Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 54(6); 2020 > Article
-
Original Article
Primary squamous cell carcinoma of the salivary gland: immunohistochemical analysis and comparison with metastatic squamous cell carcinoma -
Uiree Jo1
 , Joon Seon Song1
, Joon Seon Song1 , Seung-Ho Choi2
, Seung-Ho Choi2 , Soon Yuhl Nam2
, Soon Yuhl Nam2 , Sang Yoon Kim2
, Sang Yoon Kim2 , Kyung-Ja Cho,1
, Kyung-Ja Cho,1
-
Journal of Pathology and Translational Medicine 2020;54(6):489-496.
DOI: https://doi.org/10.4132/jptm.2020.07.19
Published online: August 31, 2020
1Department of Pathology, Asan Medical Center, University of Ulsan College Medicine, Seoul, Korea
2Department of Otorhinolaryngology-Head and Neck Surgery, Asan Medical Center, University of Ulsan College Medicine, Seoul, Korea
- Corresponding Author: Kyung-Ja Cho, MD, PhD, Departments of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-4545, Fax: +82-2-472-7898, E-mail: kjc@amc.seoul.kr
© 2020 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- Primary salivary gland squamous cell carcinoma with sialolithiasis in the submandibular gland: A case report and literature review
Sawako Ono, Katsutoshi Hirose, Yuji Hirata, Marie Yamada, Satoko Nakamura, Hidetaka Yamamoto
Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology.2024; 36(5): 768. CrossRef - A case of primary squamous cell carcinoma of the parotid gland and review of the literature
Jingli Zhao, Xinrong Nan, Chuhuan Zhou, Nan Jiang, Liangliang Tian
Journal of Case Reports and Images in Oncology.2024; 10(1): 7. CrossRef - Metastatic cutaneous squamous cell carcinoma accounts for nearly all squamous cell carcinomas of the parotid gland
Patrick J. Bradley, Göran Stenman, Lester D. R. Thompson, Alena Skálová, Roderick H. W. Simpson, Pieter J. Slootweg, Alessandro Franchi, Nina Zidar, Alfons Nadal, Henrik Hellquist, Michelle D. Williams, Ilmo Leivo, Abbas Agaimy, Alfio Ferlito
Virchows Archiv.2024; 485(1): 3. CrossRef - Common skin cancers and their association with other non-cutaneous primary malignancies: a review of the literature
Lindsay Holic
Medical Oncology.2024;[Epub] CrossRef - Salivary duct carcinoma with squamous differentiation: histomorphological and immunophenotypical analysis of six cases
Melad N Dababneh, Christopher C Griffith, Kelly R Magliocca, Ivan J Stojanov
Histopathology.2024; 85(4): 590. CrossRef - Comprehensive Next Generation Sequencing Reveals that Purported Primary Squamous Cell Carcinomas of the Parotid Gland are Genetically Heterogeneous
Justin A. Bishop, Masato Nakaguro, Ilan Weinreb, Doreen Palsgrove, Lisa M. Rooper, Travis W. Vandergriff, Brian Carlile, Jeffrey A. Sorelle, Jeffrey Gagan, Toshitaka Nagao
Head and Neck Pathology.2024;[Epub] CrossRef - Salivary gland fine needle aspiration: a focus on diagnostic challenges and tips for achieving an accurate diagnosis
Carla Saoud, Hansen Lam, Sandra I. Sanchez, Zahra Maleki
Diagnostic Histopathology.2023; 29(8): 357. CrossRef - Salivary gland pathologies: evolution in classification and association with unique genetic alterations
Michał Żurek, Łukasz Fus, Kazimierz Niemczyk, Anna Rzepakowska
European Archives of Oto-Rhino-Laryngology.2023; 280(11): 4739. CrossRef - A retrospective study of nonneoplastic and neoplastic disorders of the salivary glands
Sorin Vamesu, Oana Andreea Ursica, Ana Maria Gurita, Raluca Ioana Voda, Mariana Deacu, Mariana Aschie, Madalina Bosoteanu, Georgeta Camelia Cozaru, Anca Florentina Mitroi, Cristian Ionut Orasanu
Medicine.2023; 102(42): e35751. CrossRef - Pembrolizumab as a first line therapy in a patient with extensive mucoepidermoid salivary gland carcinoma. A complete clinical, radiological and pathological response. A very specific case
Raed Farhat, Noam Asna, Yaniv Avraham, Ashraf Khater, Majd Asakla, Alaa Safia, Sergio Szvalb, Nidal Elkhatib, Shlomo Merchavy
Discover Oncology.2022;[Epub] CrossRef - Morphologic CT and MRI features of primary parotid squamous cell carcinoma and its predictive factors for differential diagnosis with mucoepidermoid carcinoma
Xiaohua Ban, Huijun Hu, Yue Li, Lingjie Yang, Yu Wang, Rong Zhang, Chuanmiao Xie, Cuiping Zhou, Xiaohui Duan
Insights into Imaging.2022;[Epub] CrossRef - A Rare Case of Primary Squamous Cell Carcinoma of the Submandibular Salivary Gland: Brief Overview of Diagnostic Ambiguity and Treatment Challenges
Pawan Hingnikar, Anendd Jadhav, Nitin D Bhola
Cureus.2022;[Epub] CrossRef - Necrotizing Sialometaplasia of the Hard Palate: Diagnosis and
Treatment
Sangeun Lee, Yun Sung Lim, Kyuho Lee, Bo Hae Kim
Journal of Clinical Otolaryngology Head and Neck Surgery.2022; 33(4): 236. CrossRef - Parotid Salivary Duct Carcinoma With a Prominent Squamous Component: Immunohistochemical Profile, Diagnostic Pitfalls, and Therapeutic Implications
Naomi Hardy, Joshua Thompson, Ranee Mehra, Cinthia B. Drachenberg, Kyle Hatten, John C. Papadimitriou
International Journal of Surgical Pathology.2021; 29(7): 726. CrossRef - Intrasalivary Thymic Carcinoma: A Case Report and Literature Review
Michał Kunc, Alexandra Kamieniecki, Grzegorz Walczak, Tomasz Nowicki, Bartosz Wasąg, Bogusław Mikaszewski, Dominik Stodulski, Wojciech Biernat
Head and Neck Pathology.2021; 16(3): 857. CrossRef - Cancer Stem Cell Markers in Squamous Cell Carcinomas of the Salivary Glands
Mattis Bertlich, Julia Kitz, Marie Kruizenga, Jennifer Lee Spiegel, Martin Canis, Friedrich Ihler, Frank Haubner, Bernhard G. Weiss, Mark Jakob
Oncology.2021; 99(6): 402. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


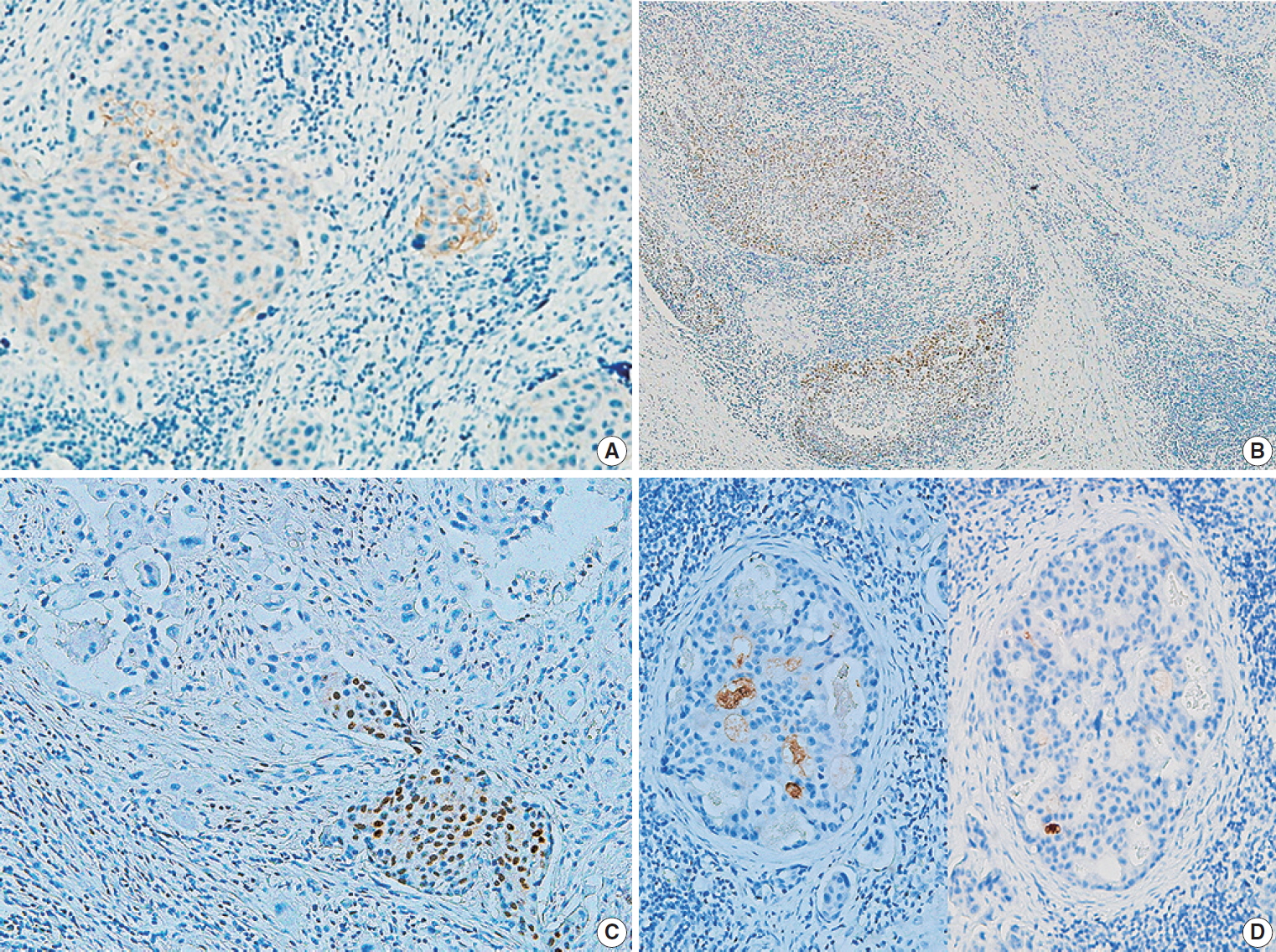
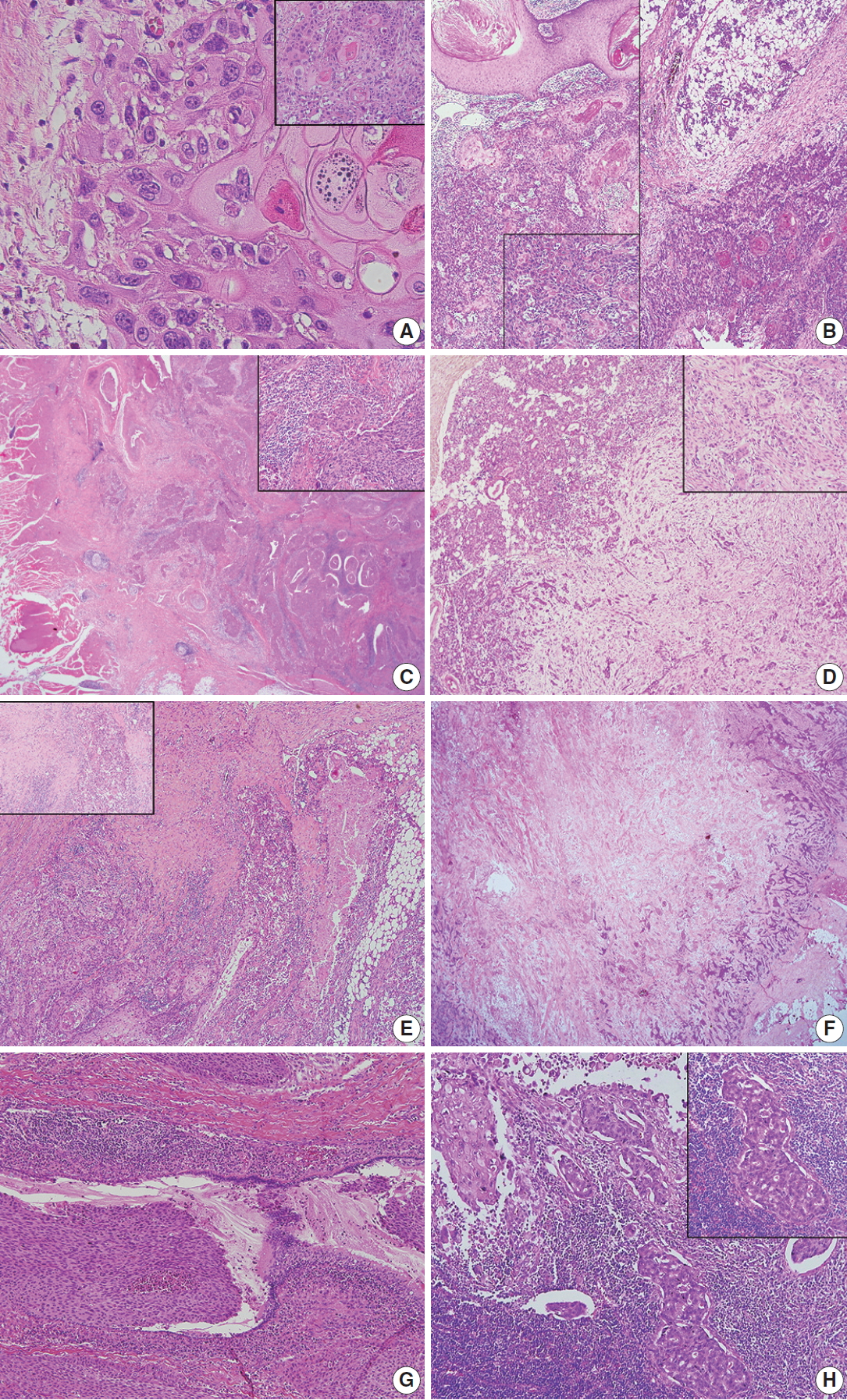
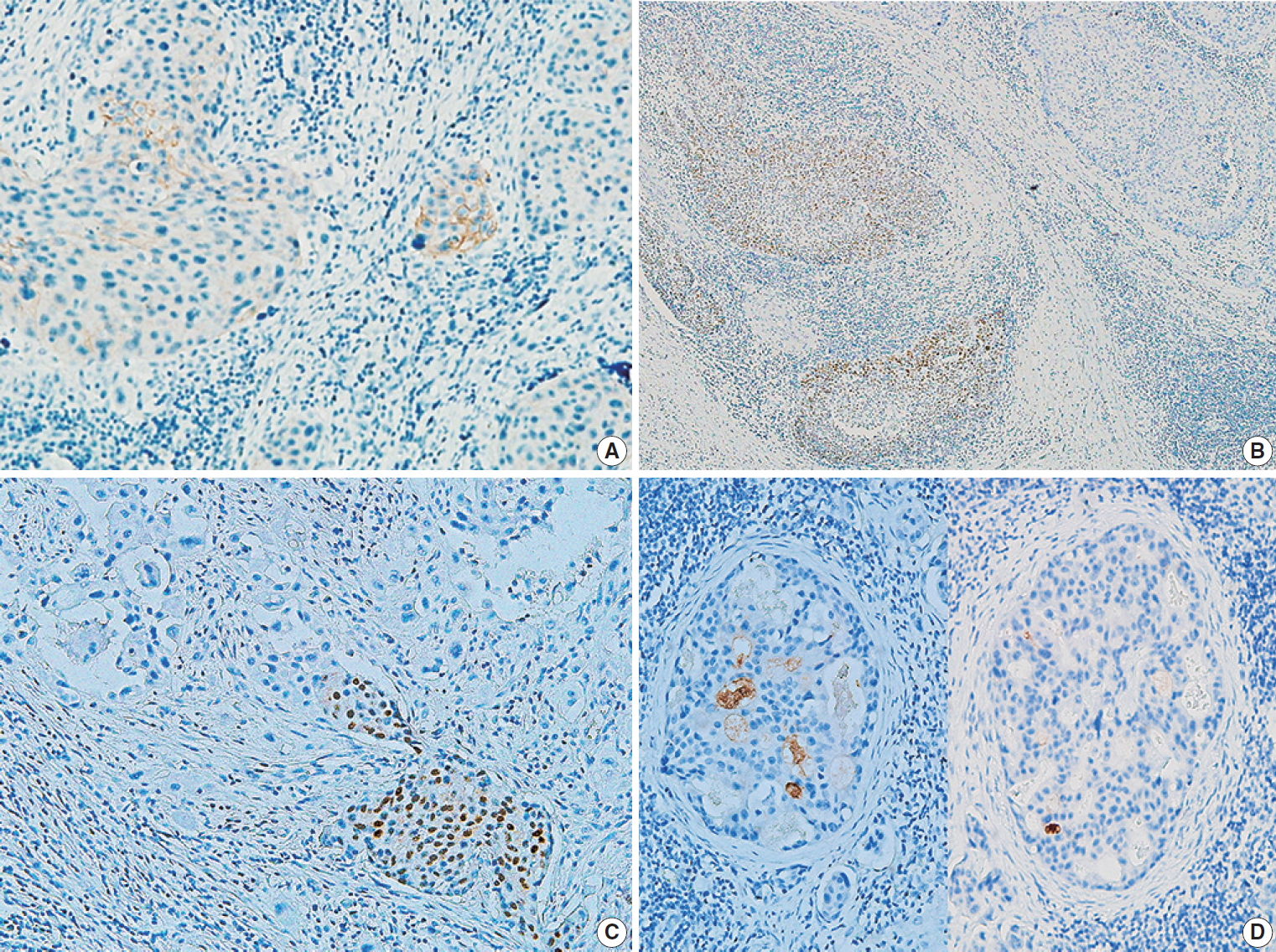
Fig. 1.
Fig. 2.
| Case No. | Sex/Age (yr) | Location of tumor | Therapy | Survival (mo) | Smoking Hx (PY) | Previous radiation Hx |
|---|---|---|---|---|---|---|
| 1 | M/71 | Parotid gland | Parotidectomy and adjuvant RT | Expired (32.2) | 45 | None |
| 2 | M/62 | Parotid gland | Parotidectomy and adjuvant CCRT | Alive (97) | 40 | None |
| 3 | M/58 | Parotid gland | Parotidectomy and adjuvant RT | Expired (21.7) | 30 | None |
| 4 | F/62 | Submandibular gland | Excision | Alive (67) | None | None |
| 5 | M/62 | Parotid gland | Parotidectomy and adjuvant CCRT | Alive (58.7) | 40 | None |
| 6 | M/65 | Parotid gland | Parotidectomy | Alive (48.7) | 30 | None |
| 7 | M/62 | Submandibular gland | Excision and adjuvant CCRT | Expired (11) | 20 | None |
| 8 | F/77 | Submandibular gland | Excision and adjuvant RT | Expired (16.3) | None | None |
| Case No. | Sex/Age | Origin site of SCC | Location of metastatic tumor | Therapy for metastatic SCC | Survival after metastasis (mo) | Smoking Hx (PY) | Radiation Hx for original SCC |
|---|---|---|---|---|---|---|---|
| 9 | M/70 | Cheek skin | Parotid gland | Parotidectomy | Expired (9.8) | 50 | None |
| 10 | F/76 | Preauricular skin | Parotid gland | Parotidectomy and adjuvant RT | Expired (142) | None | None |
| 11 | M/69 | EAC | Parotid gland | Parotidectomy and adjuvant RT | Expired (16.1) | None | None |
| 12 | F/75 | Eyelid | Parotid gland | Parotidectomy and adjuvant RT | Expired (98.9) | None | None |
| 13 | F/57 | Tongue | Parotid gland | Parotidectomy and adjuvant RT | Expired (8.7) | 5 | Present |
| 14 | M/85 | EAC | Parotid gland | Wide excision | Alive (26.7) | 30 | None |
| 15 | M/76 | Cheek skin | Parotid gland | Excision and adjuvant RT | Alive (24.2) | 60 | None |
| 16 | F/45 | Scalp | Parotid gland | Parotidectomy and adjuvant CTx | Alive (24.2) | None | None |
| Primary SCC | Metastatic SCC | |
|---|---|---|
| Keratinization and keratin pearls | Present (6 of 8 cases) | Present (7 of 8 cases) |
| Intercellular bridges with large nuclei and eosinophil cytoplasm | Present | Present |
| Tumor border | Serrated and pointed | Bosselated and expansile |
| Peritumoral inflammation | Abundant | Variable |
| Necrosis | Variable | Central tumor necrosis |
| Desmoplasia | Abundant | Variable |
| Extraparenchymal extension | Present (8 of 8 cases) | Present (7 of 8 cases) |
| Ductal involvement | Present (2 of 8 cases) | Absent |
| Ductal differentiation | Present (1 of 8 cases) | Absent |
| Lymphatic invasion | Abundant (7 of 8 cases) | Variable (4 of 8 cases) |
| Vascular invasiona | Present (1 of 8 cases) | Present (1 of 8 cases) |
| Lymph node metastasis | Present (5 of 8 cases) | Present (3 of 8 cases) |
| Ipsilateral | 4 of 8 cases | 3 of 8 cases |
| Bilateral | 1 of 8 cases | None |
| Primary SCC of salivary gland | Metastatic SCC of salivary gland | |
|---|---|---|
| Staining | ||
| Expression | 8 (100) | 8 (100) |
| p53 | ||
| Positive | 7 (88) | 5 (63) |
| Negative | 1 (12) | 3 (37) |
| p16 | ||
| Positive | 5 (63) | 6 (75) |
| Negative | 3 (37) | 2 (25) |
| Androgen receptor | ||
| Positive | 2 (25) |
0 |
| Negative | 6 (75) | 8 (100) |
| GCDFP-15 | ||
| Positive | 1 (12) | 0 |
| Negative | 7 (88) | 8 (100) |
| c-erb B2 | ||
| Positive | 1 (12) | 0 |
| Negative | 7 (88) | 8 (100) |
| Mucicarmine | ||
| Positive | 0 | 0 |
| Negative | 8 (100) | 8 (100) |
SCC, squamous cell carcinoma; Hx, history; PY, pack years; M, male; RT, radiotherapy; CCRT, combined chemoradiotherapy; F, female.
SCC, squamous cell carcinoma; Hx, history; PY, pack years; M, male; F, female; RT, radiotherapy; EAC, external auditory canal; CTx, chemotherapy.
The case of vascular invasion was concurrently observed with lymphatic invasion in both primary and metastatic squamous cell carcinomas (SCCs).
Values are presented as number (%). SCC, squamous cell carcinoma; GCDFP-15, gross cystic disease fluid protein 15. One of two cases expressing androgen receptor was found in metastatic tumors of primary SCC in lymph nodes.

 E-submission
E-submission