Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 55(1); 2021 > Article
-
Original Article
Interobserver diagnostic reproducibility in advanced-stage endometrial carcinoma -
Ho Jin Jung1
 , Soo Yeon Lee1
, Soo Yeon Lee1 , Jin Hwa Hong2
, Jin Hwa Hong2 , Yi Kyeong Chun1
, Yi Kyeong Chun1
-
Journal of Pathology and Translational Medicine 2021;55(1):43-52.
DOI: https://doi.org/10.4132/jptm.2020.10.04
Published online: December 3, 2020
1Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
2Department of Obstetrics and Gynecology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- Corresponding Author: Yi Kyeong Chun, MD, Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 08308, Korea, Tel: +82-2-2626-1472, Fax: +82-2-2626-1486, E-mail: ykcmd@naver.com
© 2021 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- The accurate pathologic diagnosis and subtyping of high-grade endometrial carcinoma are often problematic, due to its atypical and overlapping histopathological features.
-
Methods
- Three pathologists reviewed 21 surgically resected cases of advanced-stage endometrial carcinoma. The primary diagnosis was based only on hematoxylin and eosin stained slides. When a discrepancy arose, a secondary diagnosis was made by additional review of immunohistochemical (IHC) stains. Finally, three pathologists discussed all cases and rendered a consensus diagnosis.
-
Results
- The primary diagnoses were identical in 13/21 cases (62%). The secondary diagnosis based on the addition of IHC results was concordant in four of eight discrepant cases. Among four cases with discrepancies occurring in this step, two cases subsequently reached a consensus diagnosis after a thorough discussion between three reviewers. Next-generation sequencing (NGS) study was performed in two cases in which it was difficult to distinguish between serous carcinoma and endometrioid carcinoma. Based on the sequencing results, a final diagnosis of serous carcinoma was rendered. The overall kappa for concordance between the original and consensus diagnosis was 0.566 (moderate agreement).
-
Conclusions
- We investigated stepwise changes in interobserver diagnostic reproducibility in advanced-stage endometrial carcinoma. We demonstrated the utility of IHC and NGS study results in the histopathological diagnosis of advanced-stage endometrial carcinoma.
- Patients and cases
- Twenty-one patients with high International Federation of Gynecology and Obstetrics (FIGO) stage (stage III or IV) endometrial carcinoma who had undergone total hysterectomy in Korea University Guro Hospital (KUGH) between 2008 and 2017 were included in this study. A number of general pathologists performed the original diagnosis. Three pathologists (Y.K.C., S.Y.L., and H.J.J.) reviewed the cases; Y.K.C. is a gynecology specialty pathologist, S.Y.L. is a board-certified general pathologist, and H.J.J. is a fourth-year anatomic pathology resident.
- Three-step assessment of interobserver reproducibility
- The diagnostic process proceeded as follows. First, three reviewers reached a primary diagnosis based on the H&E-stained slides. Each case was diagnosed based on the 2014 WHO Classification of Tumors of Female Reproductive Organs [3], and was categorized into one of the following entities: endometrioid carcinoma, serous carcinoma, mucinous carcinoma, clear cell carcinoma, undifferentiated/dedifferentiated carcinoma, carcinosarcoma, and mixed carcinoma. Cases diagnosed as endometrioid carcinoma were classified as low-grade (FIGO grade 1 and 2) or high-grade (FIGO grade 3).
- In cases with discrepant original and primary diagnoses, reviewers reached a secondary diagnosis based on the H&E-stained and IHC-stained slides. Finally, all three pathologists conducted a discussion and reached a consensus diagnosis. If the discussion did not lead to a consensus diagnosis, NGS analysis was performed.
- Concordance between the original and consensus diagnoses was calculated using the kappa statistics. A kappa value of 0.4 indicates poor agreement, 0.4–0.6 indicates moderate agreement, 0.6–0.8 indicates substantial/good agreement, and 0.8–1.0 indicates near perfect/ excellent agreement. The Windows version of IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
- IHC staining and interpretation
- IHC staining was performed on whole slide sections using two automated staining systems, including a Bond-III autostainer (Leica Biosystems, Wetzlar, Germany) and a Bench-Mark ULTRA system (Ventana Medical Systems, Tucson, AZ, USA). In brief, 4-μm-thick paraffin-embedded tissue sections were deparaffinized and rehydrated across a graded series of ethyl alcohol concentrations. Heat-induced antigen retrieval was carried out in citrate buffer. Sections were incubated with the primary antibody in an automated immunostainer. Counterstaining with hematoxylin was performed. The antibodies used were as follows: anti-p53 (1:200, DO-7, Novocastra, Newcastle, UK), anti-ER (Ready-To-Use [RTU], SP1, Roche, Basel, Switzerland), anti-PR (RTU, 1E2, Roche), anti-p16 (RTU, E6H4, Roche), anti-MLH1 (1:100, ES05, Novocastra), anti-MSH2 (1:400, G219-1129, Novocastra), anti-PMS2 (1:100, MRQ-28, Cell Marque, Rocklin, CA, USA), anti-MSH6 (1:200, 44, Cell Marque), anti–hepatocyte nuclear factor-1β (HNF-1β; 1:200, polyclonal, Sigma-Aldrich, St. Louis, MO, USA), anti–napsin A (1:100, polyclonal, Cell Marque), anti-CD56 (1:200, 123 C3, DAKO, Glostrup, Denmark), anti-synaptophysin (1:200, polyclonal, Cell Marque,), anti-chromogranin (1:500, Dak-A3, DAKO), anti-GATA3 (1:50, L50-823, Cell Marque), anti-CD10 (RTU, CC1, Roche), anti–thyroid transcription factor-1 (TTF-1; 1:200, 8G7G3/1, DAKO), anti-WT1 (1:200, 6F-H2, Cell Marque), anti-cytokeratin (CK; 1:200, AE1/AE3, DAKO), anti-vimentin (1:1000, Vim 384, DAKO), anti-Ki67 (1:100, MIB-1, DAKO), anti-PTEN (1:400, 6H2-1, DAKO), and anti–c-KIT (1:200, polyclonal, Novocastra).
- Each reviewer independently assessed the results of IHC staining. When interpretation-associated discrepancies arose, all three reviewers conducted a discussion until a consensus was achieved. p53 immunostaining was interpreted as mutation type if the tumor exhibited: (1) diffuse strong nuclear positivity involving at least 80% of the tumor cells (aberrant pattern), (2) complete absence of staining with the presence of positive internal control staining of non-neoplastic cells such as lymphocytes (null pattern), or (3) unequivocal cytoplasmic staining accompanied by variable nuclear staining (cytoplasmic staining pattern). Cases were considered wild-type if any degree of non-diffuse nuclear staining (< 80%) of the tumor cells was present [12]. Immunostaining for ER and PR was scored based on the percentage of tumor cells exhibiting moderate to intense nuclear staining. p16 staining was interpreted as positive if ≥ 90% of tumor cells were stained. PTEN immunostaining was considered negative if there was a complete loss of expression in tumor cells. Immunostaining for MLH1, MSH2, MSH6, and PMS2 was considered negative (loss of expression pattern) if there was a complete absence of nuclear staining. Immunostaining for napsinA and WT1 was interpreted as positive if ≥ 1% of the tumor cells were stained, regardless of the staining intensity. HNF-1β immunostaining was interpreted as positive if there was positive staining in ≥ 50% of tumor cells. Immunostaining for neuroendocrine markers (CD56, synaptophysin, and chromogranin) was interpreted as positive if there was positive staining in ≥ 10% of tumor cells. Immunostaining for CK and vimentin was considered positive if there was diffuse staining in ≥ 50% of tumor cells. Immunostaining for CD10, GATA3, and TTF-1 was interpreted as positive if there was more than one area of focal staining. c-KIT immunostaining was considered positive if there was cytoplasmic staining in ≥ 1% of tumor cells. The Ki67 labeling index was measured by recording the tumor cell percentages that exhibited moderate to intense nuclear staining.
- Next-generation sequencing
- Up to five 10-μm-thick sections of formalin-fixed, paraffin-embedded tissue blocks were used for DNA and RNA sequencing analysis. Representative tumor areas were manually micro-dissected. DNA and RNA were extracted using the Invitrogen Recover All Total Nucleic Acid Isolation Kit (Invitrogen, Carlsbad, CA, USA). Massively parallel sequencing of cancer-related gene panels (Oncomine Comprehensive Assay v3, ThermoFisher Scientific Waltham, MA, USA) was performed using the Ion Chef and Ion Torrent sequencing platforms. Sequence data were aligned against the human reference genome (hg19 build). Bioinformatics data analysis was performed using Ion Reporter software (v.5.10.1.0) with the Oncomine Variants filter chain (v.5.6).
MATERIALS AND METHODS
- Interobserver diagnostic reproducibility
- The original diagnoses in 21 cases were endometrioid carcinoma (n = 15), serous carcinoma (n = 5), and clear cell carcinoma (n = 1). Among the 15 cases of endometrioid carcinoma, five were low grade (G1, 2 cases; G2, three cases), and 10 cases were high grade (G3). The primary diagnoses made by the three reviewers were identical in 13/21 cases (62%). These cases included endometrioid carcinoma (n = 9), serous carcinoma (n = 3), and clear cell carcinoma (n = 1). Table 1 showed a summary of the primary diagnosis.
- Secondary diagnosis due to disagreement in primary diagnosis or diagnostic difficulty was performed in 8/21 cases (38%). Table 2 summarized the IHC staining result in eight discordant cases. The original diagnosis of eight cases with discrepancies was endometrioid carcinoma in six cases (one case of G1 and five cases of G3) and serous carcinoma in two cases. Secondary diagnosis based on H&E and IHC staining was concordant in four of eight discrepant cases. The secondary diagnosis of four concordant cases was mesonephric-like adenocarcinoma (case 1), carcinosarcoma (case 2), clear cell carcinoma (case 3), and large cell neuroendocrine carcinoma (LCNEC) (case 4). The four discordant cases were as follows: mixed carcinoma vs. LCNEC (case 5), G3 endometrioid vs. dedifferentiated carcinoma (case 6), and G3 endometrioid vs. serous carcinoma (cases 7 and 8).
- Among four discordant cases, two cases reached a consensus diagnosis through review of all slides and discussion between three pathologists. The consensus diagnosis was LCNEC (case 5) and dedifferentiated carcinoma (case 6). In two cases of G3 endometrioid vs. serous carcinoma (cases 7 and 8), NGS was performed because a consensus was not achieved even after discussion. Table 3 was a sequential diagnosis summary of eight discordant cases.
- Through the above diagnostic process, three reviewers made a consensus diagnoses for 21 advanced-stage endometrial carcinoma cases. The final diagnoses included four cases of low-grade endometrioid carcinoma, five cases of high-grade (G3) endometrioid carcinoma, five cases of serous carcinoma, two cases of clear cell carcinoma, two cases of neuroendocrine carcinoma, one case of mesonephric-like adenocarcinoma, one case of carcinosarcoma, and one case of dedifferentiated carcinoma (Table 4). The overall kappa for concordance between the original diagnosis and consensus diagnosis was 0.566 (moderate agreement).
- Cases with a consensus diagnosis from IHC staining
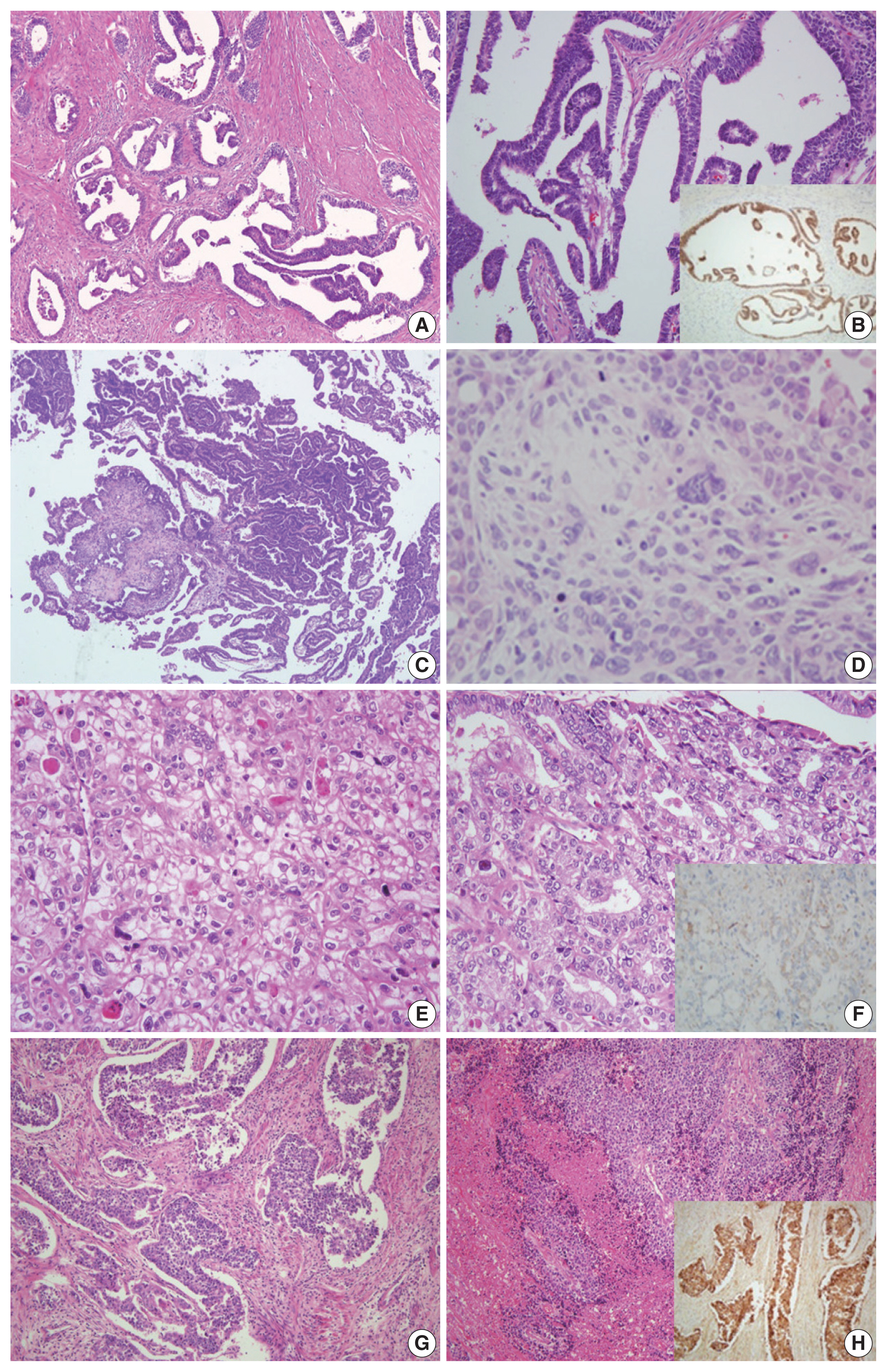
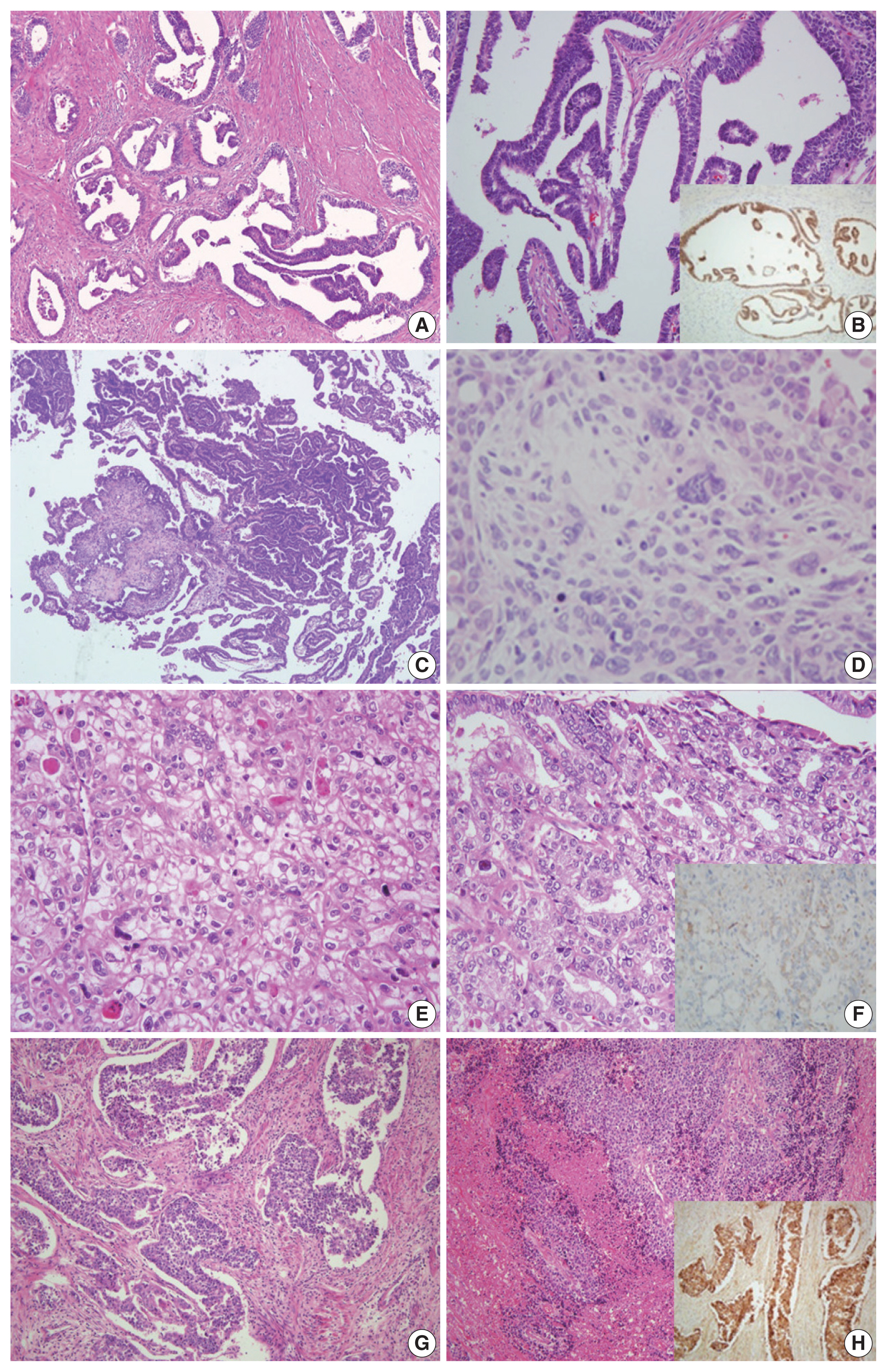
- The original diagnosis of case 1 was G1 endometrioid carcinoma. Three pathologists’ primary diagnosis were discordant, as one pathologist diagnosed this case as mesonephric-like adenocarcinoma and two others diagnosed it as low-grade endometrioid carcinoma. The tumor exhibited diverse histological morphology such as papillary and ductal/glandular patterns with a low nuclear grade. Nuclear features of the cells included nuclear overlapping, nuclear grooves, and open, vesicular chromatin, similar to those of papillary thyroid carcinoma. Although the histopathological characteristics were consistent with those of mesonephric adenocarcinoma, mesonephric remnants and uterine cervical involvement were not identified. IHC staining showed positivity in GATA3 and TTF-1, negativity in CD10 and ER, and focal positivity in PR. Based on pathologic findings and IHC results, the consensus diagnosis was mesonephric-like adenocarcinoma (Fig. 1A, B).
- In case 2, the original diagnosis was serous carcinoma. This primary diagnosis was discordant as two pathologists diagnosed this as serous carcinoma, while the remaining pathologist could not decide between serous carcinoma and carcinosarcoma. Microscopic findings showed serous and mesonephric carcinoma components. Two small foci of atypical spindle and pleomorphic cell proliferation suggested the presence of a sarcomatous component. The spindle cell component exhibited strong vimentin positivity and aberrant p53 expression, while CK expression was focal and weak. After discussion, the three pathologists agreed upon the presence of a distinct sarcomatous component, and a consensus diagnosis of carcinosarcoma was made (Fig. 1C, D).
- Case 3 showed solid or glandular proliferation patterns, and the tumor cells displayed severe nuclear atypia and abundant clear or eosinophilic cytoplasm. The primary diagnosis was discordant, with one diagnosis of clear cell carcinoma and two diagnoses of endometrioid carcinoma. On IHC stains, the tumor cells were p53 wild-type and negative for ER, PR, and HNF-1β with granular positivity for napsin A. The final consensus diagnosis was clear cell carcinoma (Fig. 1E, F).
- In case 4, large polygonal cells with prominent nucleoli and abundant cytoplasm formed well-demarcated nests, which displayed peripheral palisading. Frequent mitotic figures were observed, and geographic tumor necrosis was identified. The primary diagnosis was discordant, with two diagnoses of neuroendocrine carcinoma and one diagnosis of G3 endometrioid carcinoma. The tumor cells revealed diffuse positivity to CD56 and chromogranin. A consensus diagnosis of LCNEC was rendered (Fig. 1G, H).
- Cases confirmed by consensus discussion
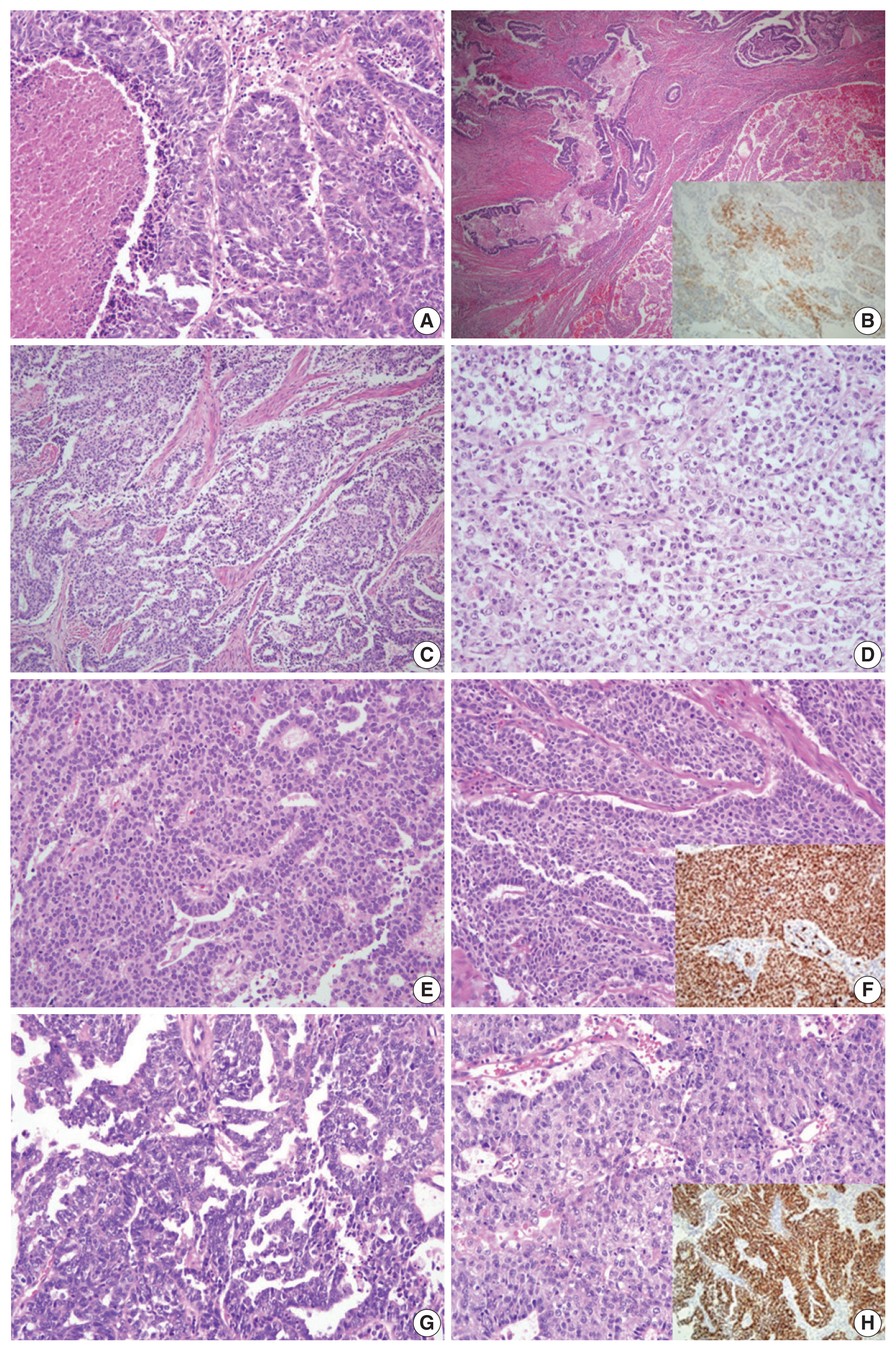
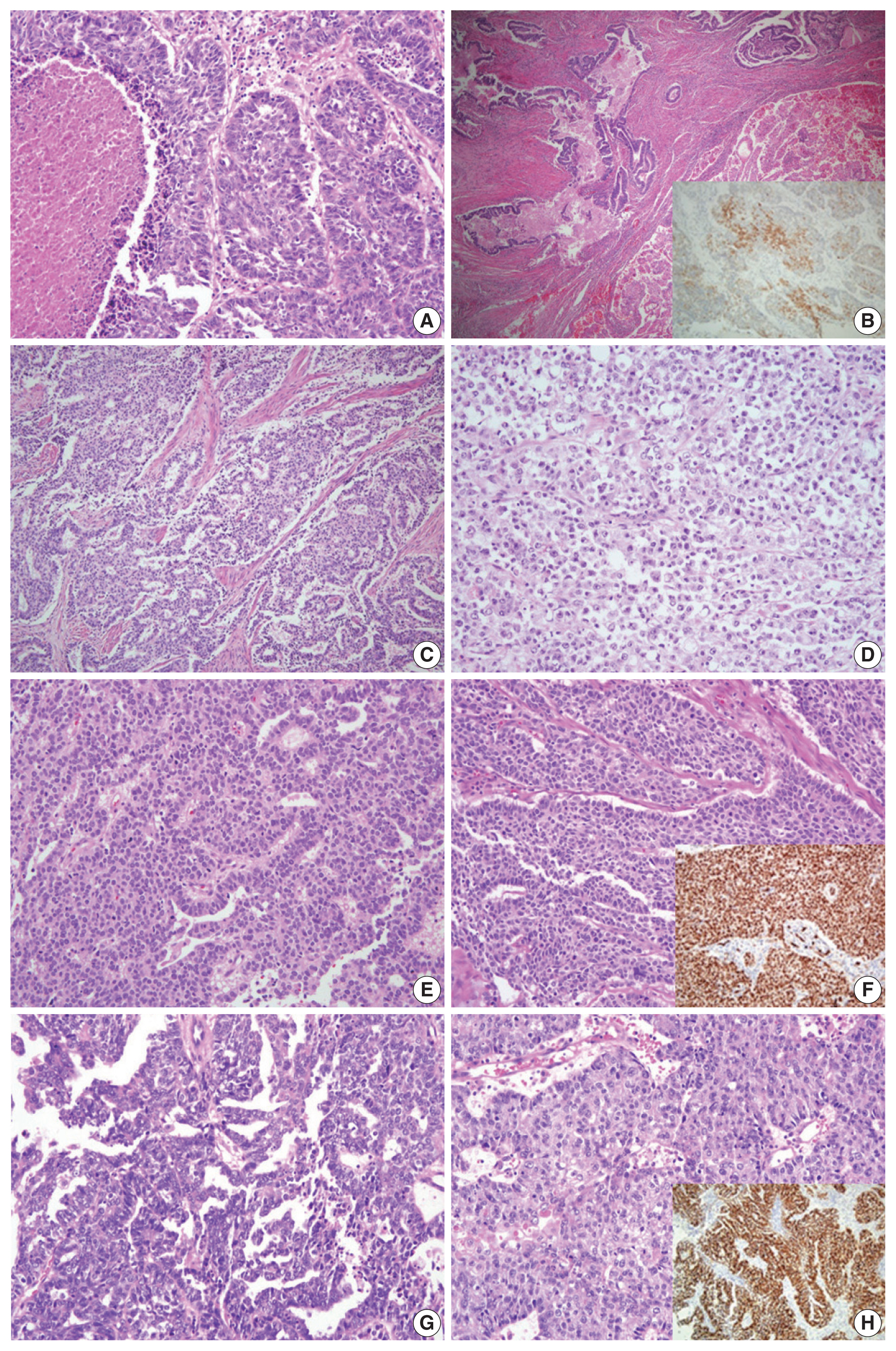
- For case 5, the primary diagnoses were mixed carcinoma (endometrioid carcinoma, clear cell carcinoma, and neuroendocrine carcinoma) vs. G3 endometrioid carcinoma vs. serous carcinoma. Histopathologic analysis showed that the tumor was composed of endometrioid carcinoma (50%), clear cell carcinoma (30%), and LCNEC (20%). The IHC staining revealed an LCNEC component showing diffuse positivity for CD56 and synaptophysin and focal positivity for chromogranin. Although the tumor consisted of various components, according to the criteria of the WHO Classification of Tumors of Female Reproductive Organs [3], case 5 was diagnosed as LCNEC (Fig. 2A, B).
- In case 6, the primary diagnosis was divided into two diagnoses of dedifferentiated carcinoma and one diagnosis of serous carcinoma. The secondary diagnosis was also discordant, as two pathologists diagnosed the case as dedifferentiated carcinoma and the remaining pathologist’s diagnosis was G3 endometrioid carcinoma. Microscopically, the tumor was composed of G1 endometrioid carcinoma and a poorly differentiated solid portion showing non-cohesive cells without readily visible gland formation. The three reviewers agreed that the solid portion was an undifferentiated carcinoma, not a high-grade endometrioid carcinoma component. A final consensus diagnosis of dedifferentiated carcinoma was reached (Fig. 2C, D).
- Cases that reached consensus diagnosis with NGS
- The microscopic features of case 7 displayed columnar tumor cells with nuclear pseudostratification and pleomorphism. While the tumor’s dominant pattern was solid proliferation, several foci showed complex papillary or glandular patterns. IHC staining revealed a complete loss of p53 staining, negativity for ER and PR, and positivity for p16, WT1, MLH1, PMS2, MSH2, and MSH6. NGS analysis was performed as histopathological features (suggestive of endometrioid carcinoma) and IHC features (suggestive of serous carcinoma) exhibited discrepancies (Fig. 2E, F). NGS revealed mutations in TP53, PIK3CA, and NF1, and copy number variations (CNVs) in MYCL, FGFR3, CDK2, CDK4, ERBB2, and CCNE1. As the molecular profile corresponded to a copy-number high group, the final diagnosis was rendered as serous carcinoma.
- Case 8 presented with high-grade tumor cells with severe nuclear pleomorphism. The tumor showed complex glandular and solid patterns. In the solid portion, nuclear pleomorphism was less severe than that of typical serous carcinoma. The primary diagnoses were divided into G3 endometrioid carcinoma versus serous carcinoma. On immunostaining, the tumor cells exhibited aberrant p53 expression, negativity for PR, diffuse positivity for p16, MLH1, PMS2, MSH2, and MSH6, and focal positivity for WT1 and ER. While the immunoprofile suggested serous carcinoma, the diagnosis of G3 endometrioid carcinoma could not be entirely excluded for the histomorphological findings (Fig. 2G, H). NGS analysis revealed mutations in TP53 and polymerase E (POLE) and CNVs involving ALK, ERBB2, CCNE1, AKT2, and AXL. Although POLE mutation was present, the total tumor mutation burden was not as high as that seen in the typical POLE-ultramutated type. Since a significant number of CNVs were detected, the case was assigned to the copy-number high group, and a final diagnosis of serous carcinoma was made.
RESULTS
- Diagnosis of high-grade endometrial carcinoma has been shown to be unreproducible, even among gynecologic pathology specialists. In several studies involving high-grade endometrial carcinoma, the consensus rate of cancer histotypes among the participating pathologists varied from 39% to 72% [7,8,13]. According to Gilks et al. [7], a diagnostic consensus among three reviewers about the exclusive or major subtype of high-grade endometrial carcinoma was reached in only 62.5% of cases. We studied diagnostic agreement in high-grade endometrial carcinoma cases to evaluate interobserver reproducibility, based on the assumption that advanced-stage endometrial carcinoma would predominantly consist of high-grade endometrial carcinomas.
- Of the advanced-stage endometrial carcinomas included in this study, the original diagnosis and the primary diagnosis by three reviewers were identical in 13/21 cases (62%). These cases had typical histopathological features, and the diagnoses included nine cases of endometrioid carcinoma, three cases of serous carcinoma, and one case of clear cell carcinoma. Among the eight cases involving discordant primary diagnoses, six cases were initially diagnosed as endometrioid carcinoma and two cases was diagnosed as serous carcinoma. We attained diagnostic agreement for six of the eight cases based on characteristic histopathological features and IHC staining results. The cases in which meticulous histologic examination alone led to an agreed diagnosis were carcinosarcoma and dedifferentiated carcinoma. The diagnosis of LCNEC, mesonephric-like adenocarcinoma, and clear cell carcinoma were achieved by panel of IHC staining. The overall kappa for concordance between the original and consensus diagnosis was 0.566 (moderate agreement). Other studies about interobserver reproducibility also show similar kappa values [8,13].
- In cases 7 and 8, it was difficult to differentiate between serous carcinoma and G3 endometrioid carcinoma. Both cases exhibited papillary growth and marked nuclear atypia, and immunostaining findings suggested a diagnosis of serous carcinoma. However, the possibility of G3 endometrioid carcinoma could not be wholly excluded. Molecular profiling by NGS was performed to reach a final diagnosis. In a comprehensive genomic analysis of endometrioid and serous carcinoma based on the The Cancer Genome Atlas database, endometrioid and serous carcinomas were divided into four molecular subgroups [11], including POLE ultramutated, microsatellite instability-hypermutated, copy-number low, and copy-number high; serous carcinoma belongs only to the copy-number high group. Endometrioid carcinoma belongs to all four groups [14]. According to this molecular classification scheme, cases 7 and 8 were designated as copy-number high group and finally diagnosed as serous carcinoma.
- In daily clinical practice settings, where molecular studies cannot be performed routinely, histopathological examination and IHC panel staining help to differentiate G3 endometrioid carcinoma from serous carcinoma. If the tumor shows papillary growth, cellular tufts, and diffuse high-grade nuclear atypia, a diagnosis of serous carcinoma is favored. When defining endometrioid features such as squamous or mucinous differentiation on a background of endometrial hyperplasia present, a diagnosis of endometrioid carcinoma can be rendered. The use of a panel of IHC stains can help to establish a confirmatory diagnosis when histomorphological findings are unclear or overlapping [6,9,15]. Biomarkers commonly used to distinguish between endometrioid and serous carcinomas are immunostaining for p53, p16, ER, PR, PTEN, and DNA mismatch repair proteins [6,9,12,16,17]. Tumors lacking aberrant p53 expression are unlikely to be serous carcinoma [17].
- The final diagnosis of case 1 was mesonephric-like adenocarcinoma, whereas the original diagnosis was G1 endometrioid carcinoma. Mesonephric carcinoma exhibits diverse morphology, such as tubular, glandular, papillary, retiform, and glomeruloid patterns, and nuclei resembling those of papillary thyroid carcinoma, with dense focal eosinophilic intraluminal secretions [17,18]. Mesonephric-like adenocarcinoma can be differentiated from low-grade endometrioid carcinoma by immunostaining for ER and PR, as mesonephric-like adenocarcinoma rarely shows immunoreactivity to these antigens. As mesonephric-like adenocarcinoma shows aggressive clinical behavior a with preponderance for pulmonary metastasis [18], accurate diagnosis of this tumor is of critical importance.
- Endometrial neuroendocrine carcinoma is a rare, highly aggressive tumor, and has a propensity for systemic spread and poor prognosis [19]. In this study, two LCNEC cases were originally diagnosed as G3 endometrioid carcinoma. Neuroendocrine carcinoma usually presents as pure neuroendocrine carcinoma or a mixture of other epithelial neoplasias, most commonly endometrioid carcinoma. The diagnosis of endometrial neuroendocrine carcinomas is challenging, due to its frequent association with low-grade endometrioid carcinoma. Awareness of these facts may help to avoid overdiagnosis or misdiagnosis of neuroendocrine carcinoma, especially in quantitatively limited samples [19].
- The dedifferentiated carcinoma identified in this study (case 6) was composed of low-grade endometrioid carcinoma and undifferentiated carcinoma. Undifferentiated carcinoma is defined as a malignant epithelial neoplasm composed of small to intermediate-sized cells arranged in sheets, without any apparent glandular differentiation; it often exhibits a characteristically ‘dyscohesive’ pattern [6,17]. When combined with low-grade endometrioid carcinoma, it is often misinterpreted as a solid growth of endometrioid carcinoma. The original diagnosis of case 6 was G3 endometrioid carcinoma. While it is easy to overlook due to its rarity, diagnosis of undifferentiated/dedifferentiated carcinoma is essential, because it has a poorer prognosis than high-grade endometrioid carcinoma [20].
- This study has a few limitations. First, the number of cases studied was small (a total of 21 cases) and the three reviewers produced diverse diagnoses, so there was no proper statistical method to calculate interobserver reproducibility. Second, only one of the three reviewers was an expert in gynecologic pathology. Since the two general pathologists were not familiar with the difficult histologic diagnosis of high-grade endometrial carcinoma, the interobserver discrepancy rate might have been measured higher than expected.
- We investigated sequential changes in interobserver reproducibility in advanced-stage and high-grade endometrial carcinoma, with the stepwise addition of IHC results and molecular data. Accurate histological diagnosis of endometrial carcinoma subtypes is critical for patient management since several rare subtypes show unfavorable prognosis and require different treatment decisions. In conclusion, we demonstrated the utility of selected IHC markers and NGS molecular profiling in the diagnosis of advanced-stage endometrial carcinoma.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board of KUGH (accession No. 2020GR0348). All the glass slides, paraffin blocks, and clinicopathological information were provided by the KUGH Biobank, which had collected patient samples and information with their informed consent.
Author Contributions
Conceptualization: HJJ, SYL, JHH, YKC. Data curation: HJJ, SYL, YKC. Formal analysis: HJJ, SYL, YKC. Investigation: HJJ, SYL, YKC. Methodology: HJJ, JHH, YKC. Supervision: YKC. Validation: HJJ, SYL, JHH, YKC. Writing—original draft: HJJ, YKC. Writing—review & editing: HJJ, SYL, YKC. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
| Case No. | Original diagnosis | Primary diagnosis | 2nd diagnosis | Consensus diagnosis | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Reviewer 1 | Reviewer 2 | Reviewer 3 | Reviewer 1 | Reviewer 2 | Reviewer 3 | |||
| 1 | G1 EC | MesL | Low-grade EC | Low-grade EC | MesL | MesL | MesL | MesL |
| 2 | SC | SC vs. CS | SC | SC | CS | CS | CS | CS |
| 3 | G3 EC | G3 EC | CC | G3 EC | CC | CC | CC | CC |
| 4 | G3 EC | LCNEC | G3 EC | LCNEC | LCNEC | LCNEC | LCNEC | LCNEC |
| 5 | G3 EC | Mixed (G3 EC + CC + LCNEC) | G3 EC | SC | LCNEC | LCNEC | Mixed (low-grade EC + LCNEC) | LCNEC |
| 6 | G3 EC | DD | DD | SC | DD | DD | G3 EC | DD |
| 7 | G3 EC | G3 EC vs. SC | SC | G3 EC | G3 EC vs. SC | SC | SC | SCa |
| 8 | SC | SC vs. G3 EC | SC | SC | G3 EC | SC | G3 EC | SCa |
G1, grade 1; G2, grade 2; G3, grade 3; EC, endometrioid carcinoma; MesL, mesonephric-like adenocarcinoma; SC, serous carcinoma; CS, carcinosarcoma; LCNEC, large cell neuroendocrine carcinoma; Mixed, mixed cell adenocarcinoma; CC, clear cell carcinoma; DD, dedifferentiated carcinoma.
aConsensus diagnosis was made after next-generation sequencing analysis.
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359-86. ArticlePubMed
- 2. Lim MC, Won YJ, Ko MJ, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999–2015. J Gynecol Oncol 2019; 30: e38.ArticlePubMedPDF
- 3. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumours of female reproductive organs. 4th ed. Lyon: IARC Press, 2014.
- 4. Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983; 15: 10-7. ArticlePubMed
- 5. Wang J, Jia N, Li Q, et al. Analysis of recurrence and survival rates in grade 3 endometrioid endometrial carcinoma. Oncol Lett 2016; 12: 2860-7. ArticlePubMedPMC
- 6. Murali R, Davidson B, Fadare O, et al. High-grade endometrial carcinomas: morphologic and immunohistochemical features, diagnostic challenges and recommendations. Int J Gynecol Pathol 2019; 38(Suppl 1):S40-63. ArticlePubMed
- 7. Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 2013; 37: 874-81. ArticlePubMed
- 8. Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 2013; 26: 1594-604. ArticlePubMedPDF
- 9. Nastic D, Shanwell E, Wallin KL, et al. A selective biomarker panel increases the reproducibility and the accuracy in endometrial biopsy diagnosis. Int J Gynecol Pathol 2017; 36: 339-47. ArticlePubMed
- 10. Yen TT, Wang TL, Fader AN, Shih IM, Gaillard S. Molecular classification and emerging targeted therapy in endometrial cancer. Int J Gynecol Pathol 2020; 39: 26-35. ArticlePubMedPMC
- 11. Hussein YR, Soslow RA. Molecular insights into the classification of high-grade endometrial carcinoma. Pathology 2018; 50: 151-61. ArticlePubMed
- 12. Kobel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol 2019; 38(Suppl 1):S123-31. ArticlePubMed
- 13. Hoang LN, Kinloch MA, Leo JM, et al. Interobserver agreement in endometrial carcinoma histotype diagnosis varies depending on The Cancer Genome Atlas (TCGA)-based molecular subgroup. Am J Surg Pathol 2017; 41: 245-52. ArticlePubMed
- 14. Soslow RA, Tornos C, Park KJ, et al. Endometrial carcinoma diagnosis: use of FIGO grading and genomic subcategories in clinical practice: recommendations of the International Society of Gynecological Pathologists. Int J Gynecol Pathol 2019; 38(Suppl 1):S64-74. ArticlePubMed
- 15. Hoang LN, McConechy MK, Kobel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 2013; 37: 1421-32. ArticlePubMed
- 16. Chen W, Husain A, Nelson GS, et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol 2017; 36: 128-39. ArticlePubMed
- 17. Carlson JW, Nastic D. High-grade endometrial carcinomas: classification with molecular insights. Surg Pathol Clin 2019; 12: 343-62. PubMed
- 18. Horn LC, Hohn AK, Krucken I, Stiller M, Obeck U, Brambs CE. Mesonephric-like adenocarcinomas of the uterine corpus: report of a case series and review of the literature indicating poor prognosis for this subtype of endometrial adenocarcinoma. J Cancer Res Clin Oncol 2020; 146: 971-83. ArticlePubMedPDF
- 19. Pocrnich CE, Ramalingam P, Euscher ED, Malpica A. Neuroendocrine carcinoma of the endometrium: a clinicopathologic study of 25 cases. Am J Surg Pathol 2016; 40: 577-86. PubMedPMC
- 20. Yokomizo R, Yamada K, Iida Y, et al. Dedifferentiated endometrial carcinoma: a report of three cases and review of the literature. Mol Clin Oncol 2017; 7: 1008-12. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Diagnostic Accuracy of Endometrial Sampling Methods for Determining Histologic Type and Grade in Endometrial Cancer: A Retrospective Cohort Study
Dina Gumin, Avishalom Sharon, Susana Mustafa Mikhail, Inshirah Sgayer, Raneen Abushqara, Lior Lowenstein, Ala Aiob
Cureus.2025;[Epub] CrossRef - Accuracy of endometrial sampling in the diagnosis of endometrial cancer: a multicenter retrospective analysis of the JAGO-NOGGO
Zaher Alwafai, Maximilian Heinz Beck, Sepideh Fazeli, Kathleen Gürtler, Christine Kunz, Juliane Singhartinger, Dominika Trojnarska, Dario Zocholl, David Johannes Krankenberg, Jens-Uwe Blohmer, Jalid Sehouli, Klaus Pietzner
BMC Cancer.2024;[Epub] CrossRef - Deep Learning for Grading Endometrial Cancer
Manu Goyal, Laura J. Tafe, James X. Feng, Kristen E. Muller, Liesbeth Hondelink, Jessica L. Bentz, Saeed Hassanpour
The American Journal of Pathology.2024; 194(9): 1701. CrossRef - Application of NGS molecular classification in the diagnosis of endometrial carcinoma: A supplement to traditional pathological diagnosis
Qunxian Rao, Jianwei Liao, Yangyang Li, Xin Zhang, Guocai Xu, Changbin Zhu, Shengya Tian, Qiuhong Chen, Hui Zhou, Bingzhong Zhang
Cancer Medicine.2023; 12(5): 5409. CrossRef - Risk Stratification of Endometrial Cancer Patients: FIGO Stage, Biomarkers and Molecular Classification
Jenneke C. Kasius, Johanna M. A. Pijnenborg, Kristina Lindemann, David Forsse, Judith van Zwol, Gunnar B. Kristensen, Camilla Krakstad, Henrica M. J. Werner, Frédéric Amant
Cancers.2021; 13(22): 5848. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1
Fig. 2
| Case No. | Original diagnosis | Primary diagnosis | ||
|---|---|---|---|---|
|
| ||||
| Reviewer 1 | Reviewer 2 | Reviewer 3 | ||
| 1 | G1 EC | MesL | Low-grade EC | Low-grade EC |
| 2 | SC | SC vs. CS | SC | SC |
| 3 | G3 EC | G3 EC | CC | G3 EC |
| 4 | G3 EC | LCNEC | G3 EC | LCNEC |
| 5 | G3 EC | Mixed (G3 EC + CC + LCNEC) | G3 EC | SC |
| 6 | G3 EC | DD | DD | SC |
| 7 | G3 EC | G3 EC vs. SC | SC | G3 EC |
| 8 | SC | G3 EC vs. SC | SC | SC |
| 9 | G1 EC | Low-grade EC | Low-grade EC | Low-grade EC |
| 10 | G2 EC | Low-grade EC | Low-grade EC | Low-grade EC |
| 11 | G2 EC | Low-grade EC | Low-grade EC | Low-grade EC |
| 12 | G2 EC | Low-grade EC | Low-grade EC | Low-grade EC |
| 13 | G3 EC | G3 EC | G3 EC | G3 EC |
| 14 | G3 EC | G3 EC | G3 EC | G3 EC |
| 15 | G3 EC | G3 EC | G3 EC | G3 EC |
| 16 | G3 EC | G3 EC | G3 EC | G3 EC |
| 17 | G3 EC | G3 EC | G3 EC | G3 EC |
| 18 | SC | SC | SC | SC |
| 19 | SC | SC | SC | SC |
| 20 | SC | SC | SC | SC |
| 21 | CC | CC | CC | CC |
| Case No. | Original diagnosis | p53 | ER | PR | Others |
|---|---|---|---|---|---|
| 1 | G1 EC | Wild type | (−) | (+), 30% | GATA3 (+), TTF-1 (+), CD10 (−) |
| 2 | SC | Aberrant | (+), 10% | (+), 5% | p16 (+, diffuse in carcinoma) CK (+, diffuse in carcinoma, focal in sarcoma) Vimentin (+ in sarcoma) WT1 (+ in carcinoma) |
| 3 | G3 EC | Wild type | (−) | (−) | Napsin A (+), HNF-1β (−) |
| 4 | G3 EC | Wild type | (+), 30% | (−) | CD56 (+), chromogranin (+), synaptophysin (−) |
| 5 | G3 EC | Wild type | (+), 20% | (+), 15% | CD56 (+), synaptophysin (+), chromogranin (+, focal) PTEN (+), WT1 (−) |
| 6 | G3 EC | Wild type | (+), 10% | (+), 5% | pMMR |
| 7 | G3 EC | Null | (−) | (−) | p16 (+, diffuse), pMMR, WT1 (+), PTEN (+) |
| 8 | SC | Aberrant | (−) | (−) | p16 (+, diffuse), pMMR, WT1 (+, focal) |
| Case No. | Original diagnosis | Primary diagnosis | 2nd diagnosis | Consensus diagnosis | ||||
|---|---|---|---|---|---|---|---|---|
|
|
| |||||||
| Reviewer 1 | Reviewer 2 | Reviewer 3 | Reviewer 1 | Reviewer 2 | Reviewer 3 | |||
| 1 | G1 EC | MesL | Low-grade EC | Low-grade EC | MesL | MesL | MesL | MesL |
| 2 | SC | SC vs. CS | SC | SC | CS | CS | CS | CS |
| 3 | G3 EC | G3 EC | CC | G3 EC | CC | CC | CC | CC |
| 4 | G3 EC | LCNEC | G3 EC | LCNEC | LCNEC | LCNEC | LCNEC | LCNEC |
| 5 | G3 EC | Mixed (G3 EC + CC + LCNEC) | G3 EC | SC | LCNEC | LCNEC | Mixed (low-grade EC + LCNEC) | LCNEC |
| 6 | G3 EC | DD | DD | SC | DD | DD | G3 EC | DD |
| 7 | G3 EC | G3 EC vs. SC | SC | G3 EC | G3 EC vs. SC | SC | SC | SC |
| 8 | SC | SC vs. G3 EC | SC | SC | G3 EC | SC | G3 EC | SC |
| Case No. | Original diagnosis | Consensus diagnosis |
|---|---|---|
| 1 | G1 EC | MesL |
| 2 | SC | CS |
| 3 | G3 EC | CC |
| 4 | G3 EC | LCNEC |
| 5 | G3 EC | LCNEC |
| 6 | G3 EC | DD |
| 7 | G3 EC | SC |
| 8 | SC | SC |
| 9 | G1 EC | Low-grade EC |
| 10 | G2 EC | Low-grade EC |
| 11 | G2 EC | Low-grade EC |
| 12 | G2 EC | Low-grade EC |
| 13 | G3 EC | G3 EC |
| 14 | G3 EC | G3 EC |
| 15 | G3 EC | G3 EC |
| 16 | G3 EC | G3 EC |
| 17 | G3 EC | G3 EC |
| 18 | SC | SC |
| 19 | SC | SC |
| 20 | SC | SC |
| 21 | CC | CC |
G1, grade 1; G2, grade 2; G3, grade 3; EC, endometrioid carcinoma; MesL, mesonephric-like adenocarcinoma; SC, serous carcinoma; CS, car-cinosarcoma; CC, clear cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; Mixed, mixed cell adenocarcinoma; DD, dedifferentiated carcinoma.
ER; estrogen receptor; PR, progesterone receptor; EC, endometrioid carcinoma; G1, grade 1; G3, grade 3; TTF-1, thyroid transcription factor-1; SC, serous carcinoma; CK, cytokeratin; pMMR, proficient mismatch repair.
G1, grade 1; G2, grade 2; G3, grade 3; EC, endometrioid carcinoma; MesL, mesonephric-like adenocarcinoma; SC, serous carcinoma; CS, carcinosarcoma; LCNEC, large cell neuroendocrine carcinoma; Mixed, mixed cell adenocarcinoma; CC, clear cell carcinoma; DD, dedifferentiated carcinoma. Consensus diagnosis was made after next-generation sequencing analysis.
G1, grade 1; G2, grade 2; G3, grade 3; EC, endometrioid carcinoma; MesL, mesonephric-like adenocarcinoma; SC, serous carcinoma; CS, carcinosarcoma; CC, clear cell carcinoma; LCNEC, large cell neuroendocrine carcinoma; DD, dedifferentiated carcinoma.

 E-submission
E-submission