Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 55(1); 2021 > Article
-
Case Study
A case of cribriform carcinoma of the skin: a newly described rare condition -
Hyun Lee1
 , Chong-Hyun Won2
, Chong-Hyun Won2 , Chan-Sik Park1
, Chan-Sik Park1
-
Journal of Pathology and Translational Medicine 2021;55(1):68-74.
DOI: https://doi.org/10.4132/jptm.2020.10.05
Published online: December 3, 2020
1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
2Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Corresponding Author: Chan-Sik Park, MD, PhD, Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea, Tel: +82-2-3010-5838, Fax: +82-2-472-7898, E-mail: csikpark@amc.seoul.kr
© 2021 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Primary cribriform carcinoma of the skin is an indolent, rare, adnexal tumor. Although its malignant potential remains uncertain, no recurrence or metastasis has been reported. A 33-year-old man presented with a solitary, erythematous, subcutaneous nodule on the right knee. The clinical impression was epidermal cyst, and the resected tumor demonstrated a well-circumscribed mass in the dermis and subcutis. The tumor was composed of two regions: a solid component and a cribriform component. The solid component (90%) showed multiple solid nests of epithelial cells. Individual cells had large, oval-to-round, hyperchromatic, pleomorphic nuclei with a nuclear groove. The cribriform component (10%) showed similar neoplastic cells with many prominent lumina. Some lumina had an eosinophilic substance that exhibited a positive periodic acid-Schiff reaction. No recurrence or metastasis was observed within a follow-up period of eight months after excision. In conclusion, we report the first case of primary cribriform carcinoma of the skin in Korea.
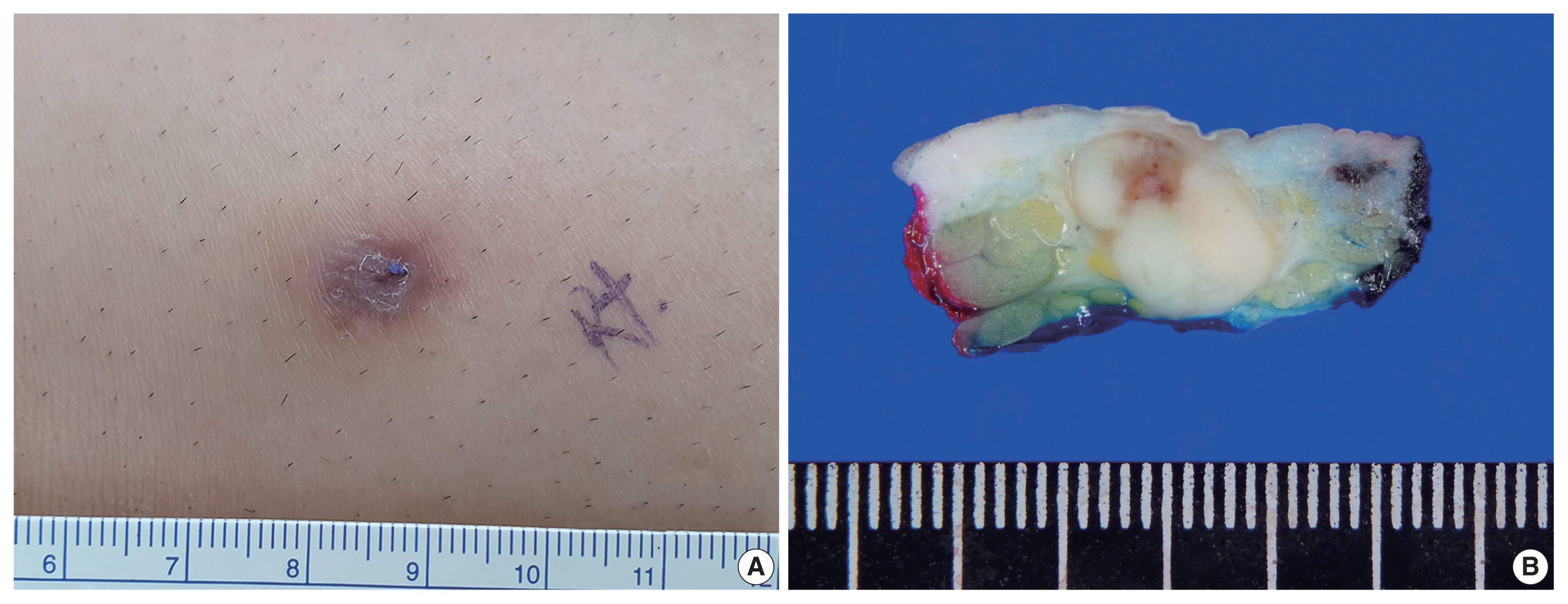
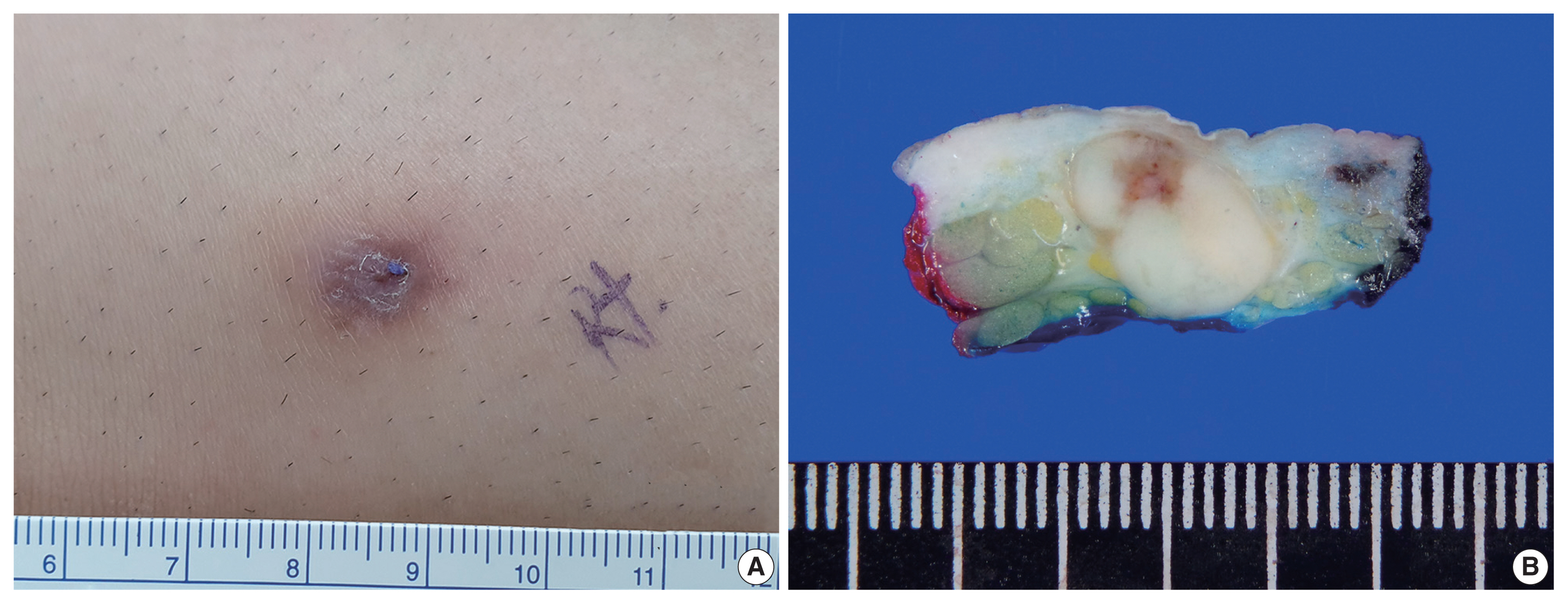
- A 33-year-old man presented with a solitary, erythematous, subcutaneous nodule on the right knee (Fig. 1A), which had developed a few months prior. The clinical impression was epidermal cyst. His past medical history comprised intracranial hemorrhage due to arteriovenous malformation 10 years previous.
- After the patient underwent systemic evaluation, including positron emission tomography–computed tomography (PET-CT) and gastric endoscopy, the mass was confirmed as a primary skin tumor, and resection was performed. The resected tumor was a well-circumscribed, yellowish-white, fibrotic, firm, 2.0 × 1.2 × 0.7-cm mass (Fig. 1B).
- Histologically, the tumor was a well-circumscribed mass of the dermis and subcutis. The tumor was composed of (Fig. 2A) a predominantly solid component (90%) and a predominantly cribriform component (10%). The solid component showed multiple solid nests of epithelial cells. Individual cells had large, oval-to-round, hyperchromatic, pleomorphic nuclei with a nuclear groove (Fig. 2B). The cytoplasm was eosinophilic and scant. The cribriform component showed similar neoplastic cells with many prominent lumina, giving rise to a cribriform pattern with a thin, thread-like, intraluminal bridge (Fig. 2C). Some lumina had an eosinophilic substance that exhibited a positive periodic acid–Schiff reaction (Fig. 2D). At the periphery of the tumor, multifocal lymphoid aggregates (Fig. 2E), desmoplastic reaction, and some infiltrative tumor cell clusters were present (Fig. 2F). The epidermis was neither involved with nor connected to the tumor.
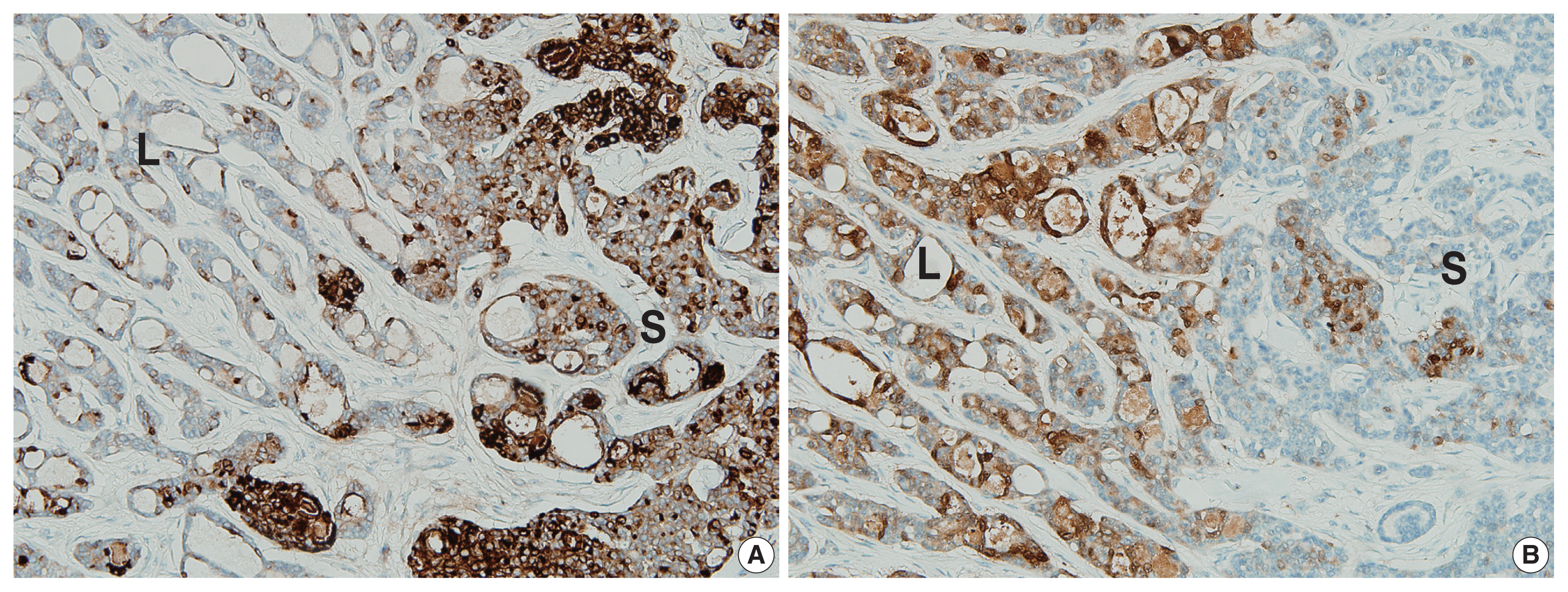
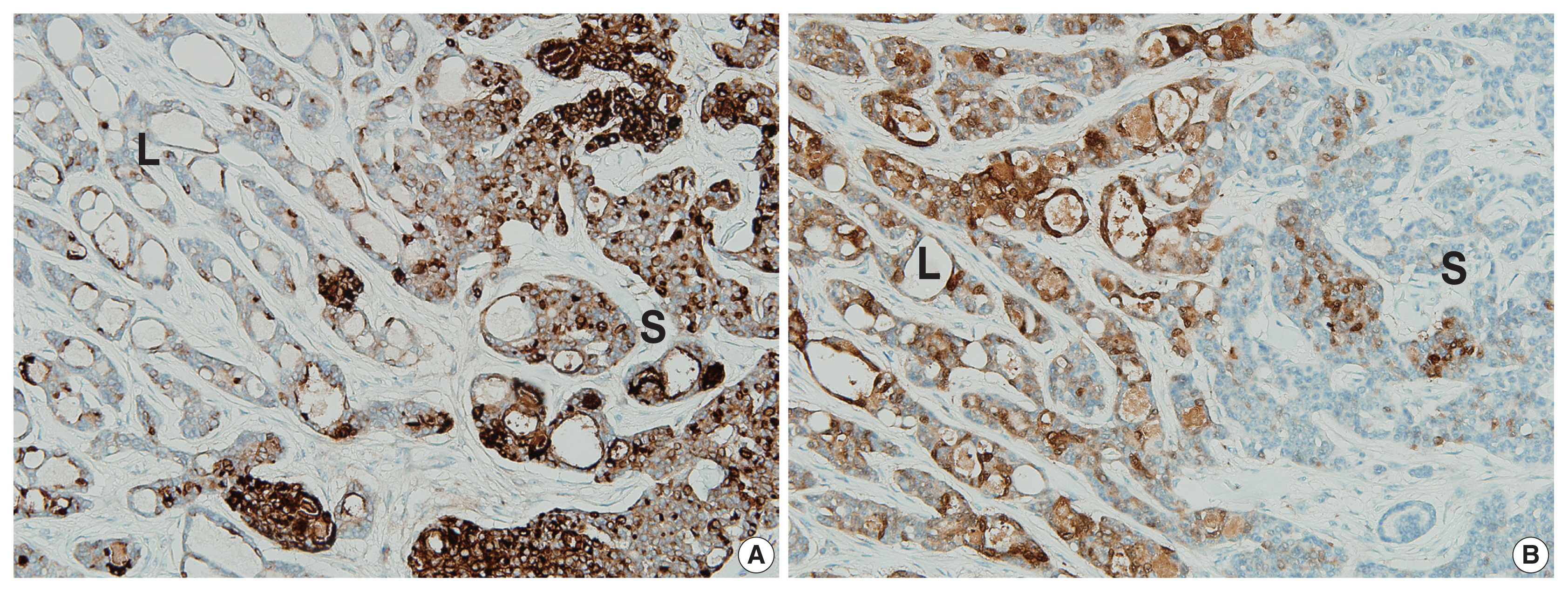
- The two components of the tumor displayed distinctive immunohistochemical staining patterns for epithelial membrane antigen (EMA) (Fig. 3A) and S-100 protein (Fig. 3B). The predominantly solid component exhibited diffuse immunopositivity for EMA and focal immunopositivity for S100 protein, while the predominantly luminal component exhibited focal immunopositivity for EMA and diffuse immunopositivity for S-100 protein.
- Given its histopathologic features, the mass was diagnosed as PCCC. No recurrence or metastasis was observed within a follow-up period of 8 months after excision.
CASE REPORT
- PCCC is a rare, newly described, unique, adnexal neoplasm with an indolent clinical course. Currently, no recurrence or metastasis has been reported. In few cases, remnants have been reported after incomplete excision [1,3]. Accurate diagnosis and exclusion of metastasis are important for avoiding over-treatment.
- In cases of primary skin neoplasms, the differential diagnosis should include tumors that can show a cribriform pattern: adenoid cystic carcinoma, secretory carcinoma, and tubular adenoma (eccrine papillary adenoma). The histopathologic features for differential diagnoses are listed in Table 2. Adenoid cystic carcinoma can be distinguished by the presence of basaloid epithelial cells with more uniform nuclei surrounding the pseudolumina. The presence of frequent perineural invasion and small true ducts with myoepithelial cell differentiation are points of differential diagnosis. Secretory carcinoma exhibits tubules and microcysts with conspicuous intraluminal secretions, but back-to-back proliferation and cuboidal neoplastic cells are characteristic compared with PCCC. Tubular adenoma can show dilated cystic spaces with attenuated epithelium, micro-papillae, and focal intraluminal bridging, mimicking PCCC. However, this condition lacks cytologic atypia and mitotic activity and involves accumulation of basal/myoepithelial cells.
- Histopathologically, metastatic tumors that show a cribriform pattern shold be excluded. A cribriform pattern can be seen in cancers of various organs, including the breast (adenoid cystic carcinoma and cribriform adenocarcinoma), prostate (ductal carcinoma and acinar carcinoma), stomach, colon, lung, thyroid (cribriform-morular variant of papillary thyroid carcinoma), uterine endometrium, and salivary gland [8–10]. To exclude metastasis, imaging studies, such as PET-CT and CT, and immunohistochemical staining are required.
- Immunohistochemical staining results are listed in Table 1. Although decapitation secretion in the luminal border supports apocrine differentiation, gross cystic disease fluid protein-15, a marker for the apocrine gland, was negative in previous reports [1–7] and in our case. The S-100 protein, a marker for the eccrine gland, demonstrated variable results (diffuse positive, focal positive, and negative) in previous reports [1–7]. Our case showed more prominent S-100 protein in the luminal component. EMA was positive in previous reports [1–7]. Rutten et al. [2] reported more prominent EMA in the luminal structures, while our case showed more prominent EMA in the solid component.
- The relatively mutually exclusive immunohistochemical staining patterns of EMA and S-100 protein may be associated with architectural differentiation, and further research is needed.
- In conclusion, we report the first case of cribriform carcinoma of the skin in Korea. Pathologists should be aware of cribriform carcinoma of the skin to avoid over-treatment.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board of Asan Medical Center (IRB 2020-0364). Formal written informed consent was not required, with a waiver from the appropriate Institutional Review Board.
Author Contributions
Conceptualization: CSP. Data curation: HL. Formal analysis: HL. Funding acquisition: CSP. Investigation: CSP. Methodology: HL. Resources: WCH. Supervision: CSP. Validation: CSP, HL. Writing—original draft: HL. Writing— review & editing: CSP. Approval of final manuscript: all authors.
Conflicts of Interest
CSP, a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.

| Case No. | Study | Year | Age (yr) | Sex | Site | Clinical diagnosis | Size (cm) | Treatment | F/U period | Prognosis | Positive IHC | Negative IHC | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Current case | 2020 | 33 | M | Lower extremity (Rt. knee) | Epidermal cyst | 2.0 | Excision | 8 mo | NROMD | S100 (diffuse, luminal component; patchy, solid component), EMA (patchy, luminal component; diffuse, solid component), CK7, CK5/6 (patchy), CD117 (patchy), EpCAM (patchy), CEA (intra-luminal), p63 (rare) | CK20, GCDFP-15, ER, calponin, SMA |
History of intracranial hemorrhage due to arteriovenous malformation No evidence of other malignancies |
| 2 | Requena et al. [1] | 1998 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA |
First description Reported as mainly women with a mean age of 44 years (range, 20 to 55) Two cases of recurrent tumor after incomplete excision |
| 3 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 4 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 5 | NA | NA | Upper extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 6 | NA | NA | Pubis | NA | NA | Excision | NA | NA | NA | NA | |||
| 7 | Adamski et al. [5] | 2005 | 37 | M | Lower extremity (Lt. popliteal fossa) | NA | 2.0 | Excision | 2 yr | NROMD | CK7 | CK20, GCDFP-15, S100 | - |
| 8 | Fernandez-Flores et al. [4] | 2007 | 62 | F | Lower extremity (Lt. popliteal fossa) | NA | 0.8 | Excision | NA | NROMD | CK AE1/AE3, CAM 5.2, CK7, EMA, ER (2+, 1%–5% cells), c-erbB-2 (2+, 50%–75% cells), p53 (1+, 1%–5% cells), S100 (2+, 50%–75% cells) | CK20, CEA, PR, GCDFP-15, CD15, SMA | - |
| 9 | Rutten et al. [2] | 2009 | 48 | M | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 18 yr | NROMD | CK MNF116, CK AE1/AE3, CAM 5.2, CK7, CEA (more prominent in the ductal structures), EMA (more prominent in the ductal structures) | CK20, GCDFP-15, S100, α-SMA, MSA, calponin, CD68, vimentin | - |
| 10 | 51 | F | Upper extremity (Rt. forearm) | Cyst | - | Excision | NA | NA | - | ||||
| 11 | 44 | F | Back | Dermatofibroma (histiocytoma) | - | Excision | 13 yr | NROMD | - | ||||
| 12 | 77 | F | Neck | NA | - | Excision | NA | NA | Recurrent tumor after incomplete excision | ||||
| 13 | 32 | F | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 9 yr | NROMD | - | ||||
| 14 | 42 | F | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 15 | 34 | F | Upper extremity (Rt. arm) | Dermatofibroma (histiocytoma) | - | Excision | 11 yr | NROMD | - | ||||
| 16 | 40 | M | Upper extremity (Lt. forearm) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 17 | 23 | M | Lower extremity (Lt. calf) | NA | - | Excision | NA | NA | - | ||||
| 18 | 20 | F | Rt. buttock | Cyst | - | Excision | NA | NA | No evidence of other malignancies | ||||
| 19 | 67 | F | Lt. preauricular area | BCC vs. adnexal tumor | - | Excision | 8 yr | NROMD | - | ||||
| 20 | 60 | M | Lower extremity (Rt. foot dorsum) | Dermatofibroma (histiocytoma) | - | Excision | 4 yr | NROMD | - | ||||
| 21 | 54 | F | Upper extremity (acral) | NA | - | Excision | NA | NA | - | ||||
| 22 | 49 | F | Lower extremity (Lt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 4 yr | NROMD | - | ||||
| 23 | 59 | F | Lt. shoulder | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 24 | 28 | M | Lower extremity (Rt. lower leg) | NA | - | Excision | NA | NA | - | ||||
| 25 | 54 | M | Upper extremity (Rt. hand) | NA | - | Excision | NA | NA | - | ||||
| 26 | 50 | F | Upper extremity (Rt. hand) | NA | - | Excision | NA | NA | - | ||||
| 27 | 70 | M | Rt. trunk | Long-standing lesion | - | Excision | 6 yr | NROMD | - | ||||
| 28 | 64 | F | NA | Dermatofibroma (histiocytoma) | - | Excision | 5 yr | NROMD | - | ||||
| 29 | 40 | F | Lower extremity (Lower arm) | NA | - | Excision | 5 yr | NROMD | - | ||||
| 30 | 64 | M | Upper back | Dermatofibroma (histiocytoma) or cyst | - | Excision | 3 yr | NROMD | - | ||||
| 31 | 54 | M | Lower extremity (Lower arm) | NA | - | Excision | 3 yr | NROMD | - | ||||
| 32 | 44 | F | Upper extremity (Lt. thumb) | NA | - | Excision | 2 yr | NROMD | - | ||||
| 33 | 36 | F | Lower extremity (Lt. anterior thigh) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 34 | 26 | F | Lower extremity (Lt. posterior leg) | Fibroma | - | Excision | NA | NA | - | ||||
| 35 | Arps et al. [3] | 2015 | 41 | F | Lower extremity (leg) | Epidermal inclusion cyst | 0.6 | Excision | NA | NROMD | S100 (diffuse in three cases, patchy in one case), CD117 (diffuse in two cases, patchy in one case), CK5/6, CK7, EpCAM, CEA (luminal), EMA (luminal), p63 (rare) | CK20, GCDFP-15, ER, PR, calponin, SMA | - |
| 36 | 32 | F | Upper extremity (elbow) | NA | 0.5 | Excision | NA | NA | - | ||||
| 37 | 35 | M | Lower extremity (leg) | NA | 0.5 | Excision | NA | NROMD | - | ||||
| 38 | 59 | F | Upper extremity (arm) | NA | 0.4 | Excision | NA | NA | - | ||||
| 39 | 61 | M | Upper extremity (arm) | Mobile nodule | 0.7 | Excision | NA | NA | - | ||||
| 40 | 31 | M | Lower extremity (leg) | Dermatofibroma | 1.2 | Excision | NA | NA | - | ||||
| 41 | Yokota et al. [7] | 2017 | 39 | F | Upper extremity (Rt. forearm) | NA | 0.5 (clinical) | Excision | 15 mo | NROMD | CK5/6, CK7, CA15-3, CA125, CD117, S100 (partially), p53 (partially), p63 (partially) | CK20, calponin, GCDFP-15, mammaglobulin, MUC1, ER, AR, D2–40 | Stable in size for more than 10 years, no evidence of other malignancies |
| 42 | Bogner et al. [6] | 2018 | 65 | M | Lt. lateral neck | NA | 0.6 (clinical) | Excision | 3 mo | NROMD | CK7 (strong), EMA (strong), CAM 5.2 (strong), EpCAM (lesser degree), CEA (in some of the lumina), p63, p40 | CK20, D2–40, TTF-1, CDX-2, hepatocyte antigen, PSA, PSAP, calponin, S100 | No evidence of other malignancies |
F/U, follow up; IHC, immunohistochemical staining; Rt., right; Lt., left; NROMD, no recurrence or metastatic disease; EMA, epithelial membrane antigen; CK, cytokeratin; EpCAM, epithelial cell adhesion molecule; CEA, carcinoembryonic antigen; GCDFP-15, gross cystic disease fluid protein-15; ER, estrogen receptor; SMA, smooth muscle actin; MSA, muscle specific antigen; NA, data not available; S100, S-100 protein; PR, progesterone receptor; BCC, basal cell carcinoma; MUC1, mucin1; AR, androgen receptor; TTF-1, thyroid transcription factor 1; PSA, prostate-specific antigen; PSAP, prostatic acid phosphatase; +, >50% of tumor cells are positive; patchy, 25%–50% of tumor cells are positive; rare, < 1% of tumor cells are positive.
| Cribriform carcinoma | Adenoid cystic carcinoma | Secretory carcinoma | Tubular adenoma | |
|---|---|---|---|---|
| Architecture |
Usually well-circumscribed Mixed variable portion of solid and cribriform |
Poorly circumscribed Composed of lobules, islands, and cords of basaloid cells with numerous cystic and ductular spaces |
Intradermal, circumscribed Back-to-back proliferation of tubules and microcysts |
Well circumscribed Variable sized tubules with attenuated epithelium |
| No back-to-back appearance | Cuboidal cells | Micro-papillae, and focal intraluminal bridging | ||
| No cuboidal cells | Sclerotic stroma | Paucicellular fibrous stroma | ||
| Desmoplastic stroma | Recognition of myoepithelial layer | |||
| Intra-(pseudo) luminal substance | Eosinophilic substance with PAS reaction | Mucin or basement membrane material that stains with mucicarmine, Alcian blue, and colloidal iron | Conspicuous intraluminal secretions | Eosinophilic proteinaceous material |
| Nuclei | Pleomorphic | Uniform | Mildly pleomorphic | Uniform |
| Mitosis | Rare | Rare | Rare to few | Absent |
| Perineural invasion | Absent | Present, frequent | Absent | Absent |
| Immunohistochemical staining | Variable CK (MNF116, AE1/AE3, CAM5.2, and CK7) | EMA and monoclonal CEA | S100 protein, mammaglobin and STAT5A | HMFG-1 and GCDFP-15 |
| EpCAM | S100, p63, GFAP, SMA, MSA and calponin: often stain peripheral cells (myoepithelial differentiation) | NTRK3: variable | EMA and CEA: luminal cells | |
| CD117, S100, and p63: variable | S100 and SMA: myoepithelial cells | |||
| CEA, EMA: highlight ductal component | ||||
| Reference | [11,12] | [12,13] | [12,14] | [3,12,13] |
PAS, periodic acid-Schiff; CK, cytokeratin; EMA, epithelial membrane antigen; CEA, carcinoembryonic antigen; S100, S-100 protein; GCDFP-15, gross cystic disease fluid protein-15; EpCAM, epithelial cell adhesion molecule; GFAP, glial fibrillary acidic protein; SMA, smooth muscle actin; MSA, muscle specific antigen.
- 1. Requena L, Kiryu H, Ackerman AB. Neoplasms with apocrine differentiation. Philadelphia: Lippincott-Raven, 1988; 879-905.
- 2. Rutten A, Kutzner H, Mentzel T, et al. Primary cutaneous cribriform apocrine carcinoma: a clinicopathologic and immunohistochemical study of 26 cases of an under-recognized cutaneous adnexal neoplasm. J Am Acad Dermatol 2009; 61: 644-51. ArticlePubMed
- 3. Arps DP, Chan MP, Patel RM, Andea AA. Primary cutaneous cribriform carcinoma: report of six cases with clinicopathologic data and immunohistochemical profile. J Cutan Pathol 2015; 42: 379-87. ArticlePubMed
- 4. Fernandez-Flores A, Pol A, Juanes F, Crespo LG. Immunohistochemical phenotype of cutaneous cribriform carcinoma with a panel of 15 antibodies. Med Mol Morphol 2007; 40: 212-7. ArticlePubMedPDF
- 5. Adamski H, Le Lan J, Chevrier S, Cribier B, Watier E, Chevrant-Breton J. Primary cutaneous cribriform carcinoma: a rare apocrine tumour. J Cutan Pathol 2005; 32: 577-80. ArticlePubMed
- 6. Bogner R, Brown T, Fearneyhough P, Gataky G. Primary cutaneous cribriform carcinoma treated with mohs micrographic surgery. Dermatol Surg 2018; 44: 583-5. ArticlePubMed
- 7. Yokota K, Kono M, Mori S, Shimizu K, Matsumoto T, Akiyama M. A solid variant of primary cutaneous cribriform carcinoma: a small, stable, long-term lesion. Eur J Dermatol 2017; 27: 419-21. ArticlePubMed
- 8. Branca G, Ieni A, Barresi V, Tuccari G, Caruso RA. An updated review of cribriform carcinomas with emphasis on histopathological diagnosis and prognostic significance. Oncol Rev 2017; 11: 317.ArticlePubMedPMCPDF
- 9. Page DL, Dixon JM, Anderson TJ, Lee D, Stewart HJ. Invasive cribriform carcinoma of the breast. Histopathology 1983; 7: 525-36. ArticlePubMed
- 10. McNeal JE, Reese JH, Redwine EA, Freiha FS, Stamey TA. Cribriform adenocarcinoma of the prostate. Cancer 1986; 58: 1714-9. ArticlePubMed
- 11. Requena L, Sangueza O. Cribriform carcinoma. In: Requena L, Sangueza O, eds. Cutaneous adnexal neoplasms. Cham: Springer, 2017; 313-20. Article
- 12. Elder DE, Massi D, Scolyer RA, Willemze R. WHO classification of skin tumours. Lyon: IARC Press, 2018.
- 13. Cassario D. Diagnostic pathology: neoplastic dermatopathology. Salt Lake City: Amirsys Publishing Inc, 2012.
- 14. Llamas-Velasco M, Mentzel T, Rutten A. Primary cutaneous secretory carcinoma: a previously overlooked low-grade sweat gland carcinoma. J Cutan Pathol 2018; 45: 240-5. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Primary cutaneous cribriform tumor: A case report and literature review
Doukou Jiang, Yongzhen Tian, Jiabin Tian, Hui Liu, Yang Guan
Journal of Cutaneous Pathology.2025; 52(1): 9. CrossRef - Primary cutaneous cribriform tumor in uncommon locations
Ting-Ting Cheng, Yu Yu
Dermatologica Sinica.2025;[Epub] CrossRef - Mohs micrographic surgery for the management of primary cutaneous cribriform carcinoma of the back
Min Jae Kim, Je‐Ho Mun
JDDG: Journal der Deutschen Dermatologischen Gesellschaft.2024; 22(4): 584. CrossRef - Mikrographische Chirurgie nach Mohs bei einem primär kutanen kribriformen Karzinom am Rücken
Min Jae Kim, Je‐Ho Mun
JDDG: Journal der Deutschen Dermatologischen Gesellschaft.2024; 22(4): 584. CrossRef - Rare skin appendage tumour on the right leg: a case of primary cutaneous cribriform carcinoma
Kashini Andrew, James M Carr, Claudia Roberts
BMJ Case Reports.2023; 16(5): e254781. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1
Fig. 2
Fig. 3
| Case No. | Study | Year | Age (yr) | Sex | Site | Clinical diagnosis | Size (cm) | Treatment | F/U period | Prognosis | Positive IHC | Negative IHC | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Current case | 2020 | 33 | M | Lower extremity (Rt. knee) | Epidermal cyst | 2.0 | Excision | 8 mo | NROMD | S100 (diffuse, luminal component; patchy, solid component), EMA (patchy, luminal component; diffuse, solid component), CK7, CK5/6 (patchy), CD117 (patchy), EpCAM (patchy), CEA (intra-luminal), p63 (rare) | CK20, GCDFP-15, ER, calponin, SMA | History of intracranial hemorrhage due to arteriovenous malformation No evidence of other malignancies |
| 2 | Requena et al. [ |
1998 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA | First description Reported as mainly women with a mean age of 44 years (range, 20 to 55) Two cases of recurrent tumor after incomplete excision |
| 3 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 4 | NA | NA | Lower extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 5 | NA | NA | Upper extremity | NA | NA | Excision | NA | NA | NA | NA | |||
| 6 | NA | NA | Pubis | NA | NA | Excision | NA | NA | NA | NA | |||
| 7 | Adamski et al. [ |
2005 | 37 | M | Lower extremity (Lt. popliteal fossa) | NA | 2.0 | Excision | 2 yr | NROMD | CK7 | CK20, GCDFP-15, S100 | - |
| 8 | Fernandez-Flores et al. [ |
2007 | 62 | F | Lower extremity (Lt. popliteal fossa) | NA | 0.8 | Excision | NA | NROMD | CK AE1/AE3, CAM 5.2, CK7, EMA, ER (2+, 1%–5% cells), c-erbB-2 (2+, 50%–75% cells), p53 (1+, 1%–5% cells), S100 (2+, 50%–75% cells) | CK20, CEA, PR, GCDFP-15, CD15, SMA | - |
| 9 | Rutten et al. [ |
2009 | 48 | M | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 18 yr | NROMD | CK MNF116, CK AE1/AE3, CAM 5.2, CK7, CEA (more prominent in the ductal structures), EMA (more prominent in the ductal structures) | CK20, GCDFP-15, S100, α-SMA, MSA, calponin, CD68, vimentin | - |
| 10 | 51 | F | Upper extremity (Rt. forearm) | Cyst | - | Excision | NA | NA | - | ||||
| 11 | 44 | F | Back | Dermatofibroma (histiocytoma) | - | Excision | 13 yr | NROMD | - | ||||
| 12 | 77 | F | Neck | NA | - | Excision | NA | NA | Recurrent tumor after incomplete excision | ||||
| 13 | 32 | F | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 9 yr | NROMD | - | ||||
| 14 | 42 | F | Lower extremity (Rt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 15 | 34 | F | Upper extremity (Rt. arm) | Dermatofibroma (histiocytoma) | - | Excision | 11 yr | NROMD | - | ||||
| 16 | 40 | M | Upper extremity (Lt. forearm) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 17 | 23 | M | Lower extremity (Lt. calf) | NA | - | Excision | NA | NA | - | ||||
| 18 | 20 | F | Rt. buttock | Cyst | - | Excision | NA | NA | No evidence of other malignancies | ||||
| 19 | 67 | F | Lt. preauricular area | BCC vs. adnexal tumor | - | Excision | 8 yr | NROMD | - | ||||
| 20 | 60 | M | Lower extremity (Rt. foot dorsum) | Dermatofibroma (histiocytoma) | - | Excision | 4 yr | NROMD | - | ||||
| 21 | 54 | F | Upper extremity (acral) | NA | - | Excision | NA | NA | - | ||||
| 22 | 49 | F | Lower extremity (Lt. thigh) | Dermatofibroma (histiocytoma) | - | Excision | 4 yr | NROMD | - | ||||
| 23 | 59 | F | Lt. shoulder | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 24 | 28 | M | Lower extremity (Rt. lower leg) | NA | - | Excision | NA | NA | - | ||||
| 25 | 54 | M | Upper extremity (Rt. hand) | NA | - | Excision | NA | NA | - | ||||
| 26 | 50 | F | Upper extremity (Rt. hand) | NA | - | Excision | NA | NA | - | ||||
| 27 | 70 | M | Rt. trunk | Long-standing lesion | - | Excision | 6 yr | NROMD | - | ||||
| 28 | 64 | F | NA | Dermatofibroma (histiocytoma) | - | Excision | 5 yr | NROMD | - | ||||
| 29 | 40 | F | Lower extremity (Lower arm) | NA | - | Excision | 5 yr | NROMD | - | ||||
| 30 | 64 | M | Upper back | Dermatofibroma (histiocytoma) or cyst | - | Excision | 3 yr | NROMD | - | ||||
| 31 | 54 | M | Lower extremity (Lower arm) | NA | - | Excision | 3 yr | NROMD | - | ||||
| 32 | 44 | F | Upper extremity (Lt. thumb) | NA | - | Excision | 2 yr | NROMD | - | ||||
| 33 | 36 | F | Lower extremity (Lt. anterior thigh) | Dermatofibroma (histiocytoma) | - | Excision | NA | NA | - | ||||
| 34 | 26 | F | Lower extremity (Lt. posterior leg) | Fibroma | - | Excision | NA | NA | - | ||||
| 35 | Arps et al. [ |
2015 | 41 | F | Lower extremity (leg) | Epidermal inclusion cyst | 0.6 | Excision | NA | NROMD | S100 (diffuse in three cases, patchy in one case), CD117 (diffuse in two cases, patchy in one case), CK5/6, CK7, EpCAM, CEA (luminal), EMA (luminal), p63 (rare) | CK20, GCDFP-15, ER, PR, calponin, SMA | - |
| 36 | 32 | F | Upper extremity (elbow) | NA | 0.5 | Excision | NA | NA | - | ||||
| 37 | 35 | M | Lower extremity (leg) | NA | 0.5 | Excision | NA | NROMD | - | ||||
| 38 | 59 | F | Upper extremity (arm) | NA | 0.4 | Excision | NA | NA | - | ||||
| 39 | 61 | M | Upper extremity (arm) | Mobile nodule | 0.7 | Excision | NA | NA | - | ||||
| 40 | 31 | M | Lower extremity (leg) | Dermatofibroma | 1.2 | Excision | NA | NA | - | ||||
| 41 | Yokota et al. [ |
2017 | 39 | F | Upper extremity (Rt. forearm) | NA | 0.5 (clinical) | Excision | 15 mo | NROMD | CK5/6, CK7, CA15-3, CA125, CD117, S100 (partially), p53 (partially), p63 (partially) | CK20, calponin, GCDFP-15, mammaglobulin, MUC1, ER, AR, D2–40 | Stable in size for more than 10 years, no evidence of other malignancies |
| 42 | Bogner et al. [ |
2018 | 65 | M | Lt. lateral neck | NA | 0.6 (clinical) | Excision | 3 mo | NROMD | CK7 (strong), EMA (strong), CAM 5.2 (strong), EpCAM (lesser degree), CEA (in some of the lumina), p63, p40 | CK20, D2–40, TTF-1, CDX-2, hepatocyte antigen, PSA, PSAP, calponin, S100 | No evidence of other malignancies |
| Cribriform carcinoma | Adenoid cystic carcinoma | Secretory carcinoma | Tubular adenoma | |
|---|---|---|---|---|
| Architecture | Usually well-circumscribed Mixed variable portion of solid and cribriform |
Poorly circumscribed Composed of lobules, islands, and cords of basaloid cells with numerous cystic and ductular spaces |
Intradermal, circumscribed Back-to-back proliferation of tubules and microcysts |
Well circumscribed Variable sized tubules with attenuated epithelium |
| No back-to-back appearance | Cuboidal cells | Micro-papillae, and focal intraluminal bridging | ||
| No cuboidal cells | Sclerotic stroma | Paucicellular fibrous stroma | ||
| Desmoplastic stroma | Recognition of myoepithelial layer | |||
| Intra-(pseudo) luminal substance | Eosinophilic substance with PAS reaction | Mucin or basement membrane material that stains with mucicarmine, Alcian blue, and colloidal iron | Conspicuous intraluminal secretions | Eosinophilic proteinaceous material |
| Nuclei | Pleomorphic | Uniform | Mildly pleomorphic | Uniform |
| Mitosis | Rare | Rare | Rare to few | Absent |
| Perineural invasion | Absent | Present, frequent | Absent | Absent |
| Immunohistochemical staining | Variable CK (MNF116, AE1/AE3, CAM5.2, and CK7) | EMA and monoclonal CEA | S100 protein, mammaglobin and STAT5A | HMFG-1 and GCDFP-15 |
| EpCAM | S100, p63, GFAP, SMA, MSA and calponin: often stain peripheral cells (myoepithelial differentiation) | NTRK3: variable | EMA and CEA: luminal cells | |
| CD117, S100, and p63: variable | S100 and SMA: myoepithelial cells | |||
| CEA, EMA: highlight ductal component | ||||
| Reference | [ |
[ |
[ |
[ |
F/U, follow up; IHC, immunohistochemical staining; Rt., right; Lt., left; NROMD, no recurrence or metastatic disease; EMA, epithelial membrane antigen; CK, cytokeratin; EpCAM, epithelial cell adhesion molecule; CEA, carcinoembryonic antigen; GCDFP-15, gross cystic disease fluid protein-15; ER, estrogen receptor; SMA, smooth muscle actin; MSA, muscle specific antigen; NA, data not available; S100, S-100 protein; PR, progesterone receptor; BCC, basal cell carcinoma; MUC1, mucin1; AR, androgen receptor; TTF-1, thyroid transcription factor 1; PSA, prostate-specific antigen; PSAP, prostatic acid phosphatase; +, >50% of tumor cells are positive; patchy, 25%–50% of tumor cells are positive; rare, < 1% of tumor cells are positive.
PAS, periodic acid-Schiff; CK, cytokeratin; EMA, epithelial membrane antigen; CEA, carcinoembryonic antigen; S100, S-100 protein; GCDFP-15, gross cystic disease fluid protein-15; EpCAM, epithelial cell adhesion molecule; GFAP, glial fibrillary acidic protein; SMA, smooth muscle actin; MSA, muscle specific antigen.

 E-submission
E-submission