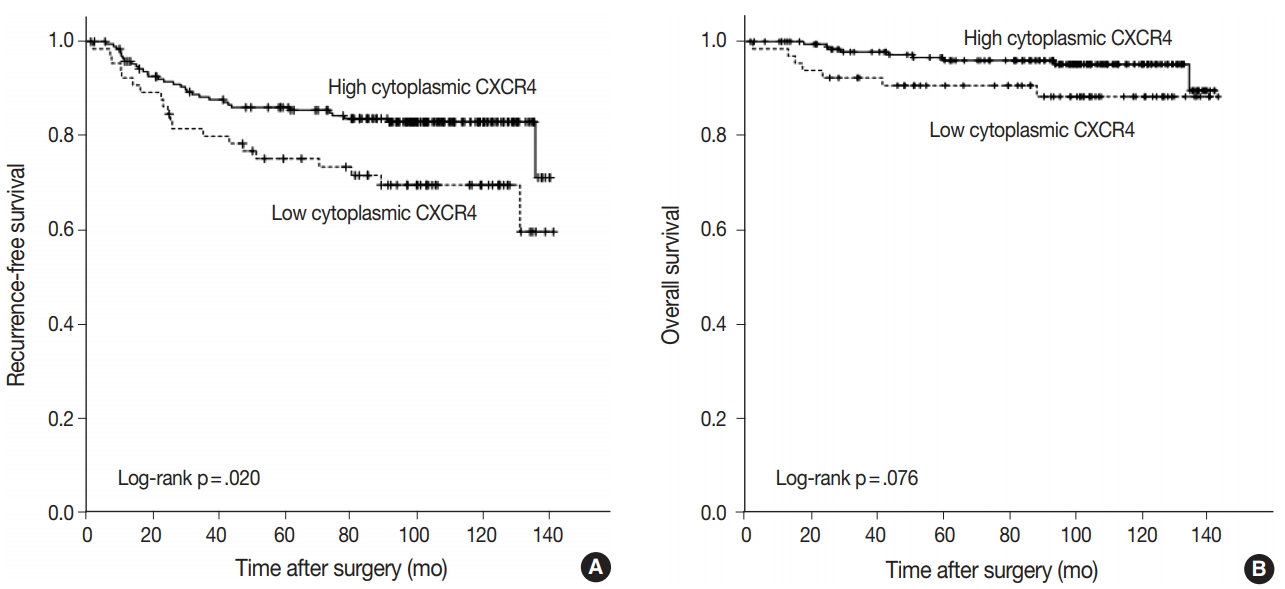
Fig. 3.
| Adjuvant chemotherapy regimen | No. of patients |
|---|---|
| Anthracycline-based | 164 |
| FAC | 132 |
| AC | 10 |
| FEC | 21 |
| EC | 1 |
| Taxane-anthracycline-based | 51 |
| AC →paclitaxel and/or docetaxel | 50 |
| FEC →paclitaxel | 1 |
| CMF | 65 |
| Change of regimen | 1 |
| Unknown | 2 |
| Total | 283 |
| Variable | Total (n = 259) | CXCR4 (cytoplasmic) |
CXCR4 (nuclear) |
||||
|---|---|---|---|---|---|---|---|
| Low (n = 65) | High (n = 194) | p-value | Low (n=144) | High (n = 115) | p-value | ||
| Age (yr) | .008 | .524 | |||||
| ≤ 50 | 152 | 29 (19.1) | 123 (80.9) | 82 (53.9) | 70 (46.1) | ||
| > 50 | 107 | 36 (33.6) | 71 (66.4) | 62 (57.9) | 45 (42.1) | ||
| Histologic grade | .007 | .310 | |||||
| I, II | 47 | 19 (40.4) | 28 (59.6) | 23 (48.9) | 24 (51.1) | ||
| III | 212 | 46 (21.7) | 166 (78.3) | 121 (57.1) | 91 (42.9) | ||
| Size (cm) | .131 |
.957 | |||||
| ≤ 5 | 243 | 58 (23.9) | 185 (76.1) | 135 (55.6) | 108 (44.4) | ||
| > 5 | 16 | 7 (43.8) | 9 (56.3) | 9 (56.3) | 7 (43.8) | ||
| Lymph node metastasis | .473 | .476 | |||||
| Negative | 165 | 39 (23.6) | 126 (76.4) | 89 (53.9) | 76 (46.1) | ||
| Positive | 94 | 26 (27.7) | 68 (72.3) | 55 (58.5) | 39 (41.5) | ||
| Stage | .045 | .087 | |||||
| I, II | 216 | 49 (22.7) | 167 (77.3) | 115 (53.2) | 101 (46.8) | ||
| III | 43 | 16 (37.2) | 27 (62.8) | 29 (67.4) | 14 (32.6) | ||
| Histologic type | .992 | .144 | |||||
| IDC | 239 | 60 (25.1) | 179 (74.9) | 136 (56.9) | 103 (43.1) | ||
| Other |
20 | 5 (25.0) | 15 (75.0) | 8 (40.0) | 12 (60.0) | ||
| Adjuvant chemotherapy regimen |
.009 | .538 | |||||
| Anthracycline-based | 149 | 27 (18.1) | 122 (81.9) | 79 (53.0) | 70 (47.0) | ||
| Taxane-anthracycline-based | 49 | 14 (28.6) | 35 (71.4) | 30 (61.2) | 19 (38.8) | ||
| CMF | 58 | 22 (37.9) | 36 (62.1) | 34 (58.6) | 24 (41.4) | ||
| Radiation therapy |
.506 | .975 | |||||
| No | 84 | 23 (27.4) | 61 (72.6) | 47 (56.0) | 37 (44.0) | ||
| Yes | 174 | 41 (23.6) | 133 (76.4) | 97 (55.7) | 77 (44.3) | ||
| Variable | Total (n = 238) | CXCL12 |
||
|---|---|---|---|---|
| Low (n = 123) | High (n = 115) | p-value | ||
| Age (yr) | .721 | |||
| ≤ 50 | 140 | 71 (50.7) | 69 (49.3) | |
| > 50 | 98 | 52 (53.1) | 46 (46.9) | |
| Histologic grade | .155 | |||
| I, II | 43 | 18 (41.9) | 25 (58.1) | |
| III | 195 | 105 (53.8) | 90 (46.2) | |
| Size (cm) | .045 | |||
| ≤ 5 | 223 | 119 (53.4) | 104 (46.6) | |
| > 5 | 15 | 4 (26.7) | 11 (73.3) | |
| Lymph node metastasis | .005 | |||
| Negative | 158 | 92 (58.2) | 66 (41.8) | |
| Positive | 80 | 31 (38.8) | 49 (61.3) | |
| Stage | .017 | |||
| I, II | 202 | 111 (55.0) | 91 (45.0) | |
| III | 36 | 12 (33.3) | 24 (66.7) | |
| Histologic type | .931 | |||
| IDC | 219 | 113 (51.6) | 106 (48.4) | |
| Other |
19 | 10 (52.6) | 9 (47.4) | |
| Adjuvant chemotherapy regimen |
.117 | |||
| Anthracycline-based | 144 | 82 (56.9) | 62 (43.1) | |
| Taxane-anthracycline-based | 38 | 15 (39.5) | 23 (60.5) | |
| CMF | 53 | 25 (47.2) | 28 (52.8) | |
| Radiation therapy |
.408 | |||
| No | 79 | 38 (48.1) | 41 (51.9) | |
| Yes | 158 | 85 (53.8) | 73 (46.2) | |
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age > 50 yr | 1.431 | 0.715–2.862 | .311 | - | - | - |
| Histologic grade III | 0.790 | 0.338–1.844 | .585 | - | - | - |
| Tumor size > 5 cm | 4.985 | 1.796–13.837 | .002 | 3.231 | 1.046–9.985 | .042 |
| Lymph node metastasis | 4.462 | 2.153–9.246 | < .001 | 3.491 | 1.630–7.478 | .001 |
| Radiation therapy | 1.029 | 0.492–2.152 | .940 | - | - | - |
| High CXCR4 (cytoplasmic) | 0.372 | 0.180–0.766 | .007 | 0.400 | 0.186–0.860 | .019 |
| High CXCR4 (nuclear) | 0.637 | 0.309–1.315 | .223 | - | - | - |
| High CXCL12 | 1.082 | 0.523–2.236 | .832 | - | - | - |
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age > 50 yr | 0.959 | 0.554–1.660 | .881 | - | - | - |
| Histologic grade III | 0.909 | 0.468–1.766 | .779 | - | - | - |
| Tumor size > 5 cm | 3.116 | 1.467–6.617 | .003 | - | - | - |
| Lymph node metastasis | 3.298 | 1.908–5.702 | < .001 | 3.005 | 1.724–5.237 | < .001 |
| Radiation therapy | 0.912 | 0.516–1.612 | .751 | - | - | - |
| High CXCR4 (cytoplasmic) | 0.521 | 0.298–0.912 | .022 | 0.552 | 0.316–0.967 | .038 |
| High CXCR4 (nuclear) | 0.875 | 0.503–1.524 | .637 | - | - | - |
| High CXCL12 | 1.199 | 0.688–2.089 | .522 | - | - | - |
FAC, 5-fluorouracil (5-FU), doxorubicin, and cyclophosphamide; AC, doxorubicin and cyclophosphamide; FEC, 5-FU, epirubicin, and cyclophosphamide; EC, epirubicin and cyclophosphamide; CMF, cyclophosphamide, methotrexate, and 5-FU. AC to CMF.
Values are presented as number (%). CXCR4, CXC chemokine receptor type 4; IDC, Invasive ductal carcinoma; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil. Fisher exact test; Invasive lobular carcinoma (2), mixed invasive ductal and lobular carcinoma (3), invasive papillary carcinoma (2), metaplastic carcinoma (8), medullary carcinoma (1), apocrine carcinoma (3), signet ring cell carcinoma (1); 3 missing values, unknown (2), change of regimen (1); 1 missing value, unknown (1).
Values are presented as number (%). CXCL12, CXC motif chemokine 12; IDC, Invasive ductal carcinoma; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil. Invasive lobular carcinoma (2), mixed invasive ductal and lobular carcinoma (2), invasive papillary carcinoma (2), metaplastic carcinoma (6), apocrine carcinoma (4), medullary carcinoma (1), signet ring cell carcinoma (1), clear cell carcinoma (1); 3 missing values, unknown (2), change of regimen (1); 1 missing value, unknown (1).
OR, odds ratio; CI, confidence interval; CXCR4, CXC chemokine receptor type 4; CXCL12, CXC motif chemokine 12.
HR, hazard ratio; CI, confidence interval; CXCR4, CXC chemokine receptor type 4; CXCL12, CXC motif chemokine 12.

 E-submission
E-submission