Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 53(1); 2019 > Article
-
Original Article
Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy -
Hyeon Jeong Oh1
 , Jung Ho Kim1
, Jung Ho Kim1 , Jeong Mo Bae1
, Jeong Mo Bae1 , Hyun Jung Kim2
, Hyun Jung Kim2 , Nam-Yun Cho2
, Nam-Yun Cho2 , Gyeong Hoon Kang1,2
, Gyeong Hoon Kang1,2
-
Journal of Pathology and Translational Medicine 2019;53(1):40-49.
DOI: https://doi.org/10.4132/jptm.2018.11.29
Published online: December 26, 2018
1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
2Laboratory of Epigenetics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
-
Corresponding Author Jung Ho Kim, MD, PhD Department of Pathology, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-2072-2828 Fax: +82-2-743-5530 E-mail: junghokim@snuh.org
Gyeong Hoon Kang, MD, PhD Department of Pathology, Seoul National University College of Medicine, 103 Daehak-ro, Jongno-gu, Seoul 03080, Korea Tel: +82-2-740-8263 Fax: +82-2-765-5600 E-mail: ghkang@snu.ac.kr
© 2019 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- This study aimed to investigate the prognostic impact of intratumoral Fusobacterium nucleatum in colorectal cancer (CRC) treated with adjuvant chemotherapy.
-
Methods
- F. nucleatum DNA was quantitatively measured in a total of 593 CRC tissues retrospectively collected from surgically resected specimens of stage III or high-risk stage II CRC patients who had received curative surgery and subsequent oxaliplatin-based adjuvant chemotherapy (either FOLFOXor CAPOX). Each case was classified into one of the three categories: F. nucleatum–high, –low, or –negative.
-
Results
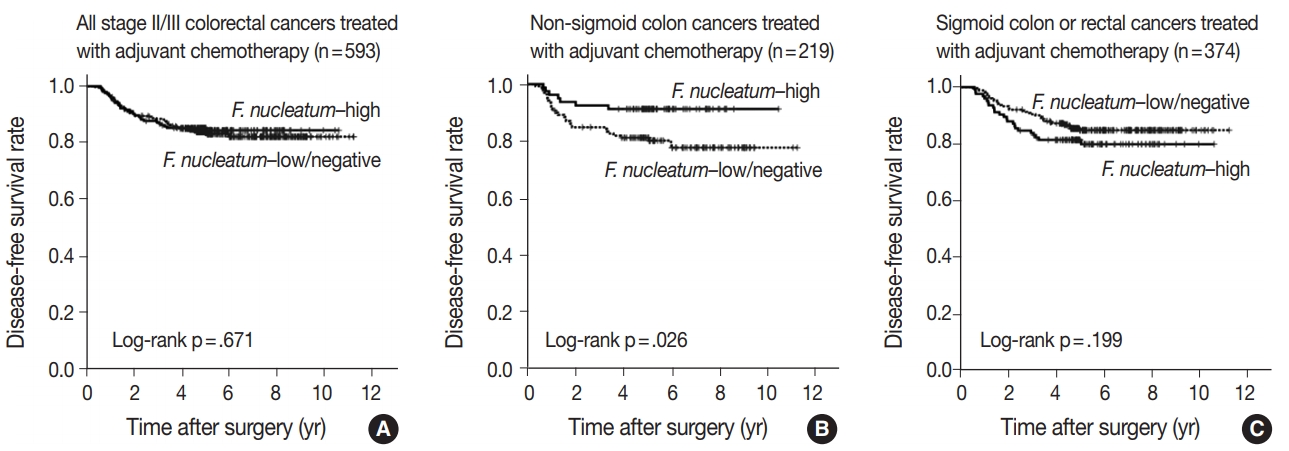
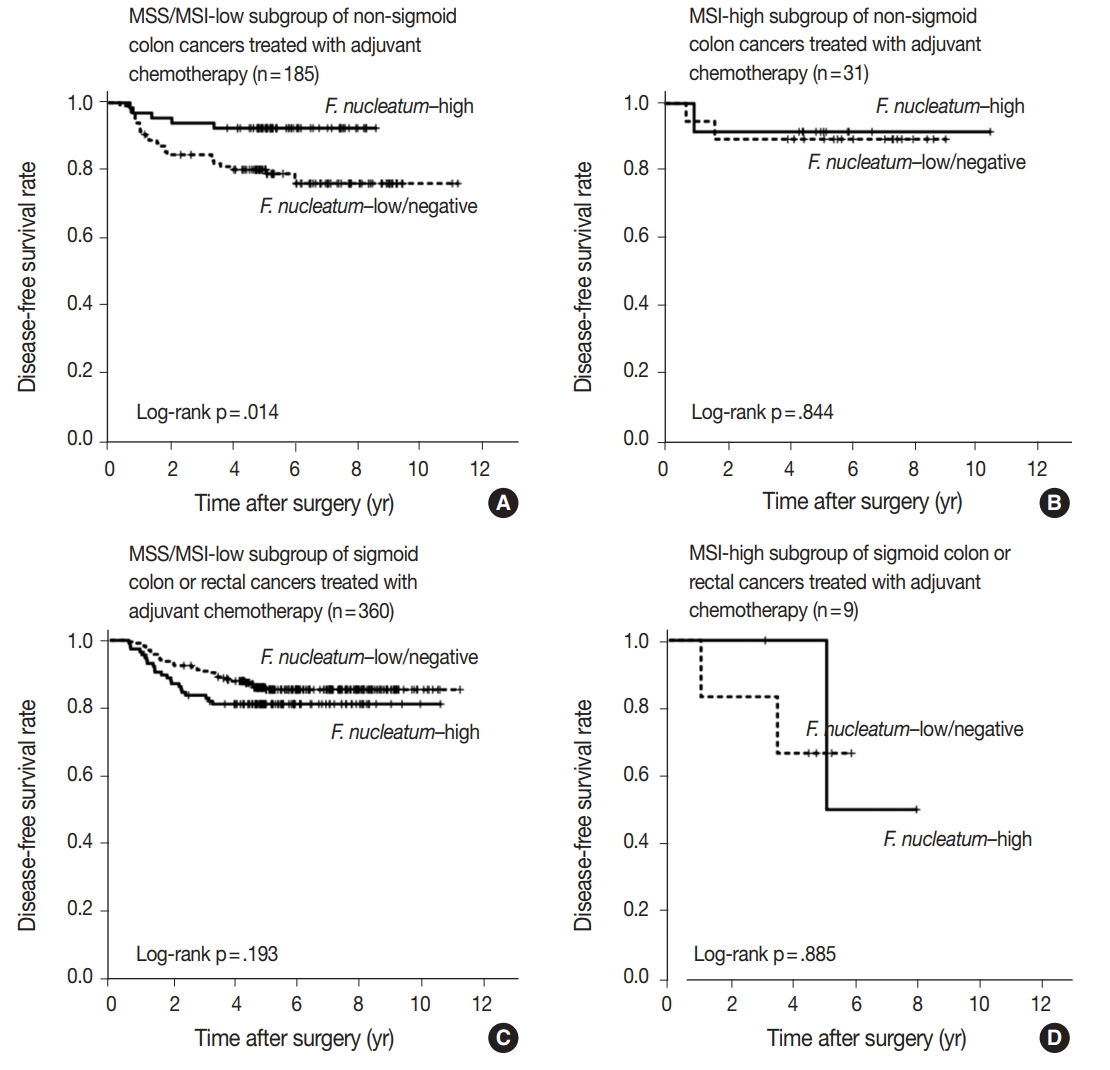
- No significant differences in survival were observed between the F.nucleatum–high and –low/negative groups in the 593 CRCs (p = .671). Subgroup analyses according to tumor location demonstrated that disease-free survival was significantly better in F.nucleatum–high than in –low/negative patients with non-sigmoid colon cancer (including cecal, ascending, transverse, and descending colon cancers; n = 219; log-rank p = .026). In multivariate analysis, F. nucleatum was determined to be an independent prognostic factor in non-sigmoid colon cancers (hazard ratio, 0.42; 95% confidence interval, 0.18 to 0.97; p = .043). Furthermore, the favorable prognostic effect of F. nucleatum–high was observed only in a non-microsatellite instability-high (non-MSI-high) subset of non-sigmoid colon cancers (log-rank p = 0.014), but not in a MSI-high subset (log-rank p = 0.844), suggesting that the combined status of tumor location and MSI may be a critical factor for different prognostic impacts of F. nucleatum in CRCs treated with adjuvant chemotherapy.
-
Conclusions
- Intratumoral F. nucleatum load is a potential prognostic factor in a non-MSI-high/non-sigmoid/non-rectal cancer subset of stage II/III CRCs treated with oxaliplatin-based adjuvant chemotherapy.
- Case selection
- Formalin-fixed, paraffin-embedded (FFPE) tissues of 747 consecutive series of primary CRCs were collected from the pathology archive of Seoul National University Hospital, Seoul, Korea. All the tissues were from surgical specimens of patients who underwent curative surgery and subsequent adjuvant chemotherapy for stage III or high-risk stage II CRC at Seoul National University Hospital from 2005 to 2012. The inclusion criteria for the case selection were age greater than 18 years, adenocarcinoma histology without neuroendocrine or squamous cell component, stage III or high-risk stage II according to pathological staging, complete resection (R0) of the primary tumor with tumorfree resection margins, and the completion of at least six cycles of adjuvant FOLFOX chemotherapy or four cycles of adjuvant CAPOX therapy. The criteria for high-risk stage II were tumor invasion into visceral peritoneum or direct invasion into adjacent organs/structures (pT4), clinically obstruction or perforation, poorly differentiated or undifferentiated histology (G3/G4), lymphovascular invasion, and perineural invasion. The patients who received pre-operative neoadjuvant chemotherapy and/or radiotherapy (especially patients with rectal cancer) and patients with a history of other malignancy within 5 years were excluded. Initially, 747 cases were subjected to quantitative polymerase chain reaction (qPCR) analysis for F. nucleatum. Among them, 154 inadequate samples determined by invalid or poor quality results from the qPCR analysis, as described subsequently, were excluded. Finally, a total of 593 CRC cases were analyzed. The Institutional Review Board of our hospital approved this study (IRB No. 1805-018-944). The Institutional Review Board exempted our study from obtaining informed consent from patients because our study was a retrospective, anonymous, tissuebased investigation.
- Clinicopathological data
- Clinical data, including age, sex, tumor location, and gross tumor type, were collected from electronic medical records. Hematoxylin and eosin-stained tissue slides of each case were independently reviewed by pathologists (J.M.B. and G.H.K.) to evaluate histopathological features, including pT/pN categories, tumor grade, lymphovascular invasion, perineural invasion, and mucinous histology.
- qPCR for F. nucleatum
- Genomic DNA extraction from FFPE tissues of the 747 CRCs and qPCR for F. nucleatum, using the 747 tumor DNA samples, were conducted as previously described [14]. In brief, the following primers and probes targeting the 16S rRNA gene DNA sequence of F. nucleatum and the reference gene (prostaglandin transporter, PGT), were used: F. nucleatum forward primer, 5'-CAACCATTACTTTAACTCTACCATGTTCA-3'; F. nucleatum reverse' primer, 5'-GTTGACTTTACAGAAGGAGATTATGTAAAAATC-3'; F. nucleatum FAM probe, 5'-GTTGACTTTACAGAAGGAGATTA-3'; PGT forward primer, 5'-ATCCCCAAAGCACCTGGTTT-3'; PGT reverse primer, 5'-AGAGGCCAAGATAGTCCTGGTAA-3'; PGT VIC probe, 5'-CCATCCATGTCCTCATCTC-3' [14]. The PCR conditions were 95°C for 10 minutes followed by 45 cycles of 95°C for 15 seconds, and 60°C for 1 minute [14]. To compare the F. nucleatum DNA amounts between tumor DNA samples, the relative values (2-ΔCt) calculated from the threshold cycle (Ct) values for F. nucleatum normalized to PGT were used. The qPCR method was validated using serially-diluted F. nucleatum genomic DNA samples (25586D-5; ATCC, Manassas, VA, USA). The results of the validation analysis are summarized in Supplementary Fig. S1. F. nucleatum–positive CRCs were further classified into two subgroups (F. nucleatum–high or F. nucleatum–low) using a cut-off median value of 2-ΔCt. Among the samples of the initial 747 cases subjected to F. nucleatum qPCR analysis, those of 154 cases were determined as failed or inadequate, based on non-evaluable or high Ct values of PGT. Thus, 593 cases were finally included in this study. The qPCR experiment of each sample was performed independently in triplicate.
- DNA analyses for MSI, CIMP, KRAS, and BRAF
- Major molecular factors, including MSI, CIMP, and KRAS/BRAF mutations, in the CRC samples were analyzed as previously described [18]. Genomic DNA of each tumor was isolated from representative FFPE tissue blocks by microdissection. MSI testing was performed by DNA fragment analysis using five microsatellite markers (BAT-25, BAT-26, D5S346, D17S250, and D2S123) according to the Bethesda guideline [19]. MSI status of each case was classified into one of the three categories: MSI-high, MSI-low, and microsatellite stable (MSS). CIMP analysis was carried out by the real-time PCR-based MethyLight assay using eight CIMP markers (MLH1, NEUROG1, CRABP1, CACNA1G, CDKN2A, IGF2, SOCS1, and RUNX3) as previously described [18]. CIMP status of each case was classified into one of the three categories: CIMP-high, CIMP-low, and CIMP-negative. Mutational status of KRAS exon 2 codons 12 and 13 and BRAF exon 15 codon 600 were examined by Sanger sequencing.
- Statistical analyses
- All statistical analyses in this study were performed using SPSS ver. 23 (IBM Corp., Armonk, NY, USA). Comparison analysis between categorical variables was conducted using chisquare test or Fisher exact test. Univariate and multivariate survival analyses were carried out using the Kaplan-Meier method with log-rank test and Cox proportional hazards regression model. All p-values were considered to indicate statistically significant differences if less than 0.05.
MATERIALS AND METHODS
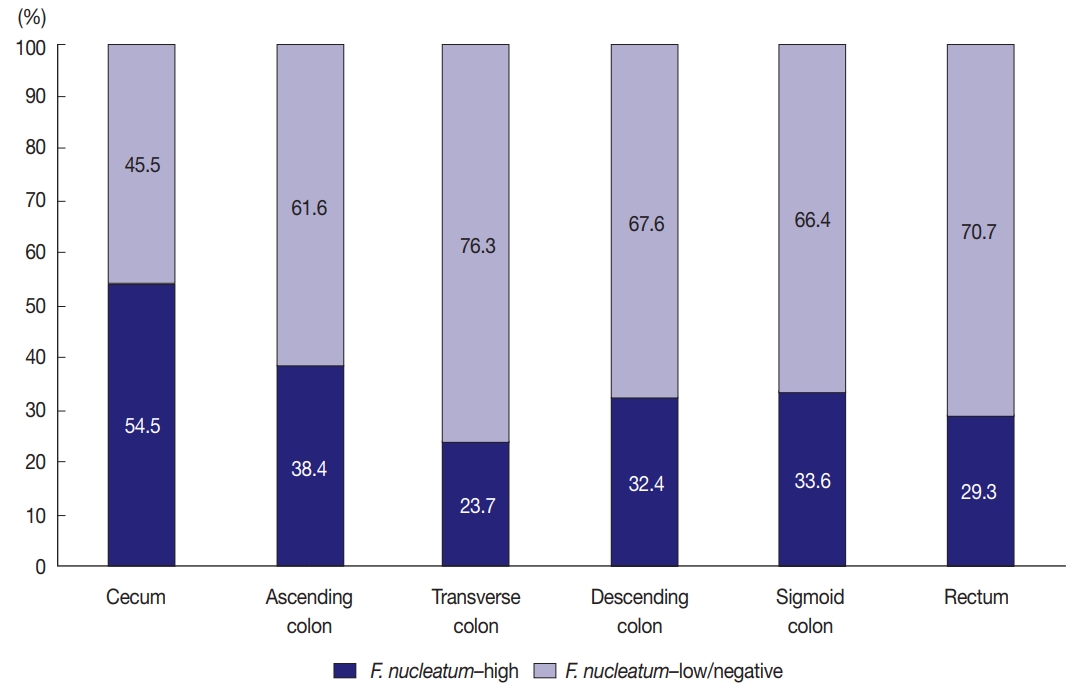
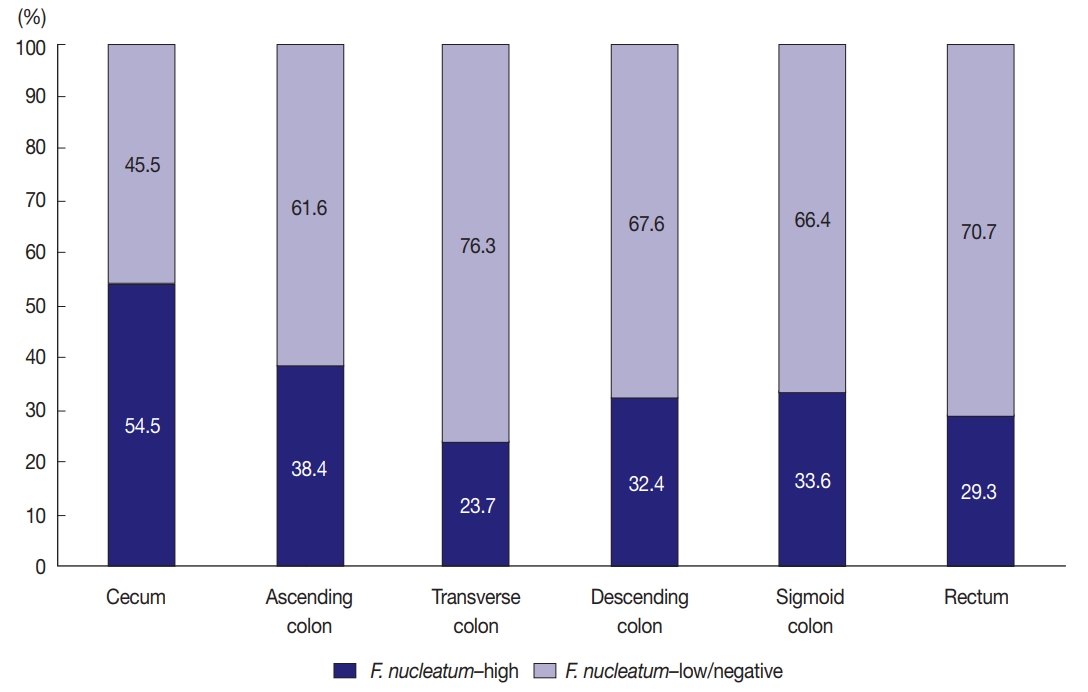
- Variable amounts of F. nucleatum according to tumor location bowel subsite in CRCs
- Among the 593 stage II/III CRCs treated with oxaliplatinbased adjuvant chemotherapy (FOLFOX or CAPOX), intratumoral F. nucleatum DNA was detected in 408 cases (68.8%). Each F. nucleatum-positive CRC was classified as F. nucleatum–high or –low based on F. nucleatum DNA load, using a cut-off median value of 2-ΔCt. The proportions of F. nucleatum–high, –low, and –negative CRCs along the tumor location bowel subsite varied (Fig. 1). The proportion of F. nucleatum–high tumors was highest among cecal cancers, whereas that of F. nucleatum–high tumors was lowest among transverse colon cancers (54.5% and 23.7%, respectively) (Fig. 1).
- Clinicopathological and molecular associations of F. nucleatum in CRCs
- We analyzed the relationship between F. nucleatum status (high vs. low/negative) and clinicopathological (age, sex, tumor sidedness, pT/pN categories, tumor grade, lymphovascular and perineural invasions, and mucinous histology) and molecular characteristics (MSI, CIMP, and KRAS/BRAF mutations) in overall stage II/III CRCs treated with oxaliplatin-based adjuvant chemotherapy (n = 593). The results are summarized in Table 1. Among the variables, the pT category was the only factor with statistical significance. F. nucleatum–high was significantly associated with advanced pT stage (pT3/pT4) (p = .005) (Table 1). CIMP-high and KRAS mutations were more frequent in F. nucleatum–high CRCs than in F. nucleatum–low/negative CRCs, without statistical significance (p = .174 and p = .093, respectively) (Table 1).
- Prognostic impact of F. nucleatum in CRCs treated with adjuvant chemotherapy
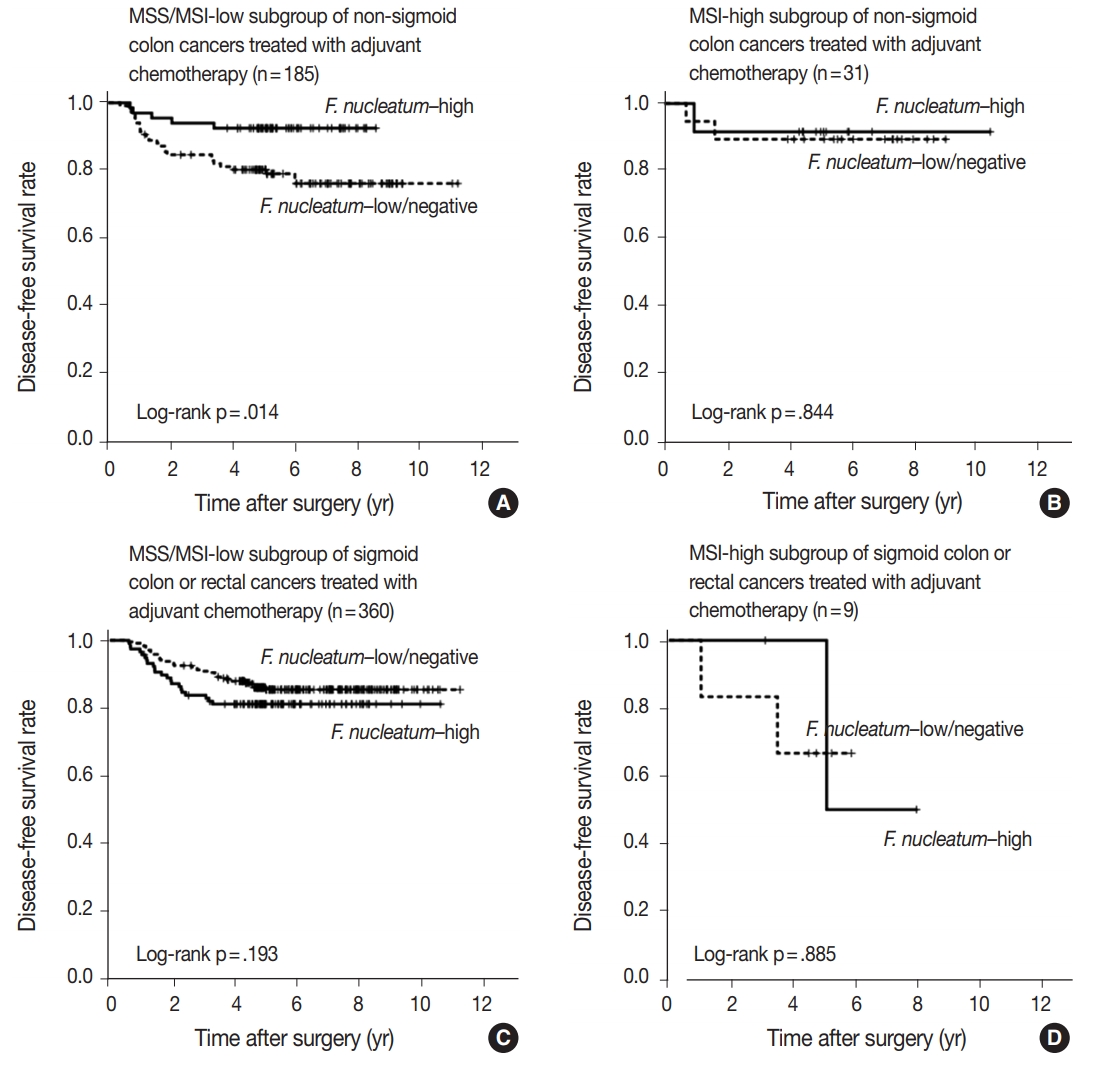
- In survival analysis, no significant difference in disease-free survival (DFS) was evident between the F. nucleatum high and F. nucleatum low/negative groups in overall 593 stage II/III CRC patients treated with oxaliplatin-based adjuvant chemotherapy (log-rank p = .671) (Fig. 2A). In addition, the prognostic significance of F. nucleatum was not identified in subgroups stratified by MSI status (log-rank p = .858 in MSI-high CRCs (n = 40), log-rank p = .625 in MSS/MSI-low CRCs (n = 545) (Supplementary Fig. S2). However, subgroup analyses according to tumor location demonstrated that DFS of the F. nucleatum–high group was significantly better than that of the F. nucleatum–low/negative group in patients with adjuvant FOLFOX or CAPOX-treated colon cancer located in the non-sigmoid colon (from cecum to descending colon, n = 219) (log-rank p = .026) (Fig. 2B). In sigmoid colon and rectal cancer patients treated with oxaliplatinbased adjuvant chemotherapy (n = 374), the F. nucleatum–high group showed a tendency toward worse DFS compared to the F. nucleatum–low/negative group, but this survival difference was not statistically significant (log-rank p = .199) (Fig. 2C). In multivariate analysis, F. nucleatum–high was an independently favorable prognostic factor in non-sigmoid colon cancer patients treated with oxaliplatin-based adjuvant chemotherapy (hazard ratio, 0.42; 95% confidence interval, 0.18 to 0.97; p = .043) (Table 2). To further identify the molecular basis of the favorable prognostic effect of F. nucleatum observed in non-sigmoid colon cancers, we analyzed the prognostic impact of F. nucleatum in subsets of nonsigmoid colon cancer patients according to MSI status. In an MSS/MSI-low subset of non-sigmoid colon cancer patients treated with adjuvant chemotherapy (n = 185), DFS was significantly better in the F. nucleatum-high group than in the F. nucleatum-low/negative group (log-rank p = .014) (Fig. 3A). However, significant DFS difference according to F. nucleatum status was not observed in an MSI-high subset of non-sigmoid colon cancer patients (n = 31) (log-rank p = .844) (Fig. 3B). Finally, survival analyses in MSS/MSI-low (n = 360) and MSI-high (n = 9) subgroups of sigmoid colon or rectal cancers treated with oxaliplatin-based adjuvant chemotherapy demonstrated tendencies toward worse DFS of F. nucleatum–high group than of F. nucleatum–low/negative group, but there was no statistical significance (log-rank p = .193 in MSS/MSI-low subgroup, Fig. 3C; log-rank p = .885 in MSI-high subgroup, Fig. 3D)
RESULTS
- Direct or indirect roles of gut microbiota in the pathogenesis of a variety of human diseases have been recently proposed. The demonstration of the close association between F. nucleatum and CRC has prompted exploration of the pathogenetic, prognostic, and predictive roles of F. nucleatum in CRC. However, there are still limited data regarding the prognostic and predictive values of F. nucleatum in CRC. Several studies using clinical samples have indicated that intratumoral F. nucleatum is potentially associated with poor prognosis in CRC patients [3,11,20]. Moreover, an experimental study suggested that F. nucleatum might be able to induce resistance to chemotherapy by modulating autophagy in CRC cells [4]. Based on the emerging prognostic significance and potential predictive value of F. nucleatum in CRC, we decided to investigate the prognostic relevance of F. nucleatum in CRCs treated with adjuvant chemotherapy. Most patients with stage III or high-risk stage II CRC are treated with adjuvant chemotherapy after curative surgery to prevent tumor recurrence. Thus, we collected a large series of stage III or high-risk stage II CRCs treated with oxaliplatin-based adjuvant chemotherapy. The survival differences in patient subgroups according to DNA amount of intratumoral F. nucleatum measured by qPCR were statistically analyzed. We found that a high load of intratumoral F. nucleatum was independently correlated with improved survival in patients with stage II/III non-sigmoid colon cancer treated with oxaliplatinbased adjuvant chemotherapy (Table 2).
- There is a discrepancy between our research and previous studies. Several previous studies revealed that F. nucleatum–high CRC patients group tended to have shorter disease-specific survival than F. nucleatum–low/negative CRC patients group [3,11,20]. However, in the current study, F. nucleatum had different prognostic impacts based on tumor location in CRCs treated with adjuvant chemotherapy. In detail, tumors with high levels of F. nucleatum had better prognosis than those with low or negative levels of F. nucleatum in non-sigmoid colon cancers, including cecum, ascending colon, transverse colon, and descending colon cancers (Table 2, Fig. 2B). On the other hand, F. nucleatum–high CRCs showed a tendency toward worse prognosis compared to F. nucleatum–low/negative CRCs in sigmoid colon and rectal cancers (Fig. 2C). Since these contrasting prognostic implications of F. nucleatum according to tumor location may counterbalance the overall prognostic effect of F. nucleatum in CRCs, presently F. nucleatum displayed no association with prognosis in a total of 593 stage II/III CRC patients treated with adjuvant chemotherapy (Fig. 2A). The reason for the discrepancy between the current and prior findings may be the difference in the composition of the study populations. Yamaoka et al. [20] described that F. nucleatum was highly correlated with shorter disease specific survival especially in stage IV CRCs. In that study, in all stages of CRCs, disease-specific survival was decreased in CRCs featuring a high level of F. nucleatum compared with that in CRCs with low levels of F. nucleatum, although the survival differences according to F. nucleatum level was decreased compared to that in the stage IV CRC subgroup [20]. In addition, it cannot be excluded that there might be heterogeneities of detailed treatment approaches, such as adjuvant chemotherapy regimen, in the CRC cohorts of other studies. By contrast, our study samples were a well-selected and relatively-homogeneous cohort that contained only stage III or high-risk stage II CRCs treated with oxaliplatin-based adjuvant chemotherapy. Therefore, the prognostic implications of F. nucleatum in CRC that is evident from our study could be meaningfully different from the results of other research groups.
- In an experimental study, F. nucleatum promoted resistance to chemotherapy in CRC cells [4]. However, our results indicate that influences of F. nucleatum on responses to chemotherapy might be diverse in the context of tumor location of CRCs. In sigmoid colon and rectal cancers, the expected chemoresistant effect of F. nucleatum seems to have occurred because F. nucleatum–high was linked with poor prognosis in sigmoid colon and rectal cancer patients treated with adjuvant chemotherapy, although statistical significance was not reached (Fig. 2C). Nevertheless, in nonsigmoid colon cancers, a chemoresistant role of F. nucleatum seems to be attenuated. Rather, F. nucleatum might induce a chemoresponsive effect because F. nucleatum–high was significantly associated with favorable prognosis in non-sigmoid colon cancers treated with adjuvant chemotherapy (Table 2, Fig. 2B). The underlying mechanism of the potential contrasting effects of F. nucleatum on the chemotherapy response depending on location of CRC is unclear. However, the idea that different tumor locations can define different prognosis and treatment responses in CRC has been increasingly addressed. In fact, based on the accumulating clinical data, primary tumor location is regarded as a prognostic factor in metastatic CRCs [21]. Stage IV CRCs primarily located in the right-sided colon are significantly associated with worse prognosis compared with left-sided stage IV CRCs. The different molecular, pathological, and clinical features between right-sided colon cancers and left-sided CRCs have been reported [21,22]. Therefore, the potential different impacts of F. nucleatum on prognosis and treatment responses according to tumor location in CRCs are not surprising. To the best of our knowledge, this study is the first report to investigate the prognostic effect of F. nucleatum according to tumor location in CRCs, especially in adjuvant chemotherapy-treated CRCs. Our study suggests that the prognostic effect of F. nucleatum should be evaluated considering the location of the tumor.
- In this study, the proportion of F. nucleatum–high CRCs differed in each tumor location bowel subsite. The proportion of F. nucleatum–high tumors in all the CRCs was 34.4% (204 of 593). Cecal cancers displayed the highest proportion of F. nucleatum-high tumors (54.5%), followed by ascending colon cancers (38.4%) (Fig. 1). It was notable that over half of the cecal cancers were F. nucleatum–high tumors. Our results are consistent with those of previous studies demonstrating the significant association of the proximal location of CRCs with a high level of intratumoral F. nucleatum. According to the study by Mima et al. [13], the proportion of F. nucleatum–high CRCs increased along the distance from the anal verge, and cecal cancers showed the highest proportion of F. nucleatum–high subtype. The underlying mechanism of the specific enrichment of F. nucleatum in cecal and ascending colon cancers is still unclear, but microenvironmental or biological factors specifically found in the cecal to ascending colon areas could influence the increase of intratumoral F. nucleatum. For example, bacterial biofilms are intensively enriched in right-sided colon tumors compared with those in left-sided colorectal tumors [23]. Based on recent experimental findings, potential molecular mechanisms can be hypothesized. According to a previous experimental study, F. nucleatum is enriched in colorectal tumor tissue by Fap2 binding to Gal-GalNAc expressed on tumor cells [24]. Thus, it can be hypothesized that Gal-GalNAc expression on tumor cells might be more upregulated in the right-sided colon than in the left-sided colon. Further investigations are needed to elucidate the biological reason of the preference of invasive F. nucleatum for right-sided colon cancers.
- According to the recent data reported by Ogino group, F. nucleatum in CRCs differentially impacts tumor-infiltrating lymphocyte (TIL) density depending on MSI status [25]. In detail, there was an inverse association between F. nucleatum load and TIL density in MSI-high CRCs, whereas a positive correlation between F. nucleatum load and TIL density was observed in non–MSI-high CRCs [25]. This finding can provide an important clue for the interpretation of our present results. It has been validated that high TIL density is strongly associated with favorable prognosis in CRCs [26]. Thus, because F. nucleatum–high tumors might be associated with increased antitumor immunity and subsequent improved prognosis in non–MSI-high CRCs, the favorable prognostic effect of F. nucleatum–high in the MSS/MSI-low subset of non-sigmoid colon cancers, which was observed in our present study, could be a reasonable finding. However, we also found that the prognostic significance of F. nucleatum was valid only in non-sigmoid colon cancers, but not in sigmoid colon/rectal cancers, suggesting that both tumor location and MSI status should be concurrently considered for understanding the prognostic implications of F. nucleatum in CRCs.
- There have been several reports regarding the poor prognostic effect of F. nucleatum in CRCs, which was mainly observed in Western CRC cohorts or stage IV CRC cohorts [3,20,27]. However, our present data indicate that high intratumoral F. nucleatum load might be associated with favorable prognosis in a limited subgroup of CRCs, a MSS/MSI-low subset of non-sigmoid colon cancers. We suspect that different compositions of tumor locations and MSI subtypes in CRC cohorts might influence the different prognostic effects of F. nucleatum in overall CRCs. Because it has been known that the frequency of MSI-high in CRCs is definitely lower in East Asia countries than in Western countries [28], the potential favorable prognostic effect of F. nucleatum in proximal colonic-located, non–MSI-high CRCs might be significantly attenuated in CRC cohorts of Western countries, which consist of relatively high numbers of MSI-high tumors. Instead, both the tendency toward worse prognosis of F. nucleatum–high in MSI-high tumors (Supplementary Fig. S2A) and the potential poor prognostic effect of F. nucleatum–high tumors observed in sigmoid colon/rectal cancers (Fig. 2C) might augment the adverse prognostic impact of F. nucleatum in overall CRCs. To confirm this hypothesis, additional investigations using various CRC cohorts having different ethnic backgrounds would be needed. Regarding the poor prognostic feature of F. nucleatum in stage IV CRCs observed in a few studies [20,27], it could be explained by relatively high proportion of distal-located CRCs as primary origin of stage IV CRCs. Thus, the potential worse prognostic effect of F. nucleatum in sigmoid colon or rectal cancers might be augmented especially in a stage IV subset of CRCs.
- Although significant associations between CIMP-high (and/or MSI-high) and F. nucleatum in CRCs were reported in several previous studies [3,8,9], significant correlation between F. nucleatum–high group and CIMP-high or MSI-high molecular subtype was not observed in our present study (Table 1). However, there was an evident tendency toward higher proportion of CIMP-high tumors in F. nucleatum-high group than in F. nucleatum–low/negative group (7.4% vs. 4.7%) (Table 1). In addition, we performed mean comparison of F. nucleatum DNA amount (2-ΔCt) between CIMP-high and CIMP-low/negative tumors, and the results indicated that mean F. nucleatum DNA amount was higher in CIMP-high tumors than in CIMP-low/negative tumors although statistical significance was not reached (0.986 vs. 0.367, p = .157) (Supplementary Fig. S3). The reason for unclear molecular association of F. nucleatum in our study samples may be explained by potential ethnic differences and biased sample composition. As mentioned above, the frequencies of MSI-high and CIMP-high in CRCs are lower in East Asian population than in Western population. If a high number of CIMP-high cases were included in our cohort, significant association between F. nucleatum–high and CIMP-high might have been observed. Moreover, our study samples were confined to selected stage III or high-risk stage II CRCs treated with adjuvant chemotherapy. Thus, molecular compositions of our CRC cohort were possibly biased. For example, the CIMP-high/non-MSI-high subtype has been known as an aggressive phenotype of CRCs and can be more enriched in stage IV tumors. Because stage IV cases were excluded from our study samples, the potential association between F. nucleatum–high and CIMP-high could be weakened. Considering that data are limited, the relationship between F. nucleatum and specific molecular phenotypes in CRCs has not been conclusive yet. Therefore, further clinical and experimental investigations are needed to elucidate whether CIMP-high and/or MSI-high molecular phenotype can significantly interact with intratumoral F. nucleatum enrichment in CRCs.
- The proportion of F. nucleatum-positive cases in CRCs by qPCR analysis has been variable according to different investigations (8.6%–74%) [29]. In our results, F. nucleatum DNA was detected in 408 out of 593 cases (68.8%). The reason for variability in the F. nucleatum-positive rate in CRCs is unclear, but tissue quality might be a critical factor for this discrepancy. Recently, Lee et al. [27] found that the tissue fixation method could affect different results of F. nucleatum qPCR analysis. We also found that when the FFPE tissues were more recent, the positive rate of F. nucleatum was increased (unpublished data). Therefore, it can be inferred that F. nucleatum-positive rate by qPCR method could be variable, depending on tissue fixation method and tissue storage time.
- There are several limitations in this study. First, we assessed the amount of F. nucleatum in genomic DNA samples extracted from FFPE tissues. The precise quantification of F. nucleatum could be disturbed owing to the degraded nature of DNA extracted from FFPE tissues although a substantial number of previous studies that analyzed F. nucleatum in clinical CRC samples also used FFPE tissue-derived DNA. Second, our study cohort was retrospectively collected. The results from our study should be validated by other prospective studies.
- In conclusion, the prognostic impact of F. nucleatum in CRCs treated with adjuvant chemotherapy may differ depending on the combined status of primary tumor location and MSI molecular phenotype. Intratumoral F. nucleatum load may be a potential prognostic factor in stage III or high-risk stage II non-sigmoid colon cancers treated with oxaliplatin-based adjuvant chemotherapy, especially in an MSS/MSI-low molecular subtype. There have been very limited data regarding the detailed prognostic implications of F. nucleatum in CRCs according to various clinicopathologic and molecular contexts. Therefore, further studies using large prospective cohorts will be necessary to validate the different location/MSI-dependent prognostic impacts of F. nucleatum in CRCs treated with adjuvant chemotherapy.
DISCUSSION
Electronic Supplementary Material
Acknowledgments
| Variable | F. nucleatum–high | F. nucleatum–low/negative | p-value |
|---|---|---|---|
| Age | .286 | ||
| Younger (< 59 yr) | 84 (41.2) | 178 (45.8) | |
| Older (≥ 59 yr) | 120 (58.8) | 211 (54.2) | |
| Sex | .925 | ||
| Male | 124 (60.8) | 238 (61.2) | |
| Female | 80 (39.2) | 151 (38.8) | |
| Tumor sidedness | .287 | ||
| Right-sided | 69 (33.8) | 115 (29.6) | |
| Left-sided | 135 (66.2) | 274 (70.4) | |
| Gross tumor type | .243 | ||
| Polypoid/fungating | 119 (58.3) | 246 (63.2) | |
| Ulceroinfiltrative | 85 (41.7) | 143 (36.8) | |
| pT category | .005 | ||
| pT1/ pT2 | 9 (4.4) | 44 (11.3) | |
| pT3/pT4 | 195 (95.6) | 345 (88.7) | |
| pN category | .464 | ||
| pN0 | 34 (16.7) | 56 (14.4) | |
| pN1/pN2 | 170 (83.3) | 333 (85.6) | |
| Tumor histological grade | .687 | ||
| G1/G2 | 188 (92.2) | 362 (93.1) | |
| G3/G4 | 16 (7.8) | 27 (6.9) | |
| Lymphovascular invasion | .419 | ||
| Absent | 112 (54.9) | 200 (51.4) | |
| Present | 92 (45.1) | 189 (48.6) | |
| Perineural invasion | .171 | ||
| Absent | 143 (70.1) | 293 (75.3) | |
| Present | 61 (29.9) | 96 (24.7) | |
| Mucinous histology | .269 | ||
| Absent | 184 (90.2) | 361 (92.8) | |
| Present | 20 (9.8) | 28 (7.2) | |
| MSI statusa | .647 | ||
| MSS/ MSI-low | 185 (92.5) | 360 (93.5) | |
| MSI-high | 15 (7.5) | 25 (6.5) | |
| CIMP statusb | .174 | ||
| CIMP-low/negative | 189 (92.6) | 369 (95.3) | |
| CIMP-high | 15 (7.4) | 18 (4.7) | |
| KRAS mutationc | .093 | ||
| Absent | 137 (67.2) | 286 (73.7) | |
| Present | 67 (32.8) | 102 (26.3) | |
| BRAF mutation | .213 | ||
| Absent | 200 (98) | 374 (96.1) | |
| Present | 4 (2) | 15 (3.9) |
Values are presented as number (%).
CRC, colorectal cancer; G1, grade 1 (well differentiated); G2, grade 2 (moderately differentiated); G3, grade 3 (poorly differentiated); G4, grade 4 (undifferentiated); MSI, microsatellite instability; MSS, microsatellite-stable; CIMP, CpG island methylator phenotype.
aAmong the 593 cases, MSI status could not be determined in eight cases due to inadequate DNA quality or quantity;
bAmong the 593 cases, CIMP status could not be determined in two cases due to inadequate DNA quality or quantity;
cAmong the 593 cases, KRAS mutation could not be determined in one case due to inadequate DNA quality or quantity.
| Variable | No. | Univariate analysis HR (95% CI) | p-value | Multivariate analysis HR (95% CI) | p-value |
|---|---|---|---|---|---|
| F. nucleatum | |||||
| F. nucleatum–low/negative | 139 | Reference | Reference | ||
| F. nucleatum–high | 80 | 0.4 (0.18–0.92) | .031 | 0.42 (0.18–0.97) | .043 |
| pT category | |||||
| pT1/pT2/pT3 | 183 | Reference | Reference | ||
| pT4 | 36 | 5.13 (2.65–9.92) | < .001 | 5.04 (2.53–10.07) | < .001 |
| pN category | |||||
| pN0/pN1 | 176 | Reference | Reference | ||
| pN2 | 43 | 2.88 (1.47–5.64) | .002 | 2.65 (1.31–5.35) | .007 |
| Lymphovascular invasion | |||||
| Absent | 129 | Reference | Reference | ||
| Present | 90 | 2.78 (1.41–5.50) | .003 | 1.39 (0.66–2.95) | .387 |
| Perineural invasion | |||||
| Absent | 169 | Reference | Reference | ||
| Present | 50 | 2.81 (1.45–5.45) | .002 | 2.92 (1.41–6.05) | .004 |
| BRAF mutation | |||||
| Absent | 204 | Reference | Reference | ||
| Present | 15 | 3.12 (1.30–7.49) | .011 | 2.21 (0.86–5.69) | .1 |
| Tumor histological grade | |||||
| G1/G2 | 190 | Reference | - | ||
| G3/G4 | 29 | 1.14 (0.44–2.92) | .791 | - | - |
| MSI statusa | |||||
| MSS/MSI-low | 185 | Reference | - | ||
| MSI-high | 31 | 0.57 (0.17–1.87) | .353 | - | - |
| CIMP statusb | |||||
| CIMP-low/negative | 192 | Reference | - | ||
| CIMP-high | 25 | 1.32 (0.51-3.40) | .567 | - | - |
| KRAS mutationc | |||||
| Absent | 148 | Reference | - | ||
| Present | 71 | 0.93 (0.46-1.89) | .844 | - | - |
HR, hazard ratio; 95% CI, 95% confidence interval of HR; G1, grade 1 (well differentiated); G2, grade 2 (moderately differentiated); G3, grade 3 (poorly differentiated); G4, grade 4 (undifferentiated); MSI, microsatellite instability; MSS, microsatellite-stable; CIMP, CpG island methylator phenotype.
aAmong the 219 cases, MSI status could not be determined in three cases due to inadequate DNA quality or quantity;
bAmong the 219 cases, CIMP status could not be determined in two cases due to inadequate DNA quality or quantity.
- 1. Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumorimmune microenvironment. Cell Host Microbe 2013; 14: 207-15. ArticlePubMedPMC
- 2. Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013; 14: 195-206. PubMedPMC
- 3. Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016; 65: 1973-80. ArticlePubMed
- 4. Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017; 170: 548-63. e16. ArticlePubMedPMC
- 5. Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017; 357: 1156-60. ArticlePubMedPMC
- 6. Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2012; 22: 292-8. ArticlePubMedPMC
- 7. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012; 22: 299-306. ArticlePubMedPMC
- 8. Tahara T, Yamamoto E, Suzuki H, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res 2014; 74: 1311-8. ArticlePubMedPMCPDF
- 9. Ito M, Kanno S, Nosho K, et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int J Cancer 2015; 137: 1258-68. ArticlePubMed
- 10. Yu J, Chen Y, Fu X, et al. Invasive Fusobacterium nucleatum may play a role in the carcinogenesis of proximal colon cancer through the serrated neoplasia pathway. Int J Cancer 2016; 139: 1318-26. ArticlePubMed
- 11. Flanagan L, Schmid J, Ebert M, et al. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis 2014; 33: 1381-90. ArticlePubMedPDF
- 12. Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol 2015; 1: 653-61. ArticlePubMedPMC
- 13. Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin Transl Gastroenterol 2016; 7: e200. ArticlePubMedPMCPDF
- 14. Park HE, Kim JH, Cho NY, Lee HS, Kang GH. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Arch 2017; 471: 329-36. ArticlePubMedPDF
- 15. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91-7. PubMed
- 16. Vetizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015; 350: 1079-84. ArticlePubMedPMC
- 17. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97-103. PubMed
- 18. Bae JM, Kim JH, Oh HJ, et al. Downregulation of acetyl-CoA synthetase 2 is a metabolic hallmark of tumor progression and aggressiveness in colorectal carcinoma. Mod Pathol 2017; 30: 267-77. ArticlePubMedPDF
- 19. Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998; 58: 5248-57. PubMed
- 20. Yamaoka Y, Suehiro Y, Hashimoto S, et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. J Gastroenterol 2018; 53: 517-24. ArticlePubMedPDF
- 21. Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer 2017; 84: 69-80. ArticlePubMedPMC
- 22. Bae JM, Kim JH, Kang GH. Molecular subtypes of colorectal cancer and their clinicopathologic features, with an emphasis on the serrated neoplasia pathway. Arch Pathol Lab Med 2016; 140: 406-12. ArticlePubMedPDF
- 23. Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A 2014; 111: 18321-6. ArticlePubMedPMC
- 24. Abed J, Emgård JE, Zamir G, et al. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumorexpressed Gal-GalNAc. Cell Host Microbe 2016; 20: 215-25. ArticlePubMedPMC
- 25. Hamada T, Zhang X, Mima K, et al. Fusobacterium nucleatum in colorectal cancer relates to immune response differentially by tumor microsatellite instability status. Cancer Immunol Res 2018; 6: 1327-36. ArticlePubMedPMCPDF
- 26. Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018; 391: 2128-39. PubMed
- 27. Lee DW, Han SW, Kang JK, et al. Association between Fusobacterium nucleatum, pathway mutation, and patient prognosis in colorectal cancer. Ann Surg Oncol 2018; 25: 3389-95. ArticlePubMedPDF
- 28. Kim JH, Kang GH. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J Gastroenterol 2014; 20: 4230-43. ArticlePubMedPMC
- 29. Shang FM, Liu HL. Fusobacterium nucleatum and colorectal cancer: a review. World J Gastrointest Oncol 2018; 10: 71-81. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Microbiota and tumor epigenetics: deep interconnections and emerging therapeutic perspectives
Lei Duan, Dan Hu, Haoling Zhang, Yan Liao
Critical Reviews in Clinical Laboratory Sciences.2026; : 1. CrossRef - Unraveling the Role of Fusobacterium nucleatum in Colorectal Cancer: Molecular Mechanisms and Pathogenic Insights
Linda Galasso, Fabrizio Termite, Irene Mignini, Giorgio Esposto, Raffaele Borriello, Federica Vitale, Alberto Nicoletti, Mattia Paratore, Maria Elena Ainora, Antonio Gasbarrini, Maria Assunta Zocco
Cancers.2025; 17(3): 368. CrossRef - Emerging roles of intratumoral microbiota: a key to novel cancer therapies
Pengzhong Fang, Jing Yang, Huiyun Zhang, Diankui Shuai, Min Li, Lin Chen, Liping Liu
Frontiers in Oncology.2025;[Epub] CrossRef - Association of Fusobacterium nucleatum with colorectal cancer molecular subtypes and its outcome: a systematic review
Luana Greco, Federica Rubbino, Clarissa Ferrari, Michela Cameletti, Fabio Grizzi, Fabrizio Bonelli, Alberto Malesci, Massimiliano Mazzone, Luigi Ricciardiello, Luigi Laghi
Gut Microbiome.2025;[Epub] CrossRef - The Interplay Between the Gut Microbiota and Colorectal Cancer: A Review of the Literature
Marco Cintoni, Marta Palombaro, Eleonora Zoli, Giuseppe D’Agostino, Gabriele Pulcini, Elena Leonardi, Pauline Raoul, Emanuele Rinninella, Flavio De Maio, Esmeralda Capristo, Antonio Gasbarrini, Maria Cristina Mele
Microorganisms.2025; 13(6): 1410. CrossRef - Harnessing intratumoral microbiota: new horizons in immune microenvironment and immunotherapy
Jinhe Zhang, Zinan You, Xinqiao Li, Jinpeng Hu, Jiamu Li, Zhitao Jing
Journal of Translational Medicine.2025;[Epub] CrossRef - Prognostic impact of Fusobacterium nucleatum on survival in colorectal cancer: A systematic review and meta-analysis
Tianyu Wang, Shengcheng Lin, Yong Ji, Ciren Puqiong, Jidong Gao, Shuluan Li
Journal of Cancer Research and Therapeutics.2025; 21(4): 796. CrossRef - Fusobacterium nucleatum in Health and Disease
Xinyi Yang, Shutian Zhang, Tingting Ning, Jing Wu
MedComm.2025;[Epub] CrossRef - Periodontitis and Oral Pathogens in Colorectal Cancer: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis
Luis Chauca-Bajaña, Andrea Ordoñez Balladares, Alejandro Ismael Lorenzo-Pouso, Rosangela Caicedo-Quiroz, Rafael Xavier Erazo Vaca, Rolando Fabricio Dau Villafuerte, Yajaira Vanessa Avila-Granizo, Carlos Hans Salazar Minda, Miguel Amador Salavarria Vélez,
Dentistry Journal.2025; 13(12): 595. CrossRef - Composition of the colon microbiota in the individuals with inflammatory bowel disease and colon cancer
Ceren Acar, Sibel Kucukyildirim Celik, H. Ozgur Ozdemirel, Beril Erdem Tuncdemir, Saadet Alan, Hatice Mergen
Folia Microbiologica.2024; 69(2): 333. CrossRef - Intratumoral microorganisms in tumors of the digestive system
Mengjuan Xuan, Xinyu Gu, Yingru Liu, Li Yang, Yi Li, Di Huang, Juan Li, Chen Xue
Cell Communication and Signaling.2024;[Epub] CrossRef - Prognostic impact of oral microbiome on survival of malignancies: a systematic review and meta-analysis
Shuluan Li, Tianyu Wang, Ya Ren, Zhou Liu, Jidong Gao, Zhi Guo
Systematic Reviews.2024;[Epub] CrossRef - Exploring the Potential of Humoral Immune Response to Commensal Bifidobacterium as a Biomarker for Human Health, including Both Malignant and Non-Malignant Diseases: A Perspective on Detection Strategies and Future Directions
Kyogo Itoh, Satoko Matsueda
Biomedicines.2024; 12(4): 803. CrossRef - Unveiling intratumoral microbiota: An emerging force for colorectal cancer diagnosis and therapy
Jinjing Zhang, Penghui Wang, Jiafeng Wang, Xiaojie Wei, Mengchuan Wang
Pharmacological Research.2024; 203: 107185. CrossRef - Spatial transcriptomic analysis reveals local effects of intratumoral fusobacterial infection on DNA damage and immune signaling in rectal cancer
William P. Duggan, Batuhan Kisakol, Ina Woods, Mohammedreza Azimi, Heiko Dussmann, Joanna Fay, Tony O’Grady, Barry Maguire, Ian S. Reynolds, Manuela Salvucci, Daniel J. Slade, Deborah A. McNamara, John P. Burke, Jochen H.M. Prehn
Gut Microbes.2024;[Epub] CrossRef - Gut microbiota characteristics of colorectal cancer patients in Hubei, China, and differences with cohorts from other Chinese regions
Jianguo Shi, Hexiao Shen, Hui Huang, Lifang Zhan, Wei Chen, Zhuohui Zhou, Yongling Lv, Kai Xiong, Zhiwei Jiang, Qiyi Chen, Lei Liu
Frontiers in Microbiology.2024;[Epub] CrossRef - The role of Fusobacterium nucleatum in cancer and its implications for clinical applications
Wanyi Luo, Juxi Han, Xian Peng, Xuedong Zhou, Tao Gong, Xin Zheng
Molecular Oral Microbiology.2024; 39(6): 417. CrossRef - Gut Microbiome and colorectal cancer: discovery of bacterial changes with metagenomics application in Turkısh population
Yakup Ulger, Anıl Delik, Hikmet Akkız
Genes & Genomics.2024; 46(9): 1059. CrossRef - Intratumoral Microbiota: Metabolic Influences and Biomarker Potential in Gastrointestinal Cancer
Xueyuan Bi, Jihan Wang, Cuicui Liu
Biomolecules.2024; 14(8): 917. CrossRef - Intratumoral Microbiota: Insights from Anatomical, Molecular, and Clinical Perspectives
Claudia Lombardo, Rosanna Fazio, Marta Sinagra, Giuseppe Gattuso, Federica Longo, Cinzia Lombardo, Mario Salmeri, Guido Nicola Zanghì, Carla Agata Erika Loreto
Journal of Personalized Medicine.2024; 14(11): 1083. CrossRef - Exploring the role of Fusobacterium nucleatum in colorectal cancer: implications for tumor proliferation and chemoresistance
Leila Dadgar-Zankbar, Zahra Elahi, Aref Shariati, Azad Khaledi, Shabnam Razavi, Amin Khoshbayan
Cell Communication and Signaling.2024;[Epub] CrossRef - Fusobacterium nucleatum Abundance is Associated with Cachexia in Colorectal Cancer Patients: The ColoCare Study
Mmadili N. Ilozumba, Tengda Lin, Sheetal Hardikar, Doratha A. Byrd, June L. Round, W. Zac Stephens, Andreana N. Holowatyj, Christy A. Warby, Victoria Damerell, Christopher I. Li, Jane C. Figueiredo, Adetunji T. Toriola, David Shibata, Gary C. Fillmore, Ba
Cancer Medicine.2024;[Epub] CrossRef - Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy
Li Yang, Aitian Li, Ying Wang, Yi Zhang
Signal Transduction and Targeted Therapy.2023;[Epub] CrossRef - Increased Fusobacterium tumoural abundance affects immunogenicity in mucinous colorectal cancer and may be associated with improved clinical outcome
William P. Duggan, Manuela Salvucci, Batuhan Kisakol, Andreas U. Lindner, Ian S. Reynolds, Heiko Dussmann, Joanna Fay, Tony O’Grady, Daniel B. Longley, Fiona Ginty, Elizabeth Mc Donough, Daniel J. Slade, John P. Burke, Jochen H. M. Prehn
Journal of Molecular Medicine.2023; 101(7): 829. CrossRef -
Fusobacterium nucleatum Load Correlates with KRAS Mutation and Sessile Serrated Pathogenesis in Colorectal Adenocarcinoma
Koki Takeda, Minoru Koi, Yoshiki Okita, Sija Sajibu, Temitope O. Keku, John M. Carethers
Cancer Research Communications.2023; 3(9): 1940. CrossRef - La asociación entre Fusobacterium nucleatum y el cáncer colorrectal: una revisión sistemática y metaanálisis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades Infecciosas y Microbiología Clínica.2022; 40(5): 224. CrossRef - The association between Fusobacterium nucleatum and cancer colorectal: A systematic review and meta-analysis
Paola Villar-Ortega, Manuela Expósito-Ruiz, Miguel Gutiérrez-Soto, Miguel Ruiz-Cabello Jiménez, José María Navarro-Marí, José Gutiérrez-Fernández
Enfermedades infecciosas y microbiologia clinica (English ed.).2022; 40(5): 224. CrossRef - Suppression of Berberine and Probiotics (in vitro and in vivo) on the Growth of Colon Cancer With Modulation of Gut Microbiota and Butyrate Production
Chao Huang, Ying Sun, Sheng-rong Liao, Zhao-xin Chen, Han-feng Lin, Wei-zeng Shen
Frontiers in Microbiology.2022;[Epub] CrossRef - Prognostic and clinicopathological significance of Fusobacterium nucleatum in colorectal cancer: a systemic review and meta-analysis
Younghoon Kim, Nam Yun Cho, Gyeong Hoon Kang
Journal of Pathology and Translational Medicine.2022; 56(3): 144. CrossRef - Iron accelerates Fusobacterium nucleatum–induced CCL8 expression in macrophages and is associated with colorectal cancer progression
Taishi Yamane, Yohei Kanamori, Hiroshi Sawayama, Hiromu Yano, Akihiro Nita, Yudai Ohta, Hironori Hinokuma, Ayato Maeda, Akiko Iwai, Takashi Matsumoto, Mayuko Shimoda, Mayumi Niimura, Shingo Usuki, Noriko Yasuda-Yoshihara, Masato Niwa, Yoshifumi Baba, Taka
JCI Insight.2022;[Epub] CrossRef - Clinicopathological differences of high Fusobacterium nucleatum levels in colorectal cancer: A review and meta-analysis
Yi Wang, Yuting Wen, Jiayin Wang, Xin Lai, Ying Xu, Xuanping Zhang, Xiaoyan Zhu, Chenglin Ruan, Yao Huang
Frontiers in Microbiology.2022;[Epub] CrossRef - Clinical Significance of Fusobacterium nucleatum and Microsatellite Instability in Evaluating Colorectal Cancer Prognosis
Yanxuan Xie, Xiaoyang Jiao, Mi Zeng, Zhiqiang Fan, Xin Li, Yumeng Yuan, Qiaoxin Zhang, Yong Xia
Cancer Management and Research.2022; Volume 14: 3021. CrossRef - Influence of the Microbiome Metagenomics and Epigenomics on Gastric Cancer
Precious Mathebela, Botle Precious Damane, Thanyani Victor Mulaudzi, Zilungile Lynette Mkhize-Khwitshana, Guy Roger Gaudji, Zodwa Dlamini
International Journal of Molecular Sciences.2022; 23(22): 13750. CrossRef - Circulating IgA Antibodies Against Fusobacterium nucleatum Amyloid Adhesin FadA are a Potential Biomarker for Colorectal Neoplasia
Jung Eun Baik, Li Li, Manish A. Shah, Daniel E. Freedberg, Zhezhen Jin, Timothy C. Wang, Yiping W. Han
Cancer Research Communications.2022; 2(11): 1497. CrossRef - Differential immune microenvironmental features of microsatellite-unstable colorectal cancers according to Fusobacterium nucleatum status
Ji Ae Lee, Seung-Yeon Yoo, Hyeon Jeong Oh, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Cancer Immunology, Immunotherapy.2021; 70(1): 47. CrossRef - Fusobacterium nucleatum and Clinicopathologic Features of Colorectal Cancer: Results From the ColoCare Study
Yannick Eisele, Patrick M. Mallea, Biljana Gigic, W. Zac Stephens, Christy A. Warby, Kate Buhrke, Tengda Lin, Juergen Boehm, Petra Schrotz-King, Sheetal Hardikar, Lyen C. Huang, T. Bartley Pickron, Courtney L. Scaife, Richard Viskochil, Torsten Koelsch, A
Clinical Colorectal Cancer.2021; 20(3): e165. CrossRef - Role of gut microbiota in epigenetic regulation of colorectal Cancer
Yinghui Zhao, Chuanxin Wang, Ajay Goel
Biochimica et Biophysica Acta (BBA) - Reviews on Cancer.2021; 1875(1): 188490. CrossRef - Fusobacterium nucleatum: caution with interpreting historical patient sample cohort
Kate L. F. Johnstone, Sinead Toomey, Stephen Madden, Brian D. P. O’Neill, Bryan T Hennessy
Journal of Pathology and Translational Medicine.2021; 55(6): 415. CrossRef -
Fusobacterium nucleatum colonization is associated with decreased survival of helicobacter pylori-positive gastric cancer patients
Yung-Yu Hsieh, Shui-Yi Tung, Hung-Yu Pan, Te-Sheng Chang, Kuo-Liang Wei, Wei-Ming Chen, Yi-Fang Deng, Chung-Kuang Lu, Yu-Hsuan Lai, Cheng-Shyong Wu, Chin Li
World Journal of Gastroenterology.2021; 27(42): 7311. CrossRef - Analysis of changes in microbiome compositions related to the prognosis of colorectal cancer patients based on tissue-derived 16S rRNA sequences
Sukjung Choi, Jongsuk Chung, Mi-La Cho, Donghyun Park, Sun Shim Choi
Journal of Translational Medicine.2021;[Epub] CrossRef - Gastrointestinal tumors and infectious agents: A wide field to explore
Miriam López-Gómez, Belén García de Santiago, Pedro-David Delgado-López, Eduardo Malmierca, Jesús González-Olmedo, César Gómez-Raposo, Carmen Sandoval, Pilar Ruiz-Seco, Nora Escribano, Jorge Francisco Gómez-Cerezo, Enrique Casado
World Journal of Meta-Analysis.2021; 9(6): 505. CrossRef - Gut Microbiota Profiles in Early- and Late-Onset Colorectal Cancer: A Potential Diagnostic Biomarker in the Future
Murdani Abdullah, Ninik Sukartini, Saskia Aziza Nursyirwan, Rabbinu Rangga Pribadi, Hasan Maulahela, Amanda Pitarini Utari, Virly Nanda Muzellina, Agustinus Wiraatmadja, Kaka Renaldi
Digestion.2021; 102(6): 823. CrossRef - The effect of periodontal bacteria infection on incidence and prognosis of cancer
Li Xiao, Qianyu Zhang, Yanshuang Peng, Daqing Wang, Ying Liu
Medicine.2020; 99(15): e19698. CrossRef - The impact of the gut microbiota on prognosis after surgery for colorectal cancer – a systematic review and meta‐analysis
Emilie Palmgren Colov, Thea Helene Degett, Hans Raskov, Ismail Gögenur
APMIS.2020; 128(2): 162. CrossRef - Can the microbiota predict response to systemic cancer therapy, surgical outcomes, and survival? The answer is in the gut
Khalid El Bairi, Rachid Jabi, Dario Trapani, Hanae Boutallaka, Bouchra Ouled Amar Bencheikh, Mohammed Bouziane, Mariam Amrani, Said Afqir, Adil Maleb
Expert Review of Clinical Pharmacology.2020; 13(4): 403. CrossRef - Predictive Values of Colon Microbiota in the Treatment Response to Colorectal Cancer
Jorge Galan-Ros, Verónica Ramos-Arenas, Pablo Conesa-Zamora
Pharmacogenomics.2020; 21(14): 1045. CrossRef - The gut microbiome and potential implications for early-onset colorectal cancer
Reetu Mukherji, Benjamin A Weinberg
Colorectal Cancer.2020;[Epub] CrossRef -
Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis
Christian Gethings-Behncke, Helen G. Coleman, Haydee W.T. Jordao, Daniel B. Longley, Nyree Crawford, Liam J. Murray, Andrew T. Kunzmann
Cancer Epidemiology, Biomarkers & Prevention.2020; 29(3): 539. CrossRef - CpG Island Methylation in Sessile Serrated Adenoma/Polyp of the Colorectum: Implications for Differential Diagnosis of Molecularly High-Risk Lesions among Non-dysplastic Sessile Serrated Adenomas/Polyps
Ji Ae Lee, Hye Eun Park, Seung-Yeon Yoo, Seorin Jeong, Nam-Yun Cho, Gyeong Hoon Kang, Jung Ho Kim
Journal of Pathology and Translational Medicine.2019; 53(4): 225. CrossRef - Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients
Andrew T. Kunzmann, Marcela Alcântara Proença, Haydee WT Jordao, Katerina Jiraskova, Michaela Schneiderova, Miroslav Levy, Václav Liska, Tomas Buchler, Ludmila Vodickova, Veronika Vymetalkova, Ana Elizabete Silva, Pavel Vodicka, David J. Hughes
European Journal of Clinical Microbiology & Infectious Diseases.2019; 38(10): 1891. CrossRef - Gut Microbiome: A Promising Biomarker for Immunotherapy in Colorectal Cancer
Sally Temraz, Farah Nassar, Rihab Nasr, Maya Charafeddine, Deborah Mukherji, Ali Shamseddine
International Journal of Molecular Sciences.2019; 20(17): 4155. CrossRef - The Four Horsemen in Colon Cancer
Marco Antonio Hernández-Luna, Sergio López-Briones, Rosendo Luria-Pérez
Journal of Oncology.2019; 2019: 1. CrossRef - The role of Fusobacterium nucleatum in colorectal cancer: from carcinogenesis to clinical management
Chun‐Hui Sun, Bin‐Bin Li, Bo Wang, Jing Zhao, Xiao‐Ying Zhang, Ting‐Ting Li, Wen‐Bing Li, Di Tang, Miao‐Juan Qiu, Xin‐Cheng Wang, Cheng‐Ming Zhu, Zhi‐Rong Qian
Chronic Diseases and Translational Medicine.2019; 5(3): 178. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Variable | F. nucleatum–high | F. nucleatum–low/negative | p-value |
|---|---|---|---|
| Age | .286 | ||
| Younger (< 59 yr) | 84 (41.2) | 178 (45.8) | |
| Older (≥ 59 yr) | 120 (58.8) | 211 (54.2) | |
| Sex | .925 | ||
| Male | 124 (60.8) | 238 (61.2) | |
| Female | 80 (39.2) | 151 (38.8) | |
| Tumor sidedness | .287 | ||
| Right-sided | 69 (33.8) | 115 (29.6) | |
| Left-sided | 135 (66.2) | 274 (70.4) | |
| Gross tumor type | .243 | ||
| Polypoid/fungating | 119 (58.3) | 246 (63.2) | |
| Ulceroinfiltrative | 85 (41.7) | 143 (36.8) | |
| pT category | .005 | ||
| pT1/ pT2 | 9 (4.4) | 44 (11.3) | |
| pT3/pT4 | 195 (95.6) | 345 (88.7) | |
| pN category | .464 | ||
| pN0 | 34 (16.7) | 56 (14.4) | |
| pN1/pN2 | 170 (83.3) | 333 (85.6) | |
| Tumor histological grade | .687 | ||
| G1/G2 | 188 (92.2) | 362 (93.1) | |
| G3/G4 | 16 (7.8) | 27 (6.9) | |
| Lymphovascular invasion | .419 | ||
| Absent | 112 (54.9) | 200 (51.4) | |
| Present | 92 (45.1) | 189 (48.6) | |
| Perineural invasion | .171 | ||
| Absent | 143 (70.1) | 293 (75.3) | |
| Present | 61 (29.9) | 96 (24.7) | |
| Mucinous histology | .269 | ||
| Absent | 184 (90.2) | 361 (92.8) | |
| Present | 20 (9.8) | 28 (7.2) | |
| MSI status |
.647 | ||
| MSS/ MSI-low | 185 (92.5) | 360 (93.5) | |
| MSI-high | 15 (7.5) | 25 (6.5) | |
| CIMP status |
.174 | ||
| CIMP-low/negative | 189 (92.6) | 369 (95.3) | |
| CIMP-high | 15 (7.4) | 18 (4.7) | |
| KRAS mutation |
.093 | ||
| Absent | 137 (67.2) | 286 (73.7) | |
| Present | 67 (32.8) | 102 (26.3) | |
| BRAF mutation | .213 | ||
| Absent | 200 (98) | 374 (96.1) | |
| Present | 4 (2) | 15 (3.9) |
| Variable | No. | Univariate analysis HR (95% CI) | p-value | Multivariate analysis HR (95% CI) | p-value |
|---|---|---|---|---|---|
| F. nucleatum | |||||
| F. nucleatum–low/negative | 139 | Reference | Reference | ||
| F. nucleatum–high | 80 | 0.4 (0.18–0.92) | .031 | 0.42 (0.18–0.97) | .043 |
| pT category | |||||
| pT1/pT2/pT3 | 183 | Reference | Reference | ||
| pT4 | 36 | 5.13 (2.65–9.92) | < .001 | 5.04 (2.53–10.07) | < .001 |
| pN category | |||||
| pN0/pN1 | 176 | Reference | Reference | ||
| pN2 | 43 | 2.88 (1.47–5.64) | .002 | 2.65 (1.31–5.35) | .007 |
| Lymphovascular invasion | |||||
| Absent | 129 | Reference | Reference | ||
| Present | 90 | 2.78 (1.41–5.50) | .003 | 1.39 (0.66–2.95) | .387 |
| Perineural invasion | |||||
| Absent | 169 | Reference | Reference | ||
| Present | 50 | 2.81 (1.45–5.45) | .002 | 2.92 (1.41–6.05) | .004 |
| BRAF mutation | |||||
| Absent | 204 | Reference | Reference | ||
| Present | 15 | 3.12 (1.30–7.49) | .011 | 2.21 (0.86–5.69) | .1 |
| Tumor histological grade | |||||
| G1/G2 | 190 | Reference | - | ||
| G3/G4 | 29 | 1.14 (0.44–2.92) | .791 | - | - |
| MSI status |
|||||
| MSS/MSI-low | 185 | Reference | - | ||
| MSI-high | 31 | 0.57 (0.17–1.87) | .353 | - | - |
| CIMP status |
|||||
| CIMP-low/negative | 192 | Reference | - | ||
| CIMP-high | 25 | 1.32 (0.51-3.40) | .567 | - | - |
| KRAS mutationc | |||||
| Absent | 148 | Reference | - | ||
| Present | 71 | 0.93 (0.46-1.89) | .844 | - | - |
Values are presented as number (%). CRC, colorectal cancer; G1, grade 1 (well differentiated); G2, grade 2 (moderately differentiated); G3, grade 3 (poorly differentiated); G4, grade 4 (undifferentiated); MSI, microsatellite instability; MSS, microsatellite-stable; CIMP, CpG island methylator phenotype. Among the 593 cases, MSI status could not be determined in eight cases due to inadequate DNA quality or quantity; Among the 593 cases, CIMP status could not be determined in two cases due to inadequate DNA quality or quantity; Among the 593 cases,
HR, hazard ratio; 95% CI, 95% confidence interval of HR; G1, grade 1 (well differentiated); G2, grade 2 (moderately differentiated); G3, grade 3 (poorly differentiated); G4, grade 4 (undifferentiated); MSI, microsatellite instability; MSS, microsatellite-stable; CIMP, CpG island methylator phenotype. Among the 219 cases, MSI status could not be determined in three cases due to inadequate DNA quality or quantity; Among the 219 cases, CIMP status could not be determined in two cases due to inadequate DNA quality or quantity.

 E-submission
E-submission