Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(2); 2019 > Article
-
Case Study
Squamous Cell Carcinoma of the Extrahepatic Common Hepatic Duct -
Myunghee Kang1
 , Na Rae Kim1
, Na Rae Kim1 , Dong Hae Chung1
, Dong Hae Chung1 , Hyun Yee Cho1
, Hyun Yee Cho1 , Yeon Ho Park2
, Yeon Ho Park2
-
Journal of Pathology and Translational Medicine 2019;53(2):112-118.
DOI: https://doi.org/10.4132/jptm.2018.09.03
Published online: October 1, 2018
1Department of Pathology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
2Department of Surgery, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- Corresponding Author Na Rae Kim, MD, PD Department of Pathology, Gil Medical Center, Gachon University College of Medicine, 21 Namdong-daero 774beon-gil, Namdong-gu, Incheon 21565, Korea Tel: +82-32-460-3073 Fax: +82-32-460-2394 E-mail: clara_nrk@gilhospital.com
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- We report a rare case of hilar squamous cell carcinoma. A 62-year-old Korean woman complaining of nausea was referred to our hospital. Her biliary computed tomography revealed a 28 mm-sized protruding solid mass in the proximal common bile duct. The patient underwent left hemihepatectomy with S1 segmentectomy and segmental excision of the common bile duct. Microscopically, the tumor was a moderately differentiated squamous cell carcinoma of the extrahepatic bile duct, without any component of adenocarcinoma or metaplastic portion in the biliary epithelium. Immunohistochemically, the tumor was positive for cytokeratin (CK) 5/6, CK19, p40, and p63. Squamous cell carcinoma of the extrahepatic bile duct is rare. To date, only 24 cases of biliary squamous cell carcinomas have been reported. Here, we provide a clinicopathologic review of previously reported extrahepatic bile duct squamous cell carcinomas.
- Clinical summary
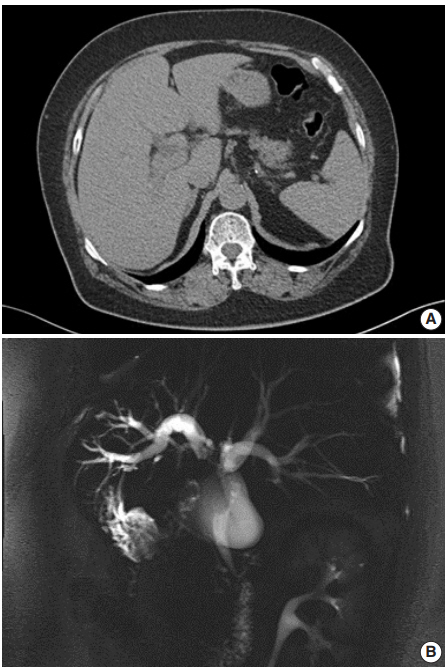
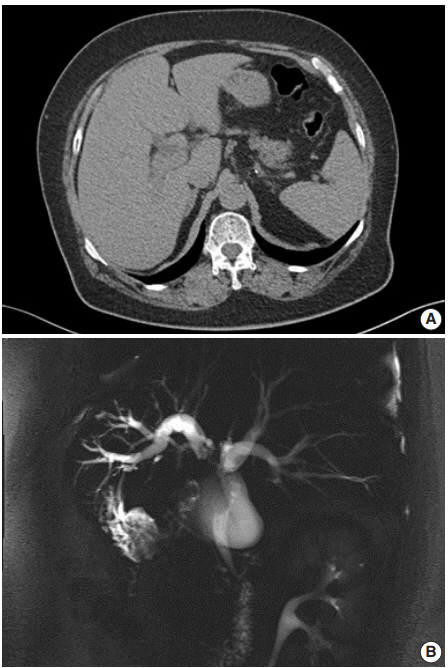
- A 62-year-old Korean woman complained of continuous nausea and abdominal discomfort for two months. Except for the diagnosis of thyroid papillary carcinoma 13 years prior to presentation, she had no history of other malignancies or cholelithiasis. Abdominal computed tomography (CT) performed at a local clinic revealed a dilated bile duct (Fig. 1A). Magnetic resonance cholangiopancreatography revealed luminal narrowing in the distal bile duct with proximal dilation (Fig. 1B). Perihilar proximal biliary cholangiocarcinoma was suspected. Liver magnetic resonance images (MRI) showed a 1 cm-sized, non-enhancing, T2 high signal intensity lesion in the left lobe, suggesting hepatic cyst or abscess. Metastasis to the common hepatic artery, portocaval lymph node, and hepatic duct ligament was also suspected. Preoperatively, an endoscopic retrograde cholangiopancreatography-assisted biopsy was performed, and a diagnosis of carcinoma with squamous differentiation was rendered. Subsequently, left hemihepatectomy with S1 segmentectomy and segmental excision of the common bile duct were performed. After surgical resection, abdominal CT revealed an enlarged common hepatic arterial lymph node, resulting in suspicion of metastasis. The patient developed ascites and a pleural effusion. In addition, a thrombus developed in the superior vena cava. Heparin was used for treatment of thrombus; however, heparin-induced thrombocytopenia was followed. The patient received 5-fluorouracil (5-FU) and cisplatin, but chemotherapy had to be stopped after the first cycle due to pancytopenia, aggravating thrombocytopenia, and persistent fever. The patient refused additional chemoradiotherapy. During the postoperative 15 months, liver MRI showed metastasis with increased size in the hepatic duct lymph nodes, portocaval, and paraduodenal areas, and the largest size increased from 1.8 to 3.1 cm in short diameter. The patient was alive over the 15-month follow-up period.
- Pathological findings
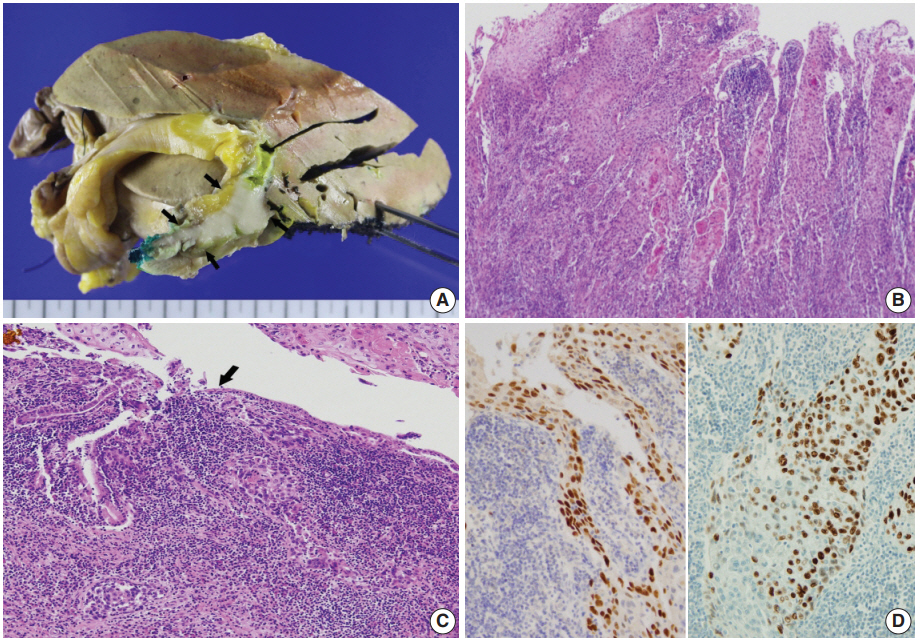
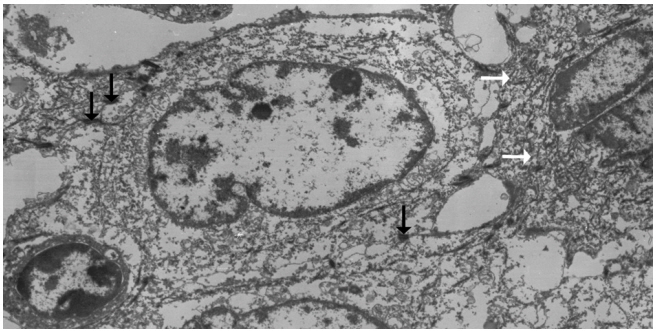
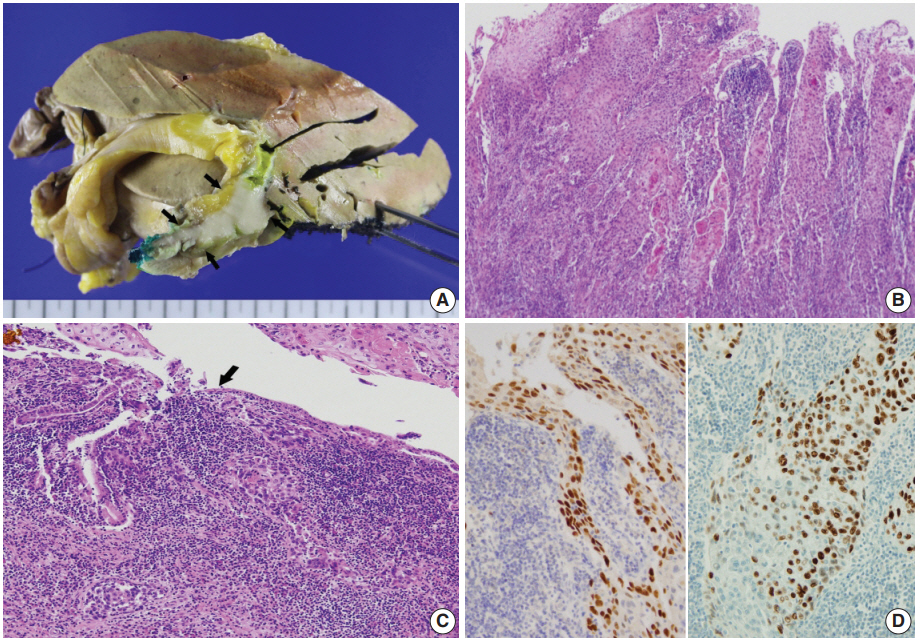
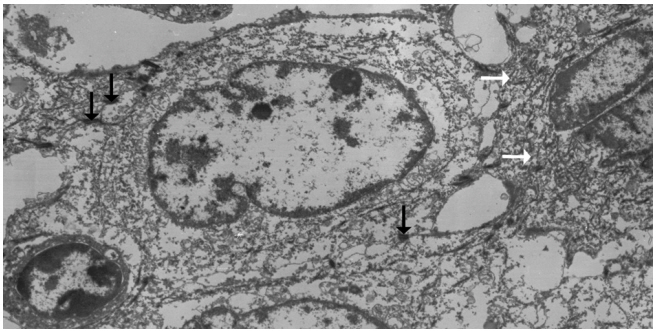
- Left hemihepatectomy with S1 segmentectomy and segmental excision of the common bile duct were performed. Serial sections revealed a firm grayish-white mass measuring 2.8 cm at the proximal common hepatic duct near the hilar region (Fig. 2A). The mass did not involve the cystic duct or the right and left hepatic ducts. Microscopically, the papillary-protruded mass was composed entirely of squamous cells with eosinophilic keratin pearls (Fig. 2B). The surface of the mass was denuded and inflamed due to preoperative stent insertion. No mucin production or duct formation was detected. There were no metaplastic or biliary intraepithelial neoplastic lesions. An abrupt transition to neoplastic squamous epithelium from the cuboidal biliary epithelium was noted (Fig. 2C). Mitosis was frequently found. The tumor extended to the pericholedochal fibroconnective tissue. Lymphovascular and perineural invasion were noted. The tumor cells were positive for cytokeratin (CK) 5/6 (CK5/6; 1:100, D5/16 B4, Dako, Glostrup, Denmark), CK19 (prediluted, B/70, Novocastra, Newcastle upon Tyne, UK), p63 (prediluted, DAK-P63, Dako) (Fig. 2D), p40 (prediluted, BC28, Dako), and Ki-67 (1:100, MIB-1, Dako). However, the tumor cells were negative for CK7 (1:100, OV-TL 12/30, Dako), CK20 (1:100, KS 20.8, Dako), periodic acid Schiff, and polyclonal carcinoembryonic antigen (prediluted, polyclonal, Dako). The tumor cells were focally non-block positive for p16 (1:200, JC8, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Entirely embedded sections of tumor and bile duct revealed no adenocarcinoma component. The tumor was diagnosed as a pure SCC with moderate differentiation. Ultrastructurally, polygonal to elongated tumor cells were filled with dilated rough endoplasmic reticulums, intermediate filaments, and primary and secondary lysosomes with prominent golgi apparatus (Fig. 3). Well-formed desmosomes were found. The gallbladder was separately submitted and showed only inflammation without any stones. A 1 cm-sized abscess with periductal inflammation was noted in the background liver parenchyma. Aspiration cytology of the enlarged common hepatic arterial lymph node showed metastatic SCC (pT2aN1M0, stage IIIc according to American Joint Committee on Cancer). Human papillomavirus was not detected using the HPV 9G DNA kit (BMT, Chuncheon, Korea) in accordance with the manufacturer’s protocol.
- Approval was obtained from our Institutional Review Board (No. GCIRB2018-066) for this case report with a waiver of informed consent.
CASE REPORT
- Histologically, the biliary mucosa is composed of a singlelayered cuboidal epithelium without squamous epithelial cells. Adenocarcinoma is the most common histologic type of biliary tract malignancies, and biliary SCC is rare. By definition, the diagnosis of adenosquamous carcinoma of the gallbladder and extrahepatic bile ducts can be made when SCC comprises more than 25% of the tumor component, but the current classification system by the World Health Organization requires that no glandular component is present for a diagnosis of biliary SCC.
- The pathogenesis of this rare biliary SCC has not been elucidated to date. It is presumed that the normal columnar epithelium undergoes squamous metaplasia by continuous irritation due to an inflammatory stimulus, which then may result in carcinomatous changes through dysplasia [1]. Predisposing conditions that can lead to squamous metaplasia of the biliary epithelium and biliary SCC include hepatolithiasis, recurrent pyogenic cholangitis, and clonorchiasis [1]. Secondly, pluripotent bile duct stem cells are known to undergo malignant transformation. Other possible theories include heterotopic squamous epithelium or squamous metaplasia of preexisting adenocarcinoma [9,13]. The second and third theories might explain biliary SCC cases that lack preexisting normal squamous epithelium, like the present case. Our patient’s histology revealed pure SCC, and there was no hepatolithiasis or choledochal cysts on imaging studies. There was no underlying squamous epithelium, but there was an abrupt transition to dysplastic squamous epithelium from the biliary mucosa. On the other hand, a previous case reported by Abbas et al. [10] showed biliary SCC associated with high-grade squamous dysplasia, similar to cervical carcinogenesis. Their finding supports the metaplasia-dysplasia-carcinoma sequence theory. However, the direct causality of inflammation-metaplasia-dysplasia should be questioned. Whether gallstones predispose to cholangiocarcinoma remain unclear, and most reported cases have not been accompanied by a metaplasia-dysplasia lesion. Another possible theory may be that SCCs are derived from undifferentiated basal cells. Immunoreactivity for CK7, CK8, CK14, CK18, and/or CK5/6 suggests the origin of the cancer cells to be the basal cells of keratinized squamous epithelium. Moreover, positive staining for biliary CK19 would confirm the bile ductular ontogeny of the neoplastic cells [19].
- The incidence of cholangiocarcinoma increases with age, and most reported cases occur in the fifth to seventh decades. Due to its rare incidence and strict diagnostic criteria, biliary SCC is rarely reported, and there are few reports to be retrieved for review. From the literature, we found 34 cases of biliary SCCs in the extrahepatic bile duct. Among the 34 reported cases of SCC of the extrahepatic duct, only 24 provided well-described clinicopathologic data [2-18]. Only one case associated with a choledochal cyst demonstrated predisposing precursors. A review of the cases revealed that age ranged from 24 to 86 years (mean, 62 years). The male-to-female ratio was 16:9. The site of occurrence of biliary SCC was the common hepatic duct region in four cases, hilar region in seven cases, proximal common bile duct region in two cases, mid portion in five cases, and distal common bile duct in seven cases.
- A review of the previously reported cases demonstrated that the prognosis of biliary SCCs is extremely grave. Cholangiocarcinoma containing a component of SCC showed the following trends: rapid progression to advanced stage, short survival time, large tumor size, aggressive intrahepatic spreading, and frequent metastasis. Findings related to poor prognosis include elevated preoperative level carbohydrate antigen 19-9, resection margin involvement, advanced T category, and metastatic lymph node [20]. The mortality rate of biliary SCCs was up to 63.6% (14/22 cases of available data) during the follow-up period (mean, 14.8 months). Twenty out of 25 cases with available data (80%) underwent surgical resection with or without chemoradiotherapy. Among them, nine cases were combined with chemoradiotherapy. Two out of 25 cases (8%) received only conservative treatment. Ten cases (40%) received chemotherapy with or without radiation. The mean survival of patients without surgery was less than 12 months. Unlike head and neck SCCs, there is no supportive evidence for radiation therapy for unresectable biliary SCC. However, there are some reports of chemotherapy’s important palliative value for painful localized metastasis or uncontrolled bleeding [20]. These results are summarized in Table 1. Patients undergoing surgery had a better prognosis than those receiving conservative, non-surgical treatments (median survival, 32 months vs 3 months, p = .009). However, age and stage at diagnosis and associated general medical condition were also influential factors. Cases with additional chemotherapy showed a tendency toward poorer prognosis than those with surgery only, although the difference was not statistically significant (median survival, 12 months vs 32 months; p = .085). Other clinical findings, including sex, age, and site of bile duct involvement, had no impact on prognosis.
- Due to the extremely rare incidence of biliary SCCs, no standardized therapeutic strategies have been established. The recommended treatment for biliary SCCs is surgical resection with or without chemoradiotherapy, and the recommended chemotherapy is GEMOX (gemcitabine plus oxaliplatin) or GP (gemcitabine plus cisplatin), as in bile duct adenocarcinomas [20]. Similar to the treatment for cancers of the gastrointestinal tract such as esophageal cancers, chemotherapy with docetaxel plus cisplatin plus 5-FU therapy or S-1 plus cisplatin therapy may be helpful. With such a regimen (S-1 plus cisplatin), one patient with biliary SCC was successfully treated [16]. Combined targeted therapy, such as epidermal growth factor receptor-targeted therapy, has shown certain benefits in other cancer types, and its effects are being investigated.
- Here, we reported a case of SCC of the hilar bile duct and reviewed previous reports regarding biliary SCCs. The poor prognosis observed in SCC patients may be attributed to its rarity, initial advanced stage, and lack of accumulated clinical data.
DISCUSSION
Author Contributions
Investigation: YHP
Supervision: DHC, HYC.
Writing—original draft: MK, NRK.
Writing—review & editing: MK, NRK.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
| No. | Age (yr)/Sex | Site | Clinical summary including tumor markers | Remarkable pathologic findings | Distant metastasis | TNM/AJCC at the diagnosis | Treatment | Outcome (follow-up) |
|---|---|---|---|---|---|---|---|---|
| 1 | 58/M | Proximal CBD (upper 1/4) | Jaundice, knife-like abdominal pain | No | Liver, retroperitoneal lymph node | Stage IVBa | No surgery | Died (23 days) |
| 2 | 24/F | Junction of proximal CBD and cystic duct | Jaundice, RUQ pain, elevated CEA | SCC, MD without lymphovascular, perineural invasion | Liver | T2aN0M1 Stage IVBa | Pancreaticoduodenectomy, CTx (cyclophosphamide, MTX, doxorubicin, procarbazine) | Died (8 mo) |
| 3 | 68/M | Mid CBD | Secondary biliary cirrhosis, portal hypertension, hepatic failure | SCC, WD | No | TxNOMO in autopsy Stage I | Cholecystectomy with T tube and wedge biopsy of liver | Died (6 mo) |
| 4 | 56/M | Hilar | Jaundice | SCC, PD | Liver | Stage IVBa | Cholecystectomy with T tube, RT | Died (3 mo) |
| 5 | 68/M | Mid CBD | Jaundice, elevated CA19-9, elastase I | SCC, WD with direct invasion of pancreas head | No | TxNOMO Stage III | Pancreaticoduodenectomy, CTx (cisplatin, 5-FU), immunotherapy (OK-432) | Alive (3 mo) |
| 6 | 68/M | Distal CBD | Jaundice, elevated CA19-9 | 1.8 cm, direct invasion of pancreas | No | T3N0M0 Stage IIIAa | Pancreaticoduodenectomy | Alive (27 mo) |
| 7 | 50/M | Hilar | Elevated CA19-9, AFR CEA, PIVKA II | 4 cm | Liver (S2,1 cm) | TxNxMl Stage IVBa | Extended left hepatic lobectomy, T tube | Died (10 mo) |
| 8 | 75/M | Distal CBD | Jaundice, elevated CA19-9 | 1.5 cm | No | TxN1M0 Stage IIIa | Pancreaticoduodenectomy | Alive (6 mo) |
| CEA+CA19-9+ | ||||||||
| 9 | 57/F | Distal CBD and ampulla of Vater | Jaundice | Invasion to pancreas and duodenum, CEA- PAS- | No | T3bN0M0 Stage IIIAa | Pylorus-preserving pancreatoduodenectomy | Not described |
| 10 | 63/M | Distal CBD | Jaundice, elevated CA19-9 | 1.5 cm invasion to pancreas and duodenum | No | T2N1M0 Stage II | Pancreaticoduodenectomy | Alive (6 mo) |
| 11 | 86/F | Junction of CBD and cystic duct | Jaundice, RUQ pain | PanCK | Not described | Not described | CTx, external beam radiation, and high-dose radiation endoluminal brachytherapy (1,800 cGy) | Died (18 mo) |
| 12 | 61/F | Mid CBD | Jaundice, WNL of CA19-9, CA125, AFP | 3 cm,CK(MNF116)+ CK10/13+ | Peritoneal carcinomatosis | T3N0M1 Stage IIIAa | Simple resection and hepatojejunal anastomosis | Died (16 mo) |
| History of cholecystectomy | ||||||||
| 13 | 60/M | Distal CBD | Recurrent episodes of cholangitis and obstructive jaundice | SCC, WD, 2 cm with metaplasia, dysplasia | No | T2N0M0 Stage lla | Pancreaticoduodenectomy | Not described |
| 14 | 28/F | Hilar | Jaundice, RUQ pain | SCC, MD with high-grade squamous dysplasia | Not described | Not described | Extended left hepatic lobectomy, RT | Alive (18 mo) |
| 15 | 41/F | Hilar | Jaundice, elevated CA19-9, choledochal cyst | Direct invasion to portal vein and duodenum | Not described | T4NxMx, Stage IVa | Endoscopic biliary stent, palliative CTx, RT | Not described |
| 16 | 64/M | Distal CBD | Abdominal discomfort, jaundice | 3 cm, CK19+ | No | T3N2M0, Stage IIIBa | Pancreaticoduodenectomy, CTx (CPT-11, PPD) | Hepatic metastasis (30 days) and died (5 mo) |
| 17 | 66/M | Hilar | Jaundice, elevated CA19-9, SPan-1, DUPAN-2 | SCC, WD, 3 cm, invasion of portal vein and liver, CK+ CAM5.2- | T4 (Stage IV) | T4N1M0 Stage IVAa | Extended right hepatic lobectomy, CTx (cisplatin+5-FU, gemcitabine+S-1) | Hepatic metastasis (6 mo) and died (12 mo) |
| 18 | 67/M | CHD | Icteric sclera, elevated CA19-9 | Synchronous double SCC, WD, 1.5 cm and adenocarcinoma | No | T1N1M0 Stage IIIBa | Pylorus-preserving pancreatoduodenectomy | Multiple hepatic metastasis (3 mo) and died (8 mo) |
| Metastatic adenocarcinoma in one regional lymph node | ||||||||
| 19 | 77/F | Mid CBD | Jaundice, WNLofCA19-9, CEA, DUPAN-2 | SCC, PD, 1.7 cm, invasion to right hepatic artery | No | T4N0M0, Stage IVAa | Pylorus-preserving pancreaticoduodenectomy, CTx (gemcitabine) | Local recurrence (20 mo) and died (32 mo) |
| CK5/6+ p53+ PAS- | ||||||||
| 20 | 78/M | Distal CBD | Jaundice, brown urine, WNL of CEA, CA19-9, DUPAN-2 | SCC, MD, 3 cm | No | T1N1M0 Stage IIIBa | Subtotal stomach-preserving pancreaticoduodenectomy, CTx (S-1, cisplatin) | Paraaortic lymph node metastasis (6 mo), alive (10 mo), |
| 21 | 62/M | CHD | Jaundice, RUQ pain, elevated CA19-9 | 1.5 cm, perineural invasion | Not described | T1N0M0 Stage la | Curative resection and choledochojejunostomy, CTx (oral fluoropyrimidine S-1) | Died (5 mo) |
| PanCK+ CAM5.2+ CK5/6+ p63+ p40+ PAS- | ||||||||
| 22 | 77/M | CHD | Elevated CA19-9, choledochal cyst | Not described | Not described | Not described | Curative resection and choledochojejunostomy | Died (32 mo) |
| 23 | 67/F | CHD | 티 evated CA19-9 | Not described | Not described | Not described | Pancreatiocoduodenectomy | Died (47 mo) |
| 24 | 73/M | Mid CBD | WNL of CA19-9 and CEA | 4 cm, CK5/6+ p63+ | No | Not described | Left hepatic lobe and caudate lobe resection, subtotal preserving pancreatoduodenectomy | Alive (45 mo) |
| 25 (present case) | 62/F | Hilar | Nausea, abdominal discomfort, elevated CA19-9 | 2.8 cm, CEA- p40+ p63+ CK5/6+ CK7- | No | T2N1M0, Stage IIIC | Cholecystectomy, left hemihepatectomy, S1 segmentectomy, CTx (5-FU, cisplatin) | Alive (9 mo) |
AJCC, American Joint Committee on Cancer; M, male; F, female; CBD, common bile duct; RUQ, right upper quadrant; CEA, carcinoembryogenic antigen; SCC, squamous cell carcinoma; MD, moderately differentiated; CTx, chemotherapy; MTX, methotrexate; WD, well differentiated; PD, poorly differentiated; RT, radiation therapy; CA 19-9, carbohydrate antigen 19-9; WNL, within normal limit; AFP, α-fetoprotein; CEA, carcinoembryonic antigen; +, positive; –, negative; CK, cytokeratin; 5-FU, 5-fluorouracil; S-1, tegafur/gimeracil/oteracil; CHD, common hepatic duct.
aThe stage was modified as the AJCC 8th edition.
- 1. Cabot RC, Painter FM. Case records of the Massachusetts General Hospital: Case 16261: four months’ jaundice and rectal pain. N Eng J Med 1930; 202: 1260-2.
- 2. Burger RE, Meeker WR, Luckett PM. Squamous cell carcinoma of the common bile duct. South Med J 1978; 71: 216-9. ArticlePubMed
- 3. Gulsrud PO, Feinberg M, Koretz RL. Rapid development of cirrhosis secondary to squamous cell carcinoma of the common bile duct. Dig Dis Sci 1979; 24: 166-9. ArticlePubMedPDF
- 4. Aranha GV, Reyes CV, Greenlee HB, Field T, Brosnan J. Squamous cell carcinoma of the proximal bile duct: a case report. J Surg Oncol 1980; 15: 29-35. ArticlePubMed
- 5. Kim KS, Park HB, Yeo HS, et al. A case of squamous cell carcinoma of the common bile duct. Korean J Gastrointest Endosc 1999; 19: 486-90.
- 6. Cho T, Nakamura J, Tomita H, et al. A case of squamous cell carcinoma of the distal extrahepatic bile duct. J Jpn Sug Assoc 2000; 61: 1853-6. Article
- 7. Gatof D, Chen YK, Shah RJ. Primary squamous cell carcinoma of the bile duct diagnosed by transpapillary cholangioscopy: case report and review. Gastrointest Endosc 2004; 60: 300-4. ArticlePubMed
- 8. La Greca G, Conti P, Urrico GS, et al. Biliary squamous cell carcinoma. Chir Ital 2004; 56: 289-95. PubMed
- 9. Sewkani A, Kapoor S, Sharma S, et al. Squamous cell carcinoma of the distal common bile duct. JOP 2005; 6: 162-5. PubMed
- 10. Abbas R, Willis J, Kinsella T, Siegel C, Sanabria J. Primary squamous cell carcinoma of the main hepatic bile duct. Can J Surg 2008; 51: E85-6. PubMedPMC
- 11. Price L, Kozarek R, Agoff N. Squamous cell carcinoma arising within a choledochal cyst. Dig Dis Sci 2008; 53: 2822-5. ArticlePubMedPDF
- 12. Kim GM, Choi GH, Kim DH, Kang CM, Lee WJ. A case of squamous cell carcinoma of the distal common bile duct. Korean J Hepatobiliary Pancreat Surg 2008; 12: 210-3.
- 13. Yamana I, Kawamoto S, Nagao S, Yoshida T, Yamashita Y. Squamous cell carcinoma of the hilar bile duct. Case Rep Gastroenterol 2011; 5: 463-70. ArticlePubMedPMC
- 14. Yoo Y, Mun S. Synchronous double primary squamous cell carcinoma and adenocarcinoma of the extrahepatic bile duct: a case report. J Med Case Rep 2015; 9: 116.ArticlePubMedPMCPDF
- 15. Goto T, Sasajima J, Koizumi K, et al. Primary poorly differentiated squamous cell carcinoma of the extrahepatic bile duct. Intern Med 2016; 55: 1581-4. ArticlePubMed
- 16. Nishiguchi R, Kim DH, Honda M, Sakamoto T. Squamous cell carcinoma of the extrahepatic bile duct with metachronous para-aortic lymph node metastasis successfully treated with S-1 plus cisplatin. BMJ Case Rep 2016; 2016: bcr2016218177.ArticlePubMedPMC
- 17. Yang G, Li J, Meng D. Primary squamous cell cholangiocarcinoma: a case report. Int J Clin Exp Pathol 2016; 9: 5772-6.
- 18. Mori H, Kaneoka Y, Maeda A, Takayama Y, Fukami Y, Onoe S. A perihilar bile duct squamous cell carcinoma treated by left hepatic lobe and caudate lobe resection, subtotal stomach preserving pancreatoduodenectomy, and portal vein resection. Jpn J Gastroenterol Surg 2017; 50: 26-32. Article
- 19. Pastuszak M, Groszewski K, Pastuszak M, Dyrla P, Wojtun´ S, Gil J. Cytokeratins in gastroenterology: systematic review. Prz Gastroenterol 2015; 10: 61-70. ArticlePubMedPMC
- 20. Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012; 61: 1657-69. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Deciphering cholangiocarcinoma heterogeneity and specific progenitor cell niche of extrahepatic cholangiocarcinoma at single-cell resolution
Chunliang Liu, Xiang Wang, Erdong Liu, Yali Zong, Wenlong Yu, Youhai Jiang, Jianan Chen, Mingye Gu, Zhengyuan Meng, Jingfeng Li, Yang Liu, Yongjie Zhang, Jing Tang, Hongyang Wang, Jing Fu
Journal of Hematology & Oncology.2025;[Epub] CrossRef - Extrahepatic cholangiocarcinoma: Current concepts in histopathology, immunohistochemistry, and molecular diagnostics
Jared Beyersdorf, M. Lisa Zhang
Seminars in Diagnostic Pathology.2025; 42(6): 150949. CrossRef - Cholangiocarcinoma With Liver Metastasis in Squamous Cell Carcinoma Type: A Case Report
Jane Chiang
Journal of Diagnostic Medical Sonography.2024; 40(6): 609. CrossRef - A Rare Case of Squamous Cell Carcinoma of the Bile Duct
Julianna Tantum, Rachael Schneider, Stefanie Gallagher, Kyley Leroy, Jared Lander, Patricia Wong
ACG Case Reports Journal.2023; 10(8): e01119. CrossRef - Metastatic Anal Squamous Cell Carcinoma Presenting as an Indeterminate Biliary Stricture Diagnosed By Cholangioscopy
Ritu Nahar, Ian Holmes, Jeffrey Baliff, Austin Chiang, Thomas Kowalski
ACG Case Reports Journal.2022; 9(6): e00785. CrossRef - Temporal Changes in Cholangiocarcinoma Incidence and Mortality in the United States from 2001 to 2017
Milind Javle, Sunyoung Lee, Nilofer S Azad, Mitesh J Borad, Robin Kate Kelley, Smitha Sivaraman, Anna Teschemaker, Ishveen Chopra, Nora Janjan, Shreekant Parasuraman, Tanios S Bekaii-Saab
The Oncologist.2022; 27(10): 874. CrossRef - PRIMARY SQUAMOUS CELL CARCINOMA OF THE COMMON BILE DUCT WITH LIVER METASTASES
Dhouha BACHA, Mohamed HAJRI, Wael FERJAOUI, Ghofrane TALBI, Lasaad GHARBI, Mohamed Taher KHALFALLAH, Sana ben SLAMA, Ahlem LAHMAR
ABCD. Arquivos Brasileiros de Cirurgia Digestiva (São Paulo).2021;[Epub] CrossRef - S1510 A Rare Case of Squamous Cell Carcinoma of the Bile Duct
Stefanie Gallagher, Kyley Leroy, Julianna Tantum, Babak Etemad
American Journal of Gastroenterology.2021; 116(1): S688. CrossRef - Heparin
Reactions Weekly.2019; 1752(1): 184. CrossRef - Carcinoma primario de células escamosas del conducto hepático común: a propósito de un caso
Ana Delgado Maroto, Andrés Barrientos Delgado, Marta Lázaro Sáez, Samia Hallouch Toutouh, Enrique Práxedes González
Gastroenterología y Hepatología.2019; 42(7): 436. CrossRef - Primary squamous cell carcinoma of the extrahepatic bile duct: A case report
Ana Delgado Maroto, Andrés Barrientos Delgado, Marta Lázaro Sáez, Samia Hallouch Toutouh, Enrique Práxedes González
Gastroenterología y Hepatología (English Edition).2019; 42(7): 436. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| No. | Age (yr)/Sex | Site | Clinical summary including tumor markers | Remarkable pathologic findings | Distant metastasis | TNM/AJCC at the diagnosis | Treatment | Outcome (follow-up) |
|---|---|---|---|---|---|---|---|---|
| 1 | 58/M | Proximal CBD (upper 1/4) | Jaundice, knife-like abdominal pain | No | Liver, retroperitoneal lymph node | Stage IVB |
No surgery | Died (23 days) |
| 2 | 24/F | Junction of proximal CBD and cystic duct | Jaundice, RUQ pain, elevated CEA | SCC, MD without lymphovascular, perineural invasion | Liver | T2aN0M1 Stage IVB |
Pancreaticoduodenectomy, CTx (cyclophosphamide, MTX, doxorubicin, procarbazine) | Died (8 mo) |
| 3 | 68/M | Mid CBD | Secondary biliary cirrhosis, portal hypertension, hepatic failure | SCC, WD | No | TxNOMO in autopsy Stage I | Cholecystectomy with T tube and wedge biopsy of liver | Died (6 mo) |
| 4 | 56/M | Hilar | Jaundice | SCC, PD | Liver | Stage IVB |
Cholecystectomy with T tube, RT | Died (3 mo) |
| 5 | 68/M | Mid CBD | Jaundice, elevated CA19-9, elastase I | SCC, WD with direct invasion of pancreas head | No | TxNOMO Stage III | Pancreaticoduodenectomy, CTx (cisplatin, 5-FU), immunotherapy (OK-432) | Alive (3 mo) |
| 6 | 68/M | Distal CBD | Jaundice, elevated CA19-9 | 1.8 cm, direct invasion of pancreas | No | T3N0M0 Stage IIIA |
Pancreaticoduodenectomy | Alive (27 mo) |
| 7 | 50/M | Hilar | Elevated CA19-9, AFR CEA, PIVKA II | 4 cm | Liver (S2,1 cm) | TxNxMl Stage IVB |
Extended left hepatic lobectomy, T tube | Died (10 mo) |
| 8 | 75/M | Distal CBD | Jaundice, elevated CA19-9 | 1.5 cm | No | TxN1M0 Stage III |
Pancreaticoduodenectomy | Alive (6 mo) |
| CEA+CA19-9+ | ||||||||
| 9 | 57/F | Distal CBD and ampulla of Vater | Jaundice | Invasion to pancreas and duodenum, CEA- PAS- | No | T3bN0M0 Stage IIIA |
Pylorus-preserving pancreatoduodenectomy | Not described |
| 10 | 63/M | Distal CBD | Jaundice, elevated CA19-9 | 1.5 cm invasion to pancreas and duodenum | No | T2N1M0 Stage II | Pancreaticoduodenectomy | Alive (6 mo) |
| 11 | 86/F | Junction of CBD and cystic duct | Jaundice, RUQ pain | PanCK | Not described | Not described | CTx, external beam radiation, and high-dose radiation endoluminal brachytherapy (1,800 cGy) | Died (18 mo) |
| 12 | 61/F | Mid CBD | Jaundice, WNL of CA19-9, CA125, AFP | 3 cm,CK(MNF116)+ CK10/13+ | Peritoneal carcinomatosis | T3N0M1 Stage IIIA |
Simple resection and hepatojejunal anastomosis | Died (16 mo) |
| History of cholecystectomy | ||||||||
| 13 | 60/M | Distal CBD | Recurrent episodes of cholangitis and obstructive jaundice | SCC, WD, 2 cm with metaplasia, dysplasia | No | T2N0M0 Stage ll |
Pancreaticoduodenectomy | Not described |
| 14 | 28/F | Hilar | Jaundice, RUQ pain | SCC, MD with high-grade squamous dysplasia | Not described | Not described | Extended left hepatic lobectomy, RT | Alive (18 mo) |
| 15 | 41/F | Hilar | Jaundice, elevated CA19-9, choledochal cyst | Direct invasion to portal vein and duodenum | Not described | T4NxMx, Stage IV |
Endoscopic biliary stent, palliative CTx, RT | Not described |
| 16 | 64/M | Distal CBD | Abdominal discomfort, jaundice | 3 cm, CK19+ | No | T3N2M0, Stage IIIB |
Pancreaticoduodenectomy, CTx (CPT-11, PPD) | Hepatic metastasis (30 days) and died (5 mo) |
| 17 | 66/M | Hilar | Jaundice, elevated CA19-9, SPan-1, DUPAN-2 | SCC, WD, 3 cm, invasion of portal vein and liver, CK+ CAM5.2- | T4 (Stage IV) | T4N1M0 Stage IVA |
Extended right hepatic lobectomy, CTx (cisplatin+5-FU, gemcitabine+S-1) | Hepatic metastasis (6 mo) and died (12 mo) |
| 18 | 67/M | CHD | Icteric sclera, elevated CA19-9 | Synchronous double SCC, WD, 1.5 cm and adenocarcinoma | No | T1N1M0 Stage IIIB |
Pylorus-preserving pancreatoduodenectomy | Multiple hepatic metastasis (3 mo) and died (8 mo) |
| Metastatic adenocarcinoma in one regional lymph node | ||||||||
| 19 | 77/F | Mid CBD | Jaundice, WNLofCA19-9, CEA, DUPAN-2 | SCC, PD, 1.7 cm, invasion to right hepatic artery | No | T4N0M0, Stage IVA |
Pylorus-preserving pancreaticoduodenectomy, CTx (gemcitabine) | Local recurrence (20 mo) and died (32 mo) |
| CK5/6+ p53+ PAS- | ||||||||
| 20 | 78/M | Distal CBD | Jaundice, brown urine, WNL of CEA, CA19-9, DUPAN-2 | SCC, MD, 3 cm | No | T1N1M0 Stage IIIB |
Subtotal stomach-preserving pancreaticoduodenectomy, CTx (S-1, cisplatin) | Paraaortic lymph node metastasis (6 mo), alive (10 mo), |
| 21 | 62/M | CHD | Jaundice, RUQ pain, elevated CA19-9 | 1.5 cm, perineural invasion | Not described | T1N0M0 Stage l |
Curative resection and choledochojejunostomy, CTx (oral fluoropyrimidine S-1) | Died (5 mo) |
| PanCK+ CAM5.2+ CK5/6+ p63+ p40+ PAS- | ||||||||
| 22 | 77/M | CHD | Elevated CA19-9, choledochal cyst | Not described | Not described | Not described | Curative resection and choledochojejunostomy | Died (32 mo) |
| 23 | 67/F | CHD | 티 evated CA19-9 | Not described | Not described | Not described | Pancreatiocoduodenectomy | Died (47 mo) |
| 24 | 73/M | Mid CBD | WNL of CA19-9 and CEA | 4 cm, CK5/6+ p63+ | No | Not described | Left hepatic lobe and caudate lobe resection, subtotal preserving pancreatoduodenectomy | Alive (45 mo) |
| 25 (present case) | 62/F | Hilar | Nausea, abdominal discomfort, elevated CA19-9 | 2.8 cm, CEA- p40+ p63+ CK5/6+ CK7- | No | T2N1M0, Stage IIIC | Cholecystectomy, left hemihepatectomy, S1 segmentectomy, CTx (5-FU, cisplatin) | Alive (9 mo) |
AJCC, American Joint Committee on Cancer; M, male; F, female; CBD, common bile duct; RUQ, right upper quadrant; CEA, carcinoembryogenic antigen; SCC, squamous cell carcinoma; MD, moderately differentiated; CTx, chemotherapy; MTX, methotrexate; WD, well differentiated; PD, poorly differentiated; RT, radiation therapy; CA 19-9, carbohydrate antigen 19-9; WNL, within normal limit; AFP, α-fetoprotein; CEA, carcinoembryonic antigen; +, positive; –, negative; CK, cytokeratin; 5-FU, 5-fluorouracil; S-1, tegafur/gimeracil/oteracil; CHD, common hepatic duct. The stage was modified as the AJCC 8th edition.

 E-submission
E-submission