Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 55(3); 2021 > Article
-
Original Article
Prognostic role of ALK-1 and h-TERT expression in glioblastoma multiforme: correlation with ALK gene alterations -
Dalia Elsers1, Doaa F. Temerik2, Alia M. Attia3, A. Hadia4, Marwa T. Hussien5

-
Journal of Pathology and Translational Medicine 2021;55(3):212-224.
DOI: https://doi.org/10.4132/jptm.2021.03.15
Published online: May 11, 2021
1Department of Pathology, Faculty of Medicine, Assiut University, Assiut, Egypt
2Department of Clinical Pathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
3Department of Radiation Oncology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
4Department of Medical Oncology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
5Department of Oncologic Pathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
- Corresponding Author: Marwa T. Hussien, MD, PhD, Department of Oncologic Pathology, South Egypt Cancer Institute, Assiut University, Assiut 171516, Egypt Tel: +20-88-208671, Fax: +20-088-2087709, E-mail: marwat.hussien@aun.edu.eg
© 20212020 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase that is expressed in the developing central and peripheral nervous systems during embryogenesis. Human telomerase reverse transcriptase (h-TERT) protein resumption is the main process of preservation of telomeres that maintains DNA integrity. The present study aims to evaluate the prognostic role of ALK-1 and h-TERT protein expression and their correlation with ALK gene alterations in glioblastoma multiforme (GBM).
-
Methods
- The current study is a retrospective study on a cohort of patients with GBM (n = 53) that attempted to detect ALK gene alterations using fluorescence in situ hybridization. ALK-1 and h-TERT proteins were evaluated using immunohistochemistry.
-
Results
- Score 3 ALK-1 expression was significantly associated with male sex, tumor multiplicity, Ki labeling index (Ki LI), and type of therapeutic modality. Score 3 h-TERT expression exhibited a significant association with Ki LI. ALK gene amplifications (ALK-A) were significantly associated with increased Ki LI and therapeutic modalities. Score 3 ALK-1 protein expression, score 3 h-TERT protein expression, and ALK-A were associated with poor overall survival (OS) and progression-free survival (PFS). Multivariate analysis for OS revealed that ALK gene alterations were an independent prognostic factor for OS and PFS.
-
Conclusions
- High protein expression of both ALK-1 and h-TERT, as well as ALK-A had a poor impact on the prognosis of GBM. Further studies are needed to establish the underlying mechanisms.
- This was a retrospective study that included 53 patients primarily diagnosed with GBM. All were recruited and diagnosed in Pathology Department, Assiut University Hospital and South Egypt Cancer Institute, between April 2014 and April 2018. Patients were followed until May 2020. All patients were eligible at age 20–80 years, if they had no previous diagnosis of cancer and no previous CNS surgery for any cause. The exclusion criteria were patients with primary malignant brain tumors other than GBM or with brain metastasis, and patients with no follow-up records.
- With regards to the treatment modalities used in the current research, all patients underwent surgical intervention, which included gross total resection, subtotal resection, or biopsy. Following surgical intervention, fractionated conformal radiation therapy was delivered to Gross tumor volume 1, which included the T2/FLAIR abnormality and the surgical cavity if present for a total dose of 46 Gy in 23 fractions at 2 Gy per fraction, once daily, for five days per week. Gross tumor volume 2 included T1 contrast enhanced abnormality and the surgical cavity if present for a boost dose of 14 Gy in seven fractions with 2 Gy per fraction, once daily, for five days per week. All patients were treated using a megavoltage linear accelerator and photon energies of 6 MV or more. Adjuvant radiotherapy only was given in 12 patients (22.6%) while the remaining patients received chemotherapy as a part of a treatment protocol. Chemotherapy treatment consisted of TMZ, which was given concomitantly with radiotherapy (75 mg/m2/day, started from the first day of radiotherapy until the end of radiation) in 27 patients (50.9%) or given as concurrent chemoradiotherapy (CCRT) and adjuvant (150–200 mg/m2/for 5 days/every 28 days for 6 or 12 cycles) in 14 patients (26.4%).
- Follow-up evaluation included history and neurological examination, laboratory investigations, assessment of treatment related toxicity, and magnetic resonance imaging (MRI) or magnetic resonance spectroscopy imaging that were available for review. Patients were evaluated for response using MRI and or magnetic resonance spectroscopy, which were performed within 48 hours of surgery, before the first cycle, after every 3 cycles of adjuvant TMZ, and every three months after termination of treatment.
- The GBM specimens used for evaluation of ALK-1, h-TERT immunohistochemical protein expression, and ALK gene alterations using the FISH technique. Clinicopathological parameters collected from the patient’s archives sheets included patient age, sex, tumor site, presence of tumor calcification, tumor multiplicity, tumor size, and Ki-67 labeling index (Ki LI) with cutoff point of 14% [15].
- Immunohistochemistry
- Formalin-fixed paraffin-embedded (FFPE) slides from the GBM tissue blocks, were retrieved from pathology lab and included for IHC study. The slides stained with hematoxylin and eosin were reviewed histologically before staining by the two pathologic consultants in this research. The FFPE blocks were cut into 3–4-μm thickness, and then put on positively charged glass slides (PCS). Sections were de-paraffinized and rehydrated, followed by antigen retrieval, which was done with Tris-EDTA in a water bath at 90°C for 45 minutes. The primary monoclonal mouse anti-Human ALK/CD246 antibody (clone ALK-1), ready-to-use (code IR641, 117498-002, CVR No. 33211317, Dako, Glostrup, Denmark), Ki-67antibody (clone MIB-1), ready-to-use (code IR626, primary mononclonal mouse, Dako), and a primary rabbit polyclonal anti–Human TERT antibody (catalog #213737, United State Biological 4 Technology, Salem, MA, USA) were applied. Anti-TERT antibody was used at a dilution of 1/75 (optimum dilution according to datasheet). Both incubated for one hour at room temperature in an airtight humid chamber. A universal staining kit “Ultra Vision Detection System Anti-Polyvalent, HRP/DAB (ready-to-use)” (catalog #TP-015-HD, LAB VISION Corp., Fremont, CA, USA) was applied following the manufacturer’s instructions.
- Evaluation of ALK-1 and h-TERT IHC expression
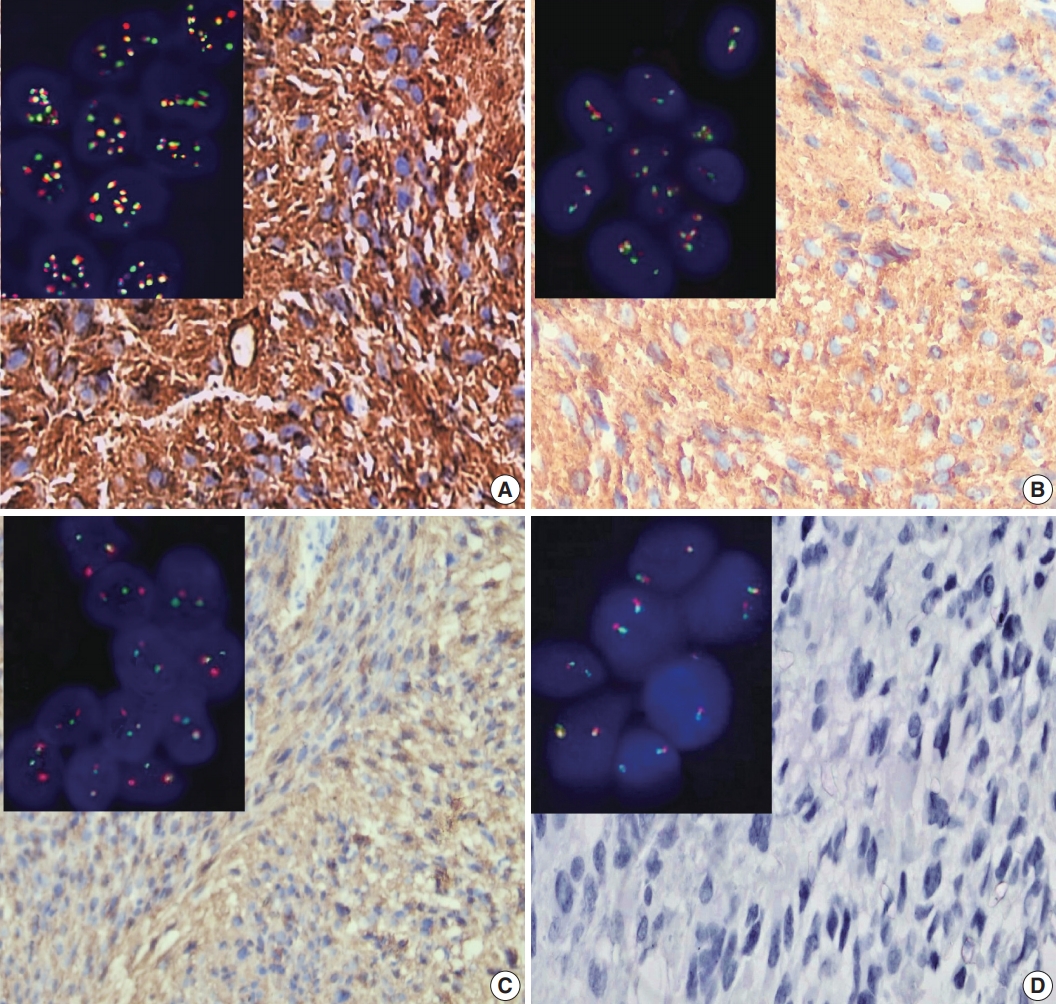
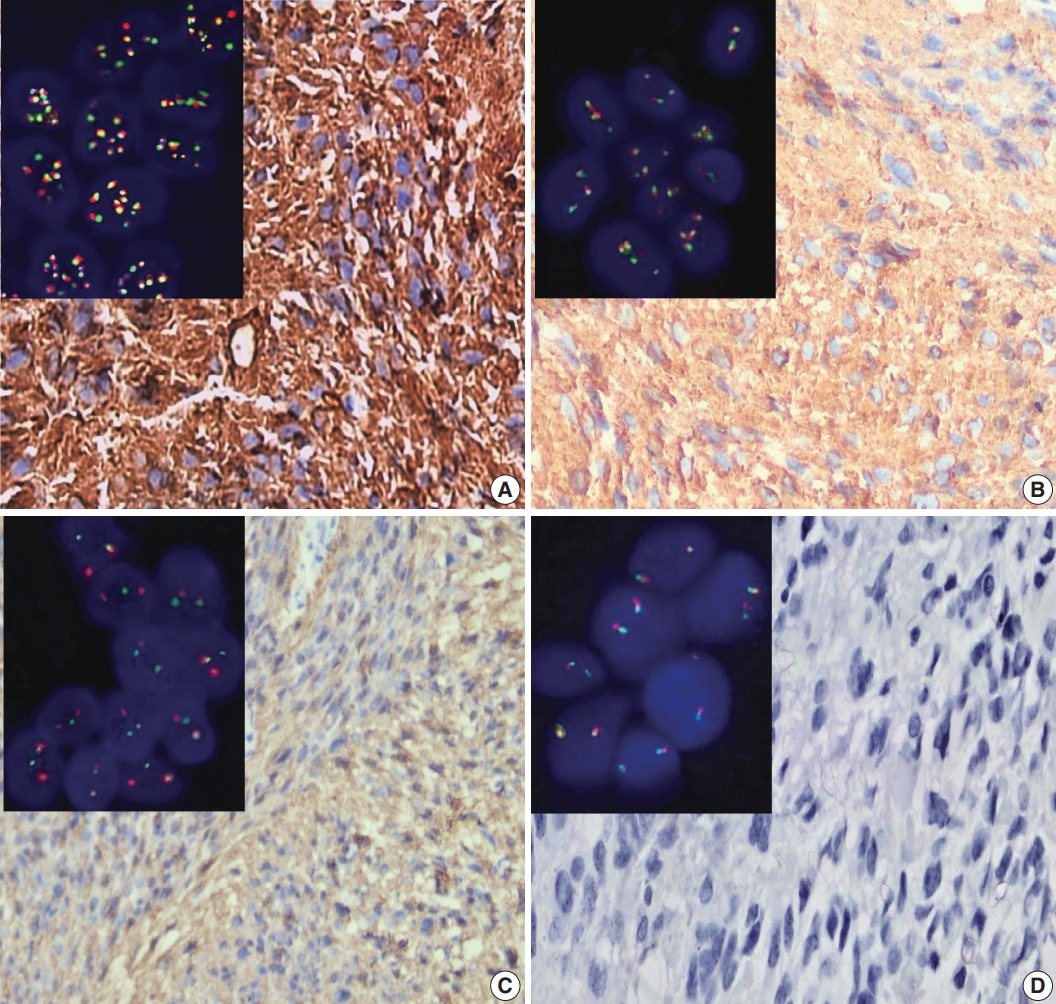
- ALK-1 positivity was identified as a brown cytoplasmic expression. A four-tier scoring system was used for evaluation of ALK-1 positivity [5]; score 1 was corresponded to weak cytoplasmic expression of ALK-1, moderate cytoplasmic expression was considered as score 2, and strong cytoplasmic expression was considered as score 3 (Fig. 1). Negative staining was scored as 0.
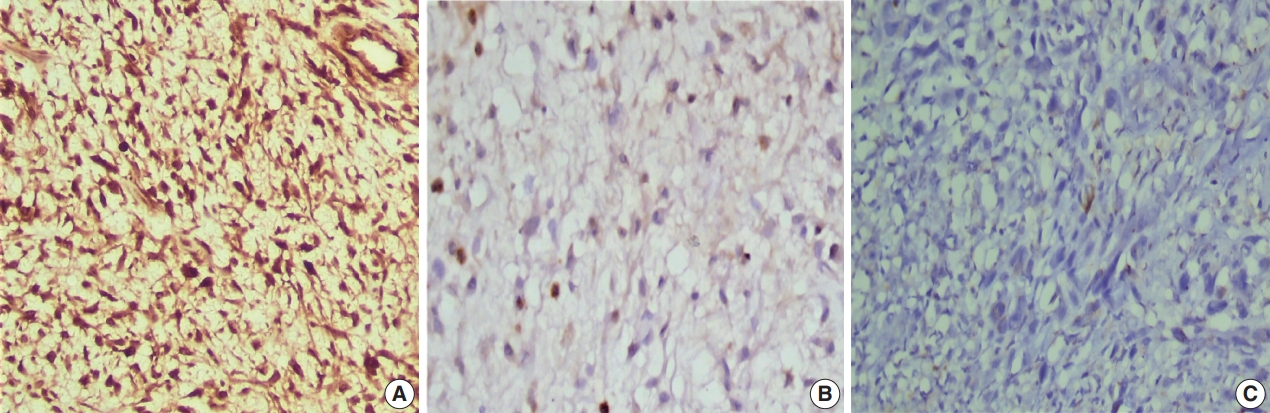
- Positive brown nuclear staining of h-TERT was deemed positive. A three-tier evaluation system was used for scoring of h-TERT IHC protein expression [11]. Score 1 corresponded to nuclear expression in < 5% of tumor cells. h-TERT expression in between 5 and 50% tumor cells was scored as 2, and expression in more than 50% of tumor cells was deemed to score 3 (Fig. 2). Positive cytoplasmic staining of ALK-1 protein in anaplastic lymphoma cells was used as a positive control. Positive nuclear staining of h-TERT in melanoma cells were used as positive control. Negative control done using the same protocol of IHC unless the addition of the primary antibody on tissue section of specific positive controls.
- Ki-67 positivity was identified as a brown nuclear expression. KI LI was defined as the percentage of positive tumor nuclei in 1,000 tumor cells with cutoff value of 14% [15].
- Fluorescence in situ hybridization
- An ALK break-apart probe set (XT ALK BA Dual Color, Break Apart Rearrangement Probe [reference number: D-6001-100-OG], Metasystems, Altlussheim, Germany) was used for FISH to detect gene rearrangements and copy number changes. In each case, we examined around 200 nuclei from at least 5–8 areas. We excluded nuclei with apparent overlapping or truncation.
- Four-micrometer-thick tissue sections were cut from FFPE GBM tissue and were put on PCS. The unstained slides were placed overnight at 60°C on a hotplate. Then, the slides were immersed 3 times in xylene for 5 minutes and dehydrated twice in 100% ethanol for 5 minutes at room temperature. In sequence, the slides were immersed for 20 minutes in 0.2 N HCl, in purified water for 3 minutes, and in 1 M sodium thiocyanate at 80°C for 30 minutes. The slides were incubated for 30 minutes in Protease Solution previously warmed to 37°C after removal of excess water and washed in purified water for 3 minutes. Then, dehydration of slides in 70%, 80%, and 100% ethanol for one minute each was done and slides were allowed to dry. Then, they were placed in a dark room. Ten microliters of probe mixture were applied to a slide, immediately covered by a coverslip, and sealed with rubber cement. They were placed in a hybridizer instrument (DakoCytomation, Glostrup, Denmark) at 73°C for 3 minutes followed by an overnight hybridization at 37°C. At the end of the hybridization period, we removed the rubber cement from the slides and placed them in 2 × saline sodium citrate (SSC; post hybridization wash) at 73°C temperature for 2 minutes, then immersed them for 1 minute in 2 × SSC at room temperature and allowed the slides to dry. Ten microliters of DAPI counterstain was applied to the target area and covered by a coverslip [6].
- Analysis of ALK gene alterations using FISH technique
- We analyzed the prepared slides under an oil immersion objective (100 ×) with a fluorescence microscope (M1, Carl Zeiss Microscopy GmbH, Gottingen, Germany) equipped with appropriate filters and a charge-coupled device camera using FISH imaging with the capturing software Metafer 5 (a Metafer slide scanning system [Metasystems]). Non-rearranged ALK showed fusion (yellow signals) or very close abutment of the probes adjacent to the 3' (red) and the 5' (green) ends of the gene. Rearranged ALK appeared as splitting of 3' and 5' signals. Tumor tissues were considered ALK-FISH positive (ALK-rearranged) if > 15% tumor cells showed splitting of red and green signals [16]. The mean cutoff copy number of 3 to 5 fusion signals in ≥ 10% of cells represented ALK–copy number gain (ALK-CNG), while the presence of ≥ 6 copies of ALK per cell in ≥ 10% of analyzed cells represented ALK-A (Fig. 1) [17].
- Statistical analysis
- The analysis for this study was done through using the SPSS ver. 21 (IBM Corp., Armonk, NY, USA). Fisher exact test was used to detect the association between ALK protein, TERT protein expression, ALK gene alterations, and various clinicopathological data. Chi-square test was used only when ≤ 20% of the cells had an expected count less than 5. Correlation between ALK gene alterations and ALK and TERT IHC protein expression were done via Spearman correlation coefficient test. OS was calculated from the date of surgical resection to the date of death from any cause or last follow-up. Progression-free survival (PFS) was calculated from the date of surgical resection to the date of progression or date of last follow-up or death.
- Kaplan-Meier curves were used to analyze OS and PFS. Comparison of survival was determined by log-rank test. Multivariate analysis using Cox proportional hazard model of predictors of outcome variables were applied. The value of significance was determined as p < .05.
MATERIALS AND METHODS
- The current study included 53 GBM patients. The study included 17 patients (32.1%) who were < 50 years of age, while 36 patients (67.9%) were ≥ 50 years old. Thirty-six cases (67.9%) were males. The most prominent tumor location was at the parieto-occipital region, constituting 28.4% of patients. The median tumor size was 5 cm. Calcification was present in 32 cases (60.4%). There were multiple tumors in six cases (11.3%).
- After a median follow-up duration of 12 months (range, 3 to 19 months), 21 out of 53 patients (39.6%) were still alive (Table 1).
- Association between ALK-1 IHC protein expression and clinicopathological parameters
- ALK-1 expression was detected in 45 cases out of 53 (84.9%). Strong ALK cytoplasmic expression (score 3) was noted in 17 GBM tumors (32.1%). Thirteen cases (24.5%) showed moderate cytoplasmic expression (score 2), while 15 cases (28.3%) showed weak cytoplasmic expression (score 1). Eight cases (15.1%) were negative (score 0).
- Score 3 ALK-1 expression showed significant association with male sex (p = .038), tumor multiplicity (p = .046), and Ki LI (p ≤ .001). The type of therapeutic modality was positively associated with ALK-1 protein expression (p = .030). The clinicopathological associations with ALK-1 protein IHC expression are summarized in Table 2.
- Association between h-TERT IHC protein expression and clinicopathological parameters
- h-TERT expression was detected in the studied cases with variable percentages and staining intensities. Strong h-TERT nuclear expression in > 50% of tumor cells (score 3) was noted in 29 (54.7%) cases, while moderate expression in 5%–50% of tumor cells (score 2) was present in 16 cases (30.2%) and eight cases (15.1%) showed weak expression in < 5% of tumor cells (score 1).
- h-TERT expression showed significant association with the presence of calcifications (p = .016). Score 3 h-TERT expression exhibited a significant association with Ki LI (p = .005). There was no significant correlation between h-TERT expression and other clinicopathological variables (Table 2).
- Association between ALK gene alterations and clinicopathological parameters
- Thirty-four cases out of 53 showed ALK gene alterations (64.2%). ALK-A was detected in four cases (7.5%), and all of these cases had strong ALK immunohistochemical expression. ALK-CNG was noted in 12 cases (22.6%), while 18 cases (34.0%) showed ALK gene rearrangement. Nineteen cases (35.9%) were negative. ALK-A was significantly associated with increased Ki LI (p = .044). The type of therapeutic modality was positively associated with ALK gene alterations (p = .027). There was no significant association between ALK gene alteration and clinicopathological variables (Table 3).
- Correlation between ALK gene alterations, ALK-1 and h-TERT IHC protein expression
- A strong positive correlation was noted between ALK protein IHC expression and ALK gene alterations (p = .001, r = 0.616). Moderate correlation was noted between ALK gene alterations and TERT IHC (p = .007, r = 0.476), as (score 2) TERT expression was associated with ALK gene rearrangement (56.2%), while (score 3) TERT expression was associated with ALK-CNG and ALK-A (51.8%). There was a strong positive correlation between ALK-1 and TERT IHC protein expression (p = .002, r = 0.602). The relationships and correlations between ALK gene alterations, ALK, and TERT IHC protein expression are presented in Tables 3 and 4, respectively.
- Outcome analysis
- The median follow-up duration of the 53 GBM patients was 12 months (range, 3 to 19 months). During follow-up, 32/53 patients (60.4%) died as a result of tumor progression. According to Kaplan-Meier analysis, the median OS was 12 months (95% confidence interval [CI], 10.449 to 13.551), while the 19-month OS rate was 39.6%. A total of 36/53 patients (67.9%) developed disease progression. The median time to progression was 10 months (range, 2 to 19 months). According to Kaplan-Meier analysis, the median PFS was 10 months (95% CI, 7.860 to 12.140). The PFS rate at 19 months was 32.1%.
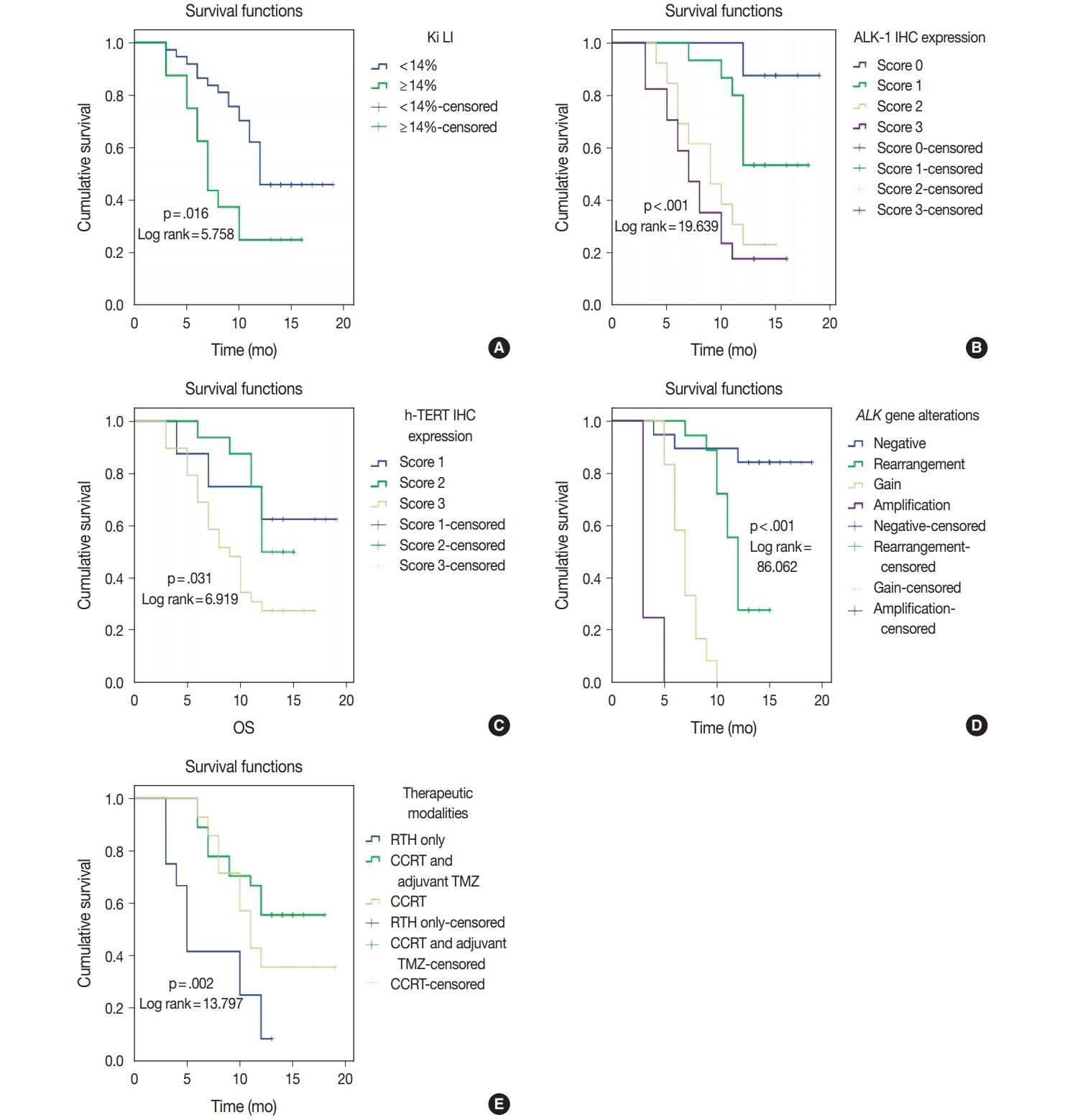
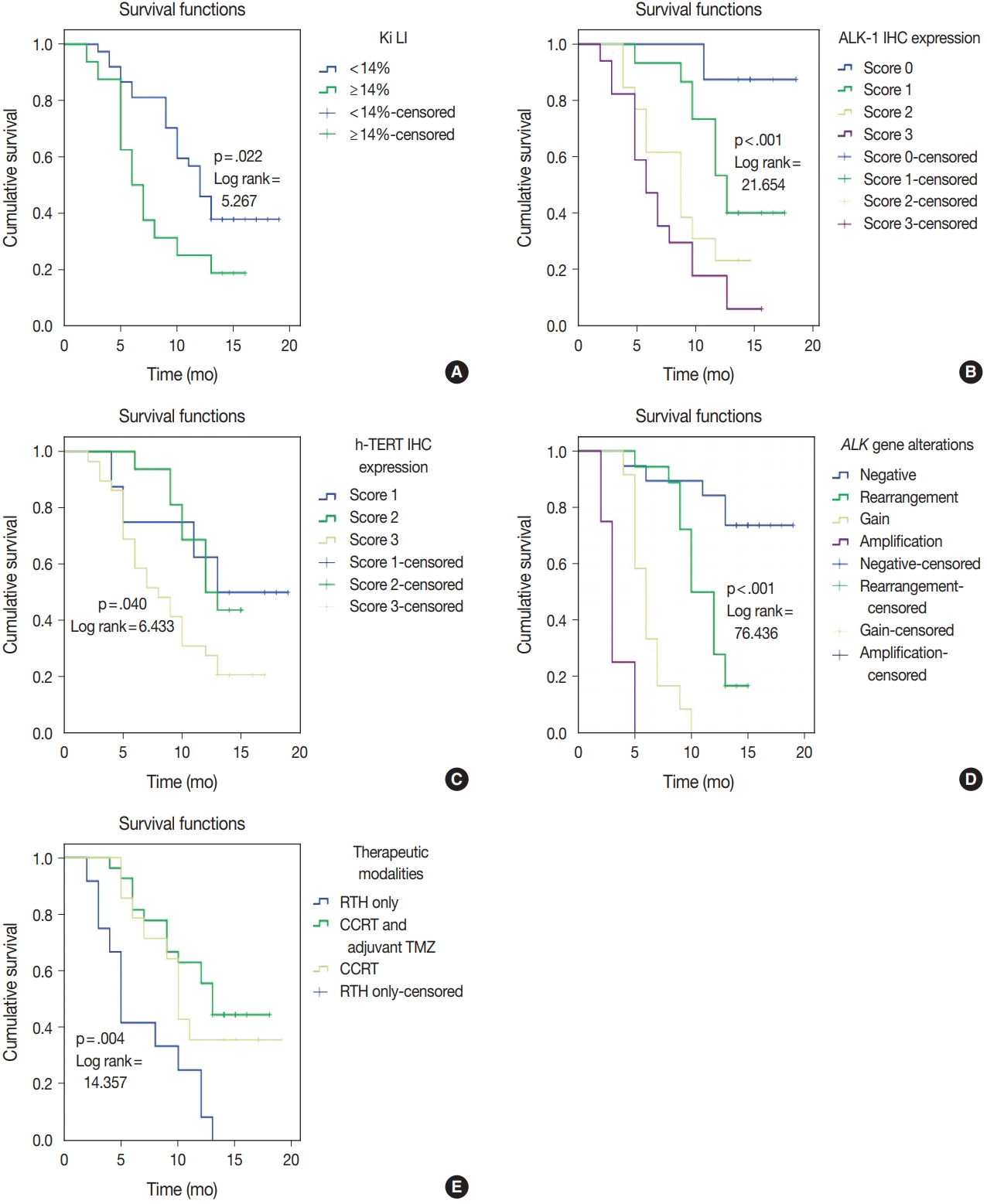
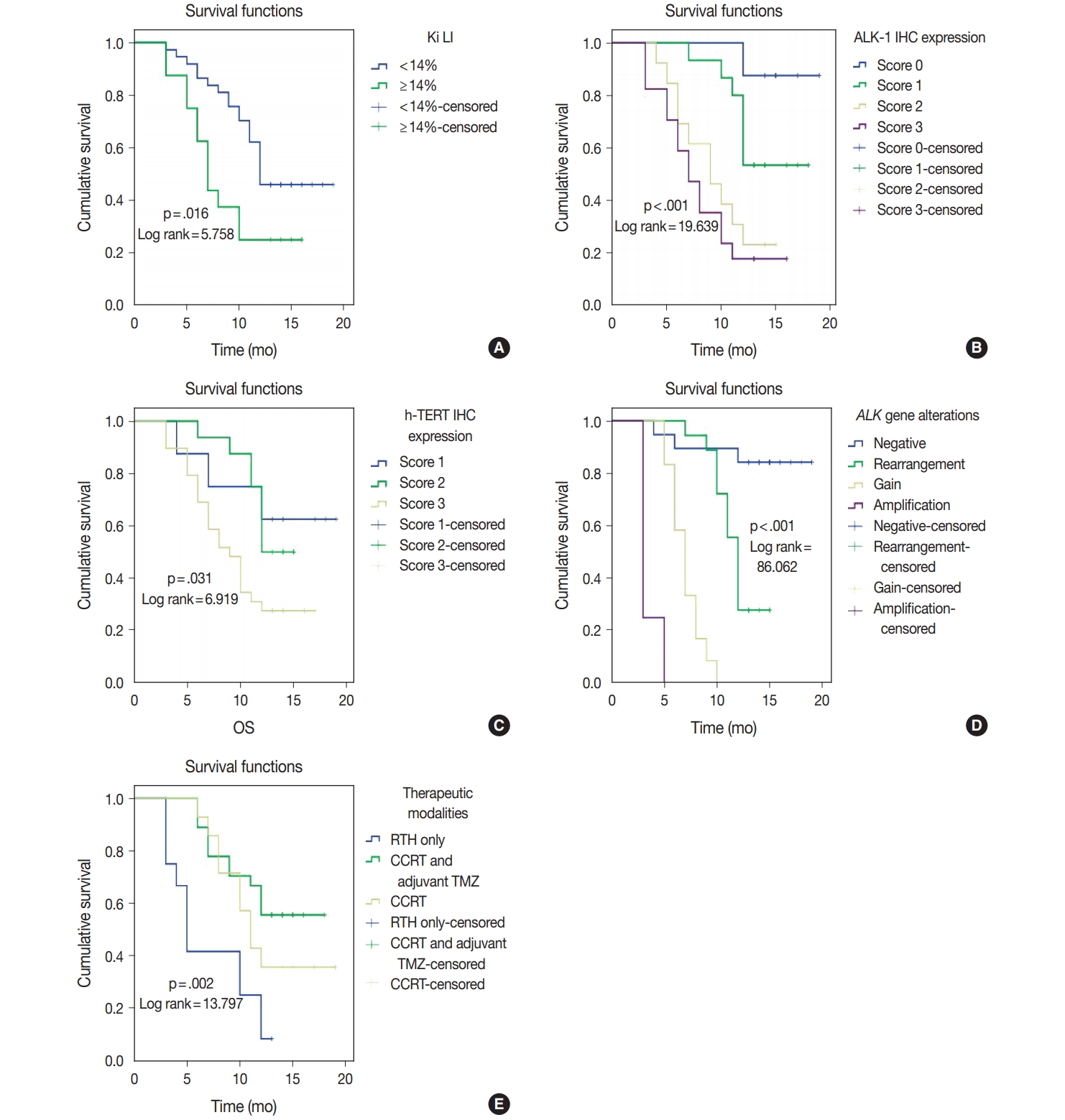
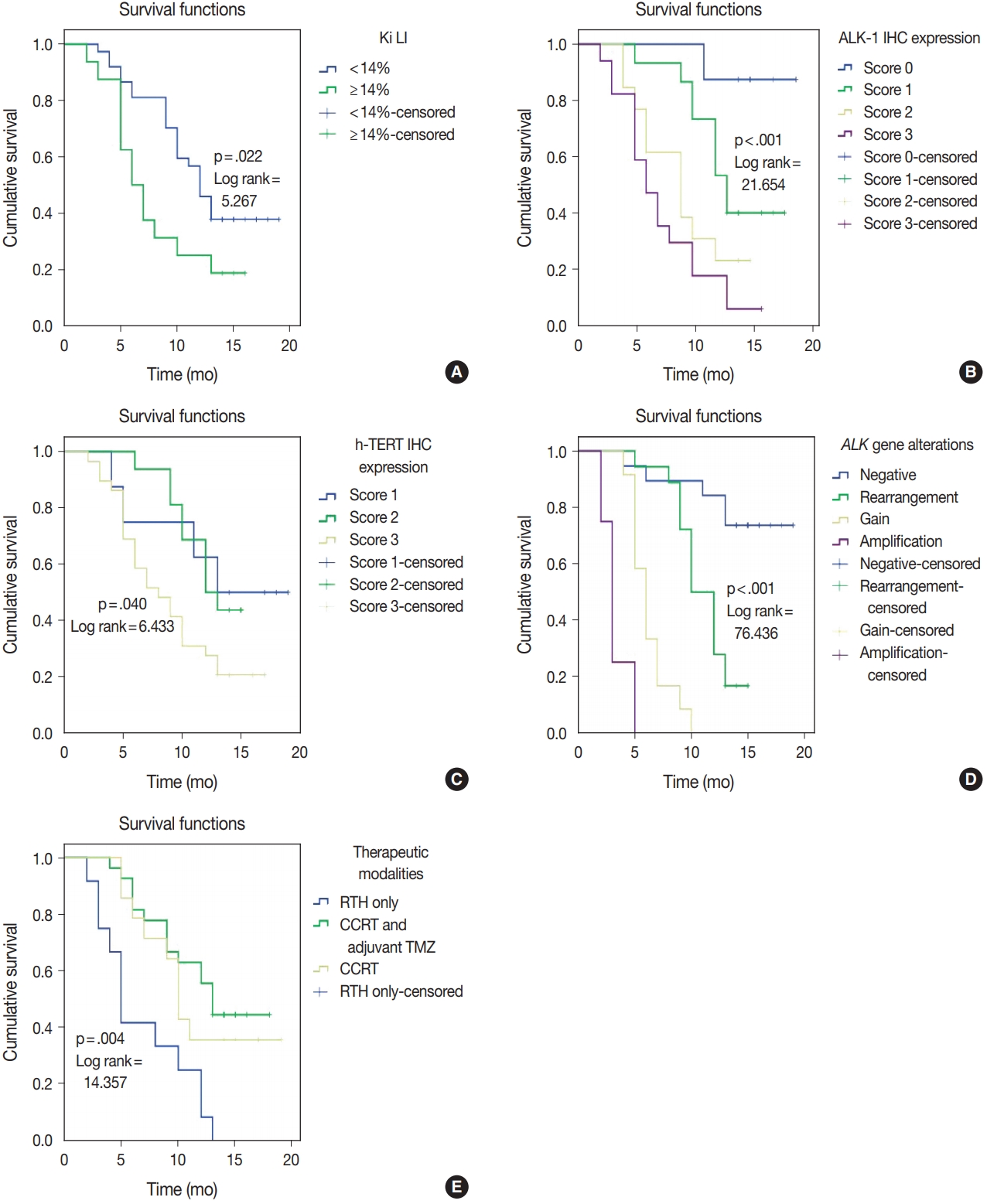
- ALK-1 protein expression (score 3), h-TERT protein expression (score 3), and ALK-A were associated with poor OS and PFS (p < .001, p = .031, and p < .001) and (p < .001, p = .040, and p < .001), respectively. Cases that exhibited high Ki LI had poor OS and short PFS (p = .016) and (p = .022), respectively (Figs. 3, 4).
- Regarding the type of therapeutic modalities, patients treated with adjuvant radiotherapy only had poor OS and PFS compared to those who were treated with CCRT or CCRT and adjuvant TMZ (p = .002) and (p = .004), respectively (Table 5, Figs. 3E, 4E).
- Multivariate analysis for OS and disease-free survival was applied to clinicopathological features that were significant in univariate analysis to adjust for confounders. Our results revealed that ALK gene alteration was the only independent prognostic factor for OS and PFS (p < .001; hazard ratio [HR], 7.514; 95% CI, 3.292 to 17.155) and (p ≤ .001; HR, 4.711; 95% CI, 2.429 to 9.136), respectively (Table 6).
RESULTS
- GBM exhibits a vast group of modifications, both genetic and epigenetic, which create a great number of mutation subsets, some of which have a proven effect in survival and therapy response [1].
- In the present study, high ALK-1 expression showed a significant association with male sex, however, no significant relation between ALK-1 expression and patient’s age. These findings are not matched with the study done by Karagkounis et al. [5], which reported that ALK overexpression is more common in older individuals (> 59 years) and that was no association between ALK expression and patient’s sex. This discrepancy was due to division of their cases into subgroups according to IDH1 protein expression with cutoff median age of 59 years, which was not implemented in the current study.
- One persistent debate is whether ALK-1 immunohistochemical overexpression was associated with ALK gene mutation or amplification in GBM cases. In the current study, we reported that ALK-A was detected in four cases out of 17 IHC stains that were strongly positive for ALK-1 expression, and ALK-CNG was noted in 12 cases. This is consistent with two early studies done by Hudson et al. [18] and Kulig et al. [19], which found that 60% and 48.2% of their GBM cases possessed ALK gene gain/amplification by FISH, respectively. On the other hand, our results are contrary to those presented by Karagkounis et al. [5] and Chiba et al. [4] with respect to the ALK gene via FISH analysis. Karagkounis et al. [5] revealed only one case of GBM showed ALK-A of the 2p domain. Likewise, Chiba et al. [4] revealed no amplification of the ALK locus.
- A study on non-small lung cancer reported that ALK gene non-translocated cancers are commonly related to unstable chromosomes and ALK-CNG, while the ALK gene translocated cancers represent a low number of ALK gene copies [20]. This may clarify our findings of the association between ALK non-rearranged tumors with increased ALK gene copies (ALK-A and ALK-CG) and moderate to strong expression of ALK-I IHC, while the ALK-rearranged tumors without ALK gene extra-copies were associated with negative to weak ALK-I IHC expression. The relationship between ALK gene extra-copies (ALK-A and ALK-CG) by FISH and ALK protein expression is still debatable. On one hand, a study revealed that ALK gene aberrations detected by FISH and ALK overexpression by IHC in rhabdomyosarcoma were significantly correlated [21]. On the other hand, other studies found no association between ALK gene aberrations detected by FISH and ALK expression by IHC in various cancer types such as esophageal squamous cell carcinoma and colorectal carcinoma [22]. Furthermore, a study of a neuroblastoma cell line harboring ALK-A was accompanied by reduction of ALK levels as a result of N-linked glycosylation inhibition with subsequent inhibition of its phosphorylated downstream molecules such as AKT and STAT3 [23]. This controversy between the relationship between ALK gene extra-copies on FISH and ALK protein expression may be attributed to either transcriptional or post-transcriptional modifications, or degradation, such as N-linked glycosylation activation or inhibition, which lead to ALK activation and reduction, respectively.
- Regarding h-TERT immunohistochemical expression, the current work revealed strong h-TERT nuclear expression in about half of the studied cases; this is compatible with the study done by Potharaju et al. [10], which showed that 60% of GBM patients expressed strong h-TERT. In spite of the use of h-TERT IHC as a mirror to detect tumors harboring TERT-mutation, h-TERT IHC was not able to identify the differences between TERT-mutated GBMs and TERT-nonmutant GBMs. h-TERT protein overexpression was noted even among gliomas with wildtype TERT. Moreover, h-TERT IHC widely varied through the TERT-mutant gliomas such as oligodendrogliomas and GBMs. This indicates that h-TERT IHC expression may be regulated by various mechanisms along with TERT promoter mutations [24].
- An interesting finding is the presence of a significant association between h-TERT expression and the presence of tumor calcification in the present study, which may be attributed to the hypoxic state in GBM that leads to activation of hypoxia-inducible factor-1α (HIF-1α) in response to hypoxic status with subsequent increase in intracellular calcium (Ca2+) and promotion of the Ca2+ signaling pathway [25]. Furthermore, activation of HIF-1α enhances h-TERT transcription [26].
- Concerning the relationship between Ki LI and ALK-1/h-TERT protein expression, all the aforementioned biomarkers had a reportedly positive association with Ki LI [4]. Persson and Englund [11] discerned that high values of Ki LI were not significantly associated with high h-TERT staining. The current study showed that high Ki LI had a poor impact on OS and PFS. This is in agreement with Persson and Englund [11], and in contrast with other studies, which noted no effect of Ki LI on OS [11,27,28]. The prognostic value of Ki LI in GBM is still uncertain, as the distribution of proliferative index was different in variable areas within the same tumor and different cutoff points are used in the literature.
- Overall, there was a strong positive correlation between ALK-1 and h-TERT IHC protein expression in the current research. All GBM cases displayed necrosis and/or microvascular proliferation, which is essential for their diagnosis [29]. These findings were corresponding to a hypoxic state of the microenvironment. ALK expression was significantly higher in tumor cells in hypervascular lesions as compared to those adjacent to necrotic foci in GBMs, and was positively correlated with the microvascular density as determined by CD34 expression. Overexpression of ALK induced an enhancement of the HIF-1α/vascular endothelial growth factor-A axis through activation of Stat3 [4]. Furthermore, a study done by Marzec et al. [30] reported that ALKpositive T-cell lymphoma expresses HIF1α. HIF1α mRNA expression is induced in ALK-positive T-cell lymphoma by the transcription of nucleophosmin/ALK tyrosine kinase. NPM/ ALK activates the HIF1α gene through the STAT3 transcription factor [30].
- GBMs contain considerable hypoxic areas within the tumor. Potharaju et al. [10] suggested that an intratumoral decrease in oxygen concentration can augment HIF-1α expression and enhance h-TERT transcription and telomerase, which in turn lead to proliferation of cancer stem cells [26]. Consequently, both ALK-1 and h-TERT expression are highly expressed in the hypervascular area of GBM, depending on HIF-1α activation. This may explain the close relationship between ALK and h-TERT expression in GBM.
- Regarding OS, high ALK-1 expression, high h-TERT expression, and ALK-A were associated with poor OS in the studied cases. This result was in congruent with a study [4] showing that loss of ALK expression had improved OS in contrast to the ALK-positive cases and another report [10] showing that patients with absent or weak h-TERT expression had significantly longer OS than patients with strong h-TERT expression. However, the current findings are inconsistent with those of Karagkounis et al. [5] and Persson and Englund [11], which noted no significant impact of ALK-1 and h-TERT expression on OS, respectively.
- Interestingly, we found that ALK gene alteration was an independent prognostic factor for OS. This in agreement with a previous observation, which revealed that patients who had ALK-A died within one month from diagnosis [5].
- In the current study, the type of therapeutic modality was positively associated with both ALK-1 protein expression and ALK gene alterations. Patients who were treated with adjuvant radiotherapy only had poor survival outcomes compared to those who were treated with CCRT or CCRT and maintenance TMZ. The best survival outcome was among patients who were treated with adjuvant CCRT and maintenance TMZ. A potential therapeutic application in patients harboring ALK-A was noted in in vitro studies that applied specific ALK inhibitors to cell lines with ALK-A [7]. Furthermore, Le Rhun et al. [13] suggested that GBM patients with ALK protein expression and ALK-CNG could benefit from novel targeted ALK inhibitors (crizotinib). Therefore, ALK-targeting therapy may be used for treatment of GBM patients with ALK gene alterations, taking into consideration the h-TERT mutation.
- The limitation of this research is that it lacks application of IDH-1 IHC for subdivision of GBM into molecular subtypes according to IDH status and for correlation with ALK-1 and h-TERT IHC, which will add to the research. This point is recommended in future studies.
- In summary, Break-Apart ALK FISH is a reliable diagnostic technique that can be applied with ease on FFPE tissue whenever the exact fusion partners are indefinite. Furthermore, ALK gene alterations have a significant prognostic impact on GBM patients. Subsequent studies with a larger number of GBM cases are recommended for better evaluation of the role of ALK-1, h-TERT, and ALK gene alterations in tumor progression and related mechanisms.
- The current work concluded that high protein expression of ALK-1, h-TERT, and ALK-A had poor impact on the prognosis of GBM. Accordingly, ALK-1 protein expression, h-TERT protein expression, and ALK gene alteration detection could be used as valuable prognostic markers in GBM patients. The type of therapeutic modality was positively associated with ALK gene alterations. The use of ALK-targeting therapy as part of treatment plan for GBM patients with ALK gene alterations is the goal, and requires further studies with a larger sample size.
DISCUSSION
Ethics Statement
The research was approved by the Committee of Medical Ethics, Faculty of Medicine, Assiut University IRB. No. 17300482. A written informed consent for participation and publication was obtained from each participant after receiving information about the details of the study. Confidentiality of patients’ records was assured and maintained throughout the study.
Availability of Data and Material
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: DE, MTH. Data curation: DE, MTH, DFT. Formal analysis: DE, MTH. Methodology: DE, MTH, DFT. Resources: DE, MTH, DFT. Writing—original draft: DE, MTH. Writing—review & editing: AMA, AH. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.

| Parameter |
ALK-1 IHC |
h-TERT IHC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | p-value | Score 1 | Score 2 | Score 3 | p-value | |
| Age (yr) | .236 | .123a | |||||||
| < 50 | 2 (11.8) | 6 (35.3) | 6 (35.3) | 3 (17.6) | 3 (17.6) | 8 (47.1) | 6 (35.3) | ||
| ≥ 50 | 6 (16.7) | 9 (25.0) | 7 (19.4) | 14 (38.9) | 5 (13.9) | 8 (22.2) | 23 (63.9) | ||
| Sex | .038 | .267a | |||||||
| Male | 7 (19.4) | 6 (16.7) | 10 (27.8) | 13 (36.1) | 6 (16.7) | 13 (36.1) | 17 (47.2) | ||
| Female | 1 (5.9) | 9 (52.9) | 3 (17.6) | 4 (23.5) | 2 (11.8) | 3 (17.6) | 12 (70.6) | ||
| Site | .425 | .119 | |||||||
| CC | 2 (50.0) | 1 (25.0) | 0 | 1 (25.0) | 0 | 3 (75.0) | 1 (25.0) | ||
| FP | 1 (8.4) | 4 (33.3) | 4 (33.3) | 3 (25.0) | 2 (16.7) | 2 (16.7) | 8 (66.6) | ||
| PO | 2 (13.3) | 7 (46.7) | 2 (13.3) | 4 (26.7) | 5 (33.3) | 2 (13.3) | 8 (53.4) | ||
| TO | 2 (28.5) | 1 (14.3) | 3 (42.9) | 1 (14.3) | 1 (14.3) | 4 (57.1) | 2 (28.6) | ||
| PS | 0 | 0 | 2 (33.3) | 4 (66.7) | 0 | 1 (16.6) | 5 (83.4) | ||
| TP | 1 (11.2) | 2 (22.2) | 2 (22.2) | 4 (44.4) | 0 | 4 (44.4) | 5 (55.6) | ||
| Calcification | .315 | .016* | |||||||
| Absent | 1 (4.8) | 6 (28.6) | 5 (23.8) | 9 (42.8) | 3 (14.3) | 2 (9.5) | 16 (76.2) | ||
| Present | 7 (21.9) | 9 (28.1) | 8 (25.0) | 8 (25.0) | 5 (15.6) | 14 (43.8) | 13 (40.6) | ||
| Multiplicity | .046 | .501 | |||||||
| Single | 8 (17.1) | 14 (29.8) | 13 (27.7) | 12 (25.5) | 8 (17.0) | 15 (31.9) | 24 (51.1) | ||
| Multiple | 0 | 1 (16.7) | 0 | 5 (83.3) | 0 | 1 (16.7) | 5 (83.3) | ||
| Tumor size | .670 | .834 | |||||||
| <Median | 4 (14.2) | 6 (21.4) | 9 (32.2) | 9 (32.2) | 5 (17.8) | 7 (25.0) | 16 (57.2) | ||
| ≥ Median | 4 (14.2) | 9 (32.2) | 7 (25.0) | 8 (28.6) | 3 (12.0) | 9 (36.0) | 13 (52.0) | ||
| Type of surgical resection | .266 | .133 | |||||||
| GTR | 3 (15.8) | 4 (21.1) | 6 (31.6) | 6 (31.6) | 5 (26.3) | 3 (15.8) | 11 (57.9) | ||
| STR | 5 (20.0) | 7 (28.0) | 7 (28.0) | 6 (24.0) | 3 (12.0) | 11 (44.0) | 11 (44.0) | ||
| Biopsy | 0 | 4 (44.4) | 0 | 5 (55.6) | 0 | 2 (22.0) | 29 (54.7) | ||
| Ki LI (%) | < .001 | .005 | |||||||
| <14 | 8 (21.6) | 15 (40.5) | 10 (27.1) | 4 (10.8) | 8 (21.6) | 14 (37.8) | 15 (40.6) | ||
| ≥14 | 0 | 0 | 3 (18.7) | 13 (81.3) | 0 | 2 (12.5) | 14 (87.5) | ||
| Therapeutic modalities | .030 | .287 | |||||||
| RTH only | 0 | 3 (25.0) | 3 (25.0) | 6 (50.0) | 1 (8.3) | 2 (16.7) | 9 (75.0) | ||
| CCRT | 5 (35.7) | 4 (28.6) | 0 | 5 (35.7) | 4 (28.6) | 3 (21.4) | 7 (50.0) | ||
| CCRT and adjuvant TMZ | 3 (11.2) | 8 (29.6) | 10 (37.0) | 6 (22.2) | 3 (11.1) | 11 (40.7) | 13 (48.2) | ||
Significant at p < .05.
ALK-1, anaplastic lymphoma kinase 1; h-TERT, human telomerase reverse transcriptase; IHC, immunohistochemistry; CC, corpus callosum; FP, fronto-parietal; PO, parieto-occipital; TO, tempero-occipital; PS, parasagittal; TP, tempero-parietal; GTR, gross total resection; STR, subtotal resection; Ki LI, Ki labeling index; RTH, radiotherapy; CCRT, concurrent chemoradiotherapy; TMZ, temozolomide.
aFisher exact test was used in this table except when ≤20% of the cells have expected count less than 5 chi-square test was used instead.
Significant at p < .05.
ALK-1, anaplastic lymphoma kinase 1; h-TERT, human telomerase reverse transcriptase; CC, corpus callosum; FP, fronto-parietal; PO, parieto-occipital; TO, tempero-occipital; PS, parasagittal; TP, tempero-parietal; GTR, gross total resection; STR, subtotal resection; Ki LI, Ki labeling index; IHC, immunohistochemistry; RTH, radiotherapy; CCRT, concurrent chemoradiotherapy; TMZ, temozolomide.
|
Spearman’s rho |
|||
|---|---|---|---|
| h-TERT expression | ALK-1 expression | ALK gene alterations | |
| h-TERT expression | |||
| Correlation coefficient | 1.000 | 0.602 | 0.476 |
| Sig. (2-tailed) | 0.002a | 0.007a | |
| No. | 53 | 53 | 53 |
| ALK-1 expression | |||
| Correlation coefficient | 0.602 | 1.000 | 0.616 |
| Sig. (2-tailed) | 0.002a | 0.001a | |
| No. | 53 | 53 | 53 |
| ALK gene alterations | |||
| Correlation coefficient | 0.476 | 0.616 | 1.000 |
| Sig. (2-tailed) | 0.007a | 0.001a | |
| No. | 53 | 53 | 53 |
Significant at p < 0.5.
OS, overall survival; PFS, progression-free survival; CC, corpus callosum; FP, fronto-parietal; PO, parieto-occipital; TO, tempro-occipital; PS, parasagittal; TP, tempro-parietal; GTR, gross total resection; STR, subtotal resection; Ki LI, Ki labeling index; ALK-1, anaplastic lymphoma kinase 1; IHC, immunohistochemistry; h-TERT, human telomerase reverse transcriptase; RTH, radiotherapy; CCRT, concurrent chemoradiotherapy; TMZ, temozolomide.
- 1. Stoyanov GS, Dzhenkov D, Ghenev P, Iliev B, Enchev Y, Tonchev AB. Cell biology of glioblastoma multiforme: from basic science to diagnosis and treatment. Med Oncol 2018; 35: 27.ArticlePubMedPDF
- 2. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803-20. ArticlePubMedPDF
- 3. Chiarle R, Voena C, Ambrogio C, Piva R, Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 2008; 8: 11-23. ArticlePubMedPDF
- 4. Chiba R, Akiya M, Hashimura M, et al. ALK signaling cascade confers multiple advantages to glioblastoma cells through neovascularization and cell proliferation. PLoS One 2017; 12: e0183516.ArticlePubMedPMC
- 5. Karagkounis G, Stranjalis G, Argyrakos T, et al. Anaplastic lymphoma kinase expression and gene alterations in glioblastoma: correlations with clinical outcome. J Clin Pathol 2017; 70: 593-9. ArticlePubMed
- 6. Wojas-Krawczyk K, Krawczyk PA, Ramlau RA, et al. The analysis of ALK gene rearrangement by fluorescence in situ hybridization in non-small cell lung cancer patients. Contemp Oncol (Pozn) 2013; 17: 484-92. PubMedPMC
- 7. Zito Marino F, Botti G, Aquino G, et al. Unproductive effects of ALK gene amplification and copy number gain in non-small-cell lung cancer: ALK gene amplification and copy gain in NSCLC. Int J Mol Sci 2020; 21: 4927.ArticlePubMedPMC
- 8. Hafezi F, Perez Bercoff D. The solo play of TERT promoter mutations. Cells 2020; 9: 749.ArticlePubMedPMC
- 9. Leao R, Apolonio JD, Lee D, Figueiredo A, Tabori U, Castelo-Branco P. Mechanisms of human telomerase reverse transcriptase (hTERT) regulation: clinical impacts in cancer. J Biomed Sci 2018; 25: 22.PubMedPMC
- 10. Potharaju M, Mathavan A, Mangaleswaran B, et al. Clinicopathological analysis of HIF-1alpha and TERT on survival outcome in glioblastoma patients: a prospective, single institution study. J Cancer 2019; 10: 2397-406. ArticlePubMedPMC
- 11. Persson A, Englund E. Different assessments of immunohistochemically stained Ki-67 and hTERT in glioblastoma multiforme yield variable results: a study with reference to survival prognosis. Clin Neuropathol 2008; 27: 224-33. ArticlePubMed
- 12. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987-96. ArticlePubMed
- 13. Le Rhun E, Chamberlain MC, Zairi F, et al. Patterns of response to crizotinib in recurrent glioblastoma according to ALK and MET molecular profile in two patients. CNS Oncol 2015; 4: 381-6. ArticlePubMedPMC
- 14. Alidousty C, Duerbaum N, Wagener-Ryczek S, et al. Prevalence and potential biological role of TERT amplifications in ALK translocated adenocarcinoma of the lung. Histopathology 2021; 78: 578-85. ArticlePubMedPDF
- 15. Saha R, Chatterjee U, Mandal S, Saha K, Chatterjee S, Ghosh SN. Expression of phosphatase and tensin homolog, epidermal growth factor receptor, and Ki-67 in astrocytoma: a prospective study in a tertiary care hospital. Indian J Med Paediatr Oncol 2014; 35: 149-55. ArticlePubMedPMC
- 16. McLeer-Florin A, Moro-Sibilot D, Melis A, et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol 2012; 7: 348-54. ArticlePubMed
- 17. Salido M, Pijuan L, Martinez-Aviles L, et al. Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J Thorac Oncol 2011; 6: 21-7. ArticlePubMedPMC
- 18. Hudson L, Kulig K, Young D, McLendon R, Abemethy A. ALK and cMET expression in glioblastoma multiforme: implications for therapeutic targeting. Mol Cancer Ther 2011; 10(11 Suppl): A42.
- 19. Kulig K, McLendon RE, Locke SC, et al. MET and ALK in glioblastoma multiforme (GBM): comparison of IHC and FISH. J Clin Oncol 2012; 30(15 Suppl): 2021.ArticlePubMed
- 20. Peretti U, Ferrara R, Pilotto S, et al. ALK gene copy number gains in non-small-cell lung cancer: prognostic impact and clinico-pathological correlations. Respir Res 2016; 17: 105.ArticlePubMedPMC
- 21. Lee JS, Lim SM, Rha SY, et al. Prognostic implications of anaplastic lymphoma kinase gene aberrations in rhabdomyosarcoma; an immunohistochemical and fluorescence in situ hybridisation study. J Clin Pathol 2014; 67: 33-9. ArticlePubMed
- 22. Schoppmann SF, Streubel B, Birner P. Amplification but not translocation of anaplastic lymphoma kinase is a frequent event in oesophageal cancer. Eur J Cancer 2013; 49: 1876-81. ArticlePubMed
- 23. Del Grosso F, De Mariano M, Passoni L, Luksch R, Tonini GP, Longo L. Inhibition of N-linked glycosylation impairs ALK phosphorylation and disrupts pro-survival signaling in neuroblastoma cell lines. BMC Cancer 2011; 11: 525.PubMedPMC
- 24. Masui K, Komori T, Kato Y, et al. Elevated TERT expression in TERT-wildtype adult diffuse gliomas: histological evaluation with a novel TERT-specific antibody. Biomed Res Int 2018; 2018: 7945845.ArticlePubMedPMCPDF
- 25. Leclerc C, Haeich J, Aulestia FJ, et al. Calcium signaling orchestrates glioblastoma development: facts and conjunctures. Biochim Biophys Acta 2016; 1863: 1447-59. ArticlePubMed
- 26. Nishi H, Nakada T, Kyo S, Inoue M, Shay JW, Isaka K. Hypoxia-inducible factor 1 mediates upregulation of telomerase (hTERT). Mol Cell Biol 2004; 24: 6076-83. ArticlePubMedPMCPDF
- 27. Alkhaibary A, Alassiri AH, AlSufiani F, Alharbi MA. Ki-67 labeling index in glioblastoma; does it really matter? Hematol Oncol Stem Cell Ther 2019; 12: 82-8. ArticlePubMed
- 28. Tsidulko AY, Kazanskaya GM, Kostromskaya DV, et al. Prognostic relevance of NG2/CSPG4, CD44 and Ki-67 in patients with glioblastoma. Tumour Biol 2017; 39: 1010428317724282.ArticlePubMedPDF
- 29. Abdelzaher E. Glioblastoma multiforme, NOS [Internet] Bingham Farms: PathologyOutlines.com, 2020 [cited 2020 May 27]. Available from: https://www.pathologyoutlines.com/topic/cnstumorglioblastomagiantcell.html.
- 30. Marzec M, Liu X, Wong W, et al. Oncogenic kinase NPM/ALK induces expression of HIF1alpha mRNA. Oncogene 2011; 30: 1372-8. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Vascular endothelial cells as signaling niches for epithelial stem cells in the skin
Tudorita Tumbar, Torsa Ganguly, Cailin E. McMahon, Mohammad A. Tavallaei
Frontiers in Cell and Developmental Biology.2026;[Epub] CrossRef - TERT Gene Mutation in Gliomas Cross‐Linked With (NTRK, PDL1, ALK, IDH, P53, EGFR, HER2): A Integrative Review TERT Gene Mutation in Gliomas
Gunter Gerson, Guilherme Nobre Nogueira, Iasmin Maria Rodrigues Saldanha, Ana Gabriela Ponte Farias, Cauan Miranda Mateus, Osvaldo Mariano Viana Neto, Maria Jânia Teixeira
Journal of Surgical Oncology.2025; 131(6): 1202. CrossRef - Mapping chromatin remodelling in glioblastoma identifies epigenetic regulation of key molecular pathways and novel druggable targets
Claire Vinel, James Boot, Weiwei Jin, Nicola Pomella, Alexandra Hadaway, Charles Mein, Nicolae Radu Zabet, Silvia Marino
BMC Biology.2025;[Epub] CrossRef - Association of human telomerase reverse transcriptase promoter mutation with unfavorable prognosis in glioma: A systematic review and meta-analysis
Rongxuan Hua, Qiuxuan Li, Han Gao, Boya Wang, Chengwei He, Ying Wang, Sitian Zhang, Lei Gao, Hongwei Shang, Wen Wang, Jingdong Xu
Journal of Research in Medical Sciences.2023;[Epub] CrossRef - Immunohistochemical surrogates for molecular alterations for the classification and grading of gliomas
Viharkumar Patel, Sanda Alexandrescu
Seminars in Diagnostic Pathology.2022; 39(1): 78. CrossRef - Meme Kanseri Hastalarında hTERT Gen Ekspresyonunun Klinikopatolojik Önemi
Ebubekir DİRİCAN, Burak KANKAYA, Zeynep TATAR
Sağlık Bilimlerinde Değer.2022; 12(1): 22. CrossRef - Prognostic and predictive markers in glioblastoma and ALK overexpression
Jang-Hee Kim
Journal of Pathology and Translational Medicine.2021; 55(3): 236. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
| Variable | No. (%) |
|---|---|
| Age (yr) | |
| < 50 | 17 (32.1) |
| ≥ 50 | 36 (67.9) |
| Sex | |
| Male | 36 (67.9) |
| Female | 17 (32.1) |
| Site | |
| CC | 4 (7.5) |
| FP | 12 (22.6) |
| PO | 15 (28.4) |
| TO | 7 (13.2) |
| PS | 6 (11.3) |
| TP | 9 (17.0) |
| Calcification | |
| Absent | 21 (39.6) |
| Present | 32 (60.4) |
| Multiplicity | |
| Single | 47 (88.7) |
| Multiple | 6 (11.3) |
| Tumor size | |
| Median (interquartile range) | 5 (4–7) |
| Type of surgical resection | |
| GTR | 19 (35.0) |
| STR | 25 (47.0) |
| Biopsy | 9 (17.0) |
| Ki LI (%) | |
| < 14 | 37 (69.8) |
| ≥ 14 | 16 (30.2) |
| Status | |
| Living | 21 (39.6) |
| Dead | 32 (60.4) |
| Therapeutic modalities | |
| RTH only | 12 (22.6) |
| CCRT | 14 (26.4) |
| CCRT and adjuvant TMZ | 27 (50.9) |
| Parameter | ALK-1 IHC |
h-TERT IHC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | p-value | Score 1 | Score 2 | Score 3 | p-value | |
| Age (yr) | .236 | .123 |
|||||||
| < 50 | 2 (11.8) | 6 (35.3) | 6 (35.3) | 3 (17.6) | 3 (17.6) | 8 (47.1) | 6 (35.3) | ||
| ≥ 50 | 6 (16.7) | 9 (25.0) | 7 (19.4) | 14 (38.9) | 5 (13.9) | 8 (22.2) | 23 (63.9) | ||
| Sex | .038 | .267 |
|||||||
| Male | 7 (19.4) | 6 (16.7) | 10 (27.8) | 13 (36.1) | 6 (16.7) | 13 (36.1) | 17 (47.2) | ||
| Female | 1 (5.9) | 9 (52.9) | 3 (17.6) | 4 (23.5) | 2 (11.8) | 3 (17.6) | 12 (70.6) | ||
| Site | .425 | .119 | |||||||
| CC | 2 (50.0) | 1 (25.0) | 0 | 1 (25.0) | 0 | 3 (75.0) | 1 (25.0) | ||
| FP | 1 (8.4) | 4 (33.3) | 4 (33.3) | 3 (25.0) | 2 (16.7) | 2 (16.7) | 8 (66.6) | ||
| PO | 2 (13.3) | 7 (46.7) | 2 (13.3) | 4 (26.7) | 5 (33.3) | 2 (13.3) | 8 (53.4) | ||
| TO | 2 (28.5) | 1 (14.3) | 3 (42.9) | 1 (14.3) | 1 (14.3) | 4 (57.1) | 2 (28.6) | ||
| PS | 0 | 0 | 2 (33.3) | 4 (66.7) | 0 | 1 (16.6) | 5 (83.4) | ||
| TP | 1 (11.2) | 2 (22.2) | 2 (22.2) | 4 (44.4) | 0 | 4 (44.4) | 5 (55.6) | ||
| Calcification | .315 | .016* | |||||||
| Absent | 1 (4.8) | 6 (28.6) | 5 (23.8) | 9 (42.8) | 3 (14.3) | 2 (9.5) | 16 (76.2) | ||
| Present | 7 (21.9) | 9 (28.1) | 8 (25.0) | 8 (25.0) | 5 (15.6) | 14 (43.8) | 13 (40.6) | ||
| Multiplicity | .046 | .501 | |||||||
| Single | 8 (17.1) | 14 (29.8) | 13 (27.7) | 12 (25.5) | 8 (17.0) | 15 (31.9) | 24 (51.1) | ||
| Multiple | 0 | 1 (16.7) | 0 | 5 (83.3) | 0 | 1 (16.7) | 5 (83.3) | ||
| Tumor size | .670 | .834 | |||||||
| <Median | 4 (14.2) | 6 (21.4) | 9 (32.2) | 9 (32.2) | 5 (17.8) | 7 (25.0) | 16 (57.2) | ||
| ≥ Median | 4 (14.2) | 9 (32.2) | 7 (25.0) | 8 (28.6) | 3 (12.0) | 9 (36.0) | 13 (52.0) | ||
| Type of surgical resection | .266 | .133 | |||||||
| GTR | 3 (15.8) | 4 (21.1) | 6 (31.6) | 6 (31.6) | 5 (26.3) | 3 (15.8) | 11 (57.9) | ||
| STR | 5 (20.0) | 7 (28.0) | 7 (28.0) | 6 (24.0) | 3 (12.0) | 11 (44.0) | 11 (44.0) | ||
| Biopsy | 0 | 4 (44.4) | 0 | 5 (55.6) | 0 | 2 (22.0) | 29 (54.7) | ||
| Ki LI (%) | < .001 | .005 | |||||||
| <14 | 8 (21.6) | 15 (40.5) | 10 (27.1) | 4 (10.8) | 8 (21.6) | 14 (37.8) | 15 (40.6) | ||
| ≥14 | 0 | 0 | 3 (18.7) | 13 (81.3) | 0 | 2 (12.5) | 14 (87.5) | ||
| Therapeutic modalities | .030 | .287 | |||||||
| RTH only | 0 | 3 (25.0) | 3 (25.0) | 6 (50.0) | 1 (8.3) | 2 (16.7) | 9 (75.0) | ||
| CCRT | 5 (35.7) | 4 (28.6) | 0 | 5 (35.7) | 4 (28.6) | 3 (21.4) | 7 (50.0) | ||
| CCRT and adjuvant TMZ | 3 (11.2) | 8 (29.6) | 10 (37.0) | 6 (22.2) | 3 (11.1) | 11 (40.7) | 13 (48.2) | ||
| ALK gene alterations |
p-value | ||||
|---|---|---|---|---|---|
| Negative | Rearrangement | Gain | Amplification | ||
| Age (yr) | .430 | ||||
| < 50 | 7 (41.2) | 7 (41.2) | 3 (17.6) | 0 | |
| ≥ 50 | 12 (33.3) | 11 (30.6) | 9 (25.0) | 4 (11.1) | |
| Sex | .181 | ||||
| Male | 10 (27.8) | 12 (33.3) | 10 (27.8) | 4 (11.1) | |
| Female | 9 (52.9) | 6 (35.3) | 2 (11.8) | 0 | |
| Site | .955 | ||||
| CC | 2 (50.0) | 1 (20.0) | 1 (20.0) | 0 | |
| FP | 4 (33.3) | 3 (25.0) | 3 (25.0) | 2 (16.7) | |
| PO | 6 (40.0) | 5 (33.3) | 3 (20.0) | 1 (6.7) | |
| TO | 2 (28.6) | 3 (42.8) | 1 (14.3) | 1 (14.3) | |
| PS | 2 (33.3) | 1 (16.7) | 3 (50.0) | 0 | |
| TP | 3 (33.3) | 5 (55.6) | 1 (11.1) | 0 | |
| Calcification | .710 | ||||
| Absent | 6 (28.6) | 7 (33.3) | 6 (28.6) | 2 (9.5) | |
| Present | 13 (40.6) | 11 (34.4) | 6 (18.8) | 2 (6.2) | |
| Multiplicity | .162 | ||||
| Single | 19 (40.4) | 15 (31.9) | 10 (21.3) | 3 (6.4) | |
| Multiple | 0 | 3 (50.0) | 2 (33.3) | 1 (16.7) | |
| Size | .836 | ||||
| < Median | 12 (38.7) | 9 (29.0) | 7 (22.6) | 3 (9.7) | |
| > Median | 7 (31.8) | 9 (40.9) | 5 (22.7) | 1 (4.6) | |
| Type of surgical resection | .125 | ||||
| GTR | 9 (47.4) | 3 (15.8) | 5 (26.3) | 2 (10.5) | |
| STR | 8 (32.0) | 9 (36.0) | 7 (28.0) | 1 (4.0) | |
| Biopsy | 2 (22.2) | 6 (66.7) | 0 | 1 (11.1) | |
| Ki- LI | .044 | ||||
| < 14% | 15 (40.5) | 15 (40.5) | 6 (16.2) | 1 (2.8) | |
| ≥ 14% | 4 (25.0) | 3 (18.8) | 6 (37.4) | 3 (18.8) | |
| ALK-1 IHC | .001 | ||||
| Score 0 | 8 (100) | 0 | 0 | 0 | |
| Score 1 | 6 (40.0) | 9 (60.0) | 0 | 0 | |
| Score 2 | 2 (15.4) | 5 (38.4) | 6 (46.2) | 0 | |
| Score 3 | 3 (17.7) | 4 (23.5) | 6 (35.3) | 4 (23.5) | |
| h-TERT IHC | .008 | ||||
| Score 1 | 6 (75.0) | 2 (25.0) | 0 | 0 | |
| Score 2 | 6 (37.5) | 9 (56.2) | 1 (6.3) | 0 | |
| Score 3 | 7 (24.1) | 7 (24.1) | 11 (38.0) | 4 (13.8) | |
| Therapeutic modalities | .027 | ||||
| RTH | 2 (16.7) | 3 (25.0) | 3 (25.0) | 4 (33.3) | |
| CCRT & adjuvant TMZ | 11 (40.7) | 10 (37.0) | 6 (22.3) | 0 | |
| CCRT | 6 (42.9) | 5 (35.7) | 3 (21.4) | 0 | |
| Spearman’s rho |
|||
|---|---|---|---|
| h-TERT expression | ALK-1 expression | ALK gene alterations | |
| h-TERT expression | |||
| Correlation coefficient | 1.000 | 0.602 | 0.476 |
| Sig. (2-tailed) | 0.002 |
0.007 |
|
| No. | 53 | 53 | 53 |
| ALK-1 expression | |||
| Correlation coefficient | 0.602 | 1.000 | 0.616 |
| Sig. (2-tailed) | 0.002 |
0.001 |
|
| No. | 53 | 53 | 53 |
| ALK gene alterations | |||
| Correlation coefficient | 0.476 | 0.616 | 1.000 |
| Sig. (2-tailed) | 0.007 |
0.001 |
|
| No. | 53 | 53 | 53 |
| Parameter | OS |
PFS |
||
|---|---|---|---|---|
| Log-rank (chi-square) | p-value | Log-rank (chi-square) | p-value | |
| Age (< 50 yr vs. ≥ 50 yr) | 3.146 | .076 | 2.419 | .120 |
| Sex (male vs. female) | 3.326 | .119 | 3.887 | .153 |
| Site (CC vs. FP vs. PO vs. TO vs. PS vs. TP) | 3.398 | .639 | 4.216 | .519 |
| Calcification (absent vs. present) | 0.666 | .414 | 0.253 | .615 |
| Multiplicity (single vs. multiple) | 3.993 | .254 | 3.505 | .190 |
| Size (<median vs. > median) | 0.010 | .921 | 0.048 | .827 |
| Type of surgical resection (GTR vs. STR vs. biopsy) | 0.066 | .967 | 0.168 | .919 |
| Ki LI (< 14% vs. ≥ 14%) | 5.758 | .016 | 5.267 | .022 |
| ALK-1 IHC (score 0 vs. score 1 vs. score 2 vs. score 3) | 19.639 | < .001 | 21.654 | < .001 |
| h-TERT IHC (score 1 vs. score 2 vs. score 3) | 6.919 | .031 | 6.433 | .040 |
| ALK gene alterations (negative rearrangement vs. gain amplification) | 86.062 | < .001 | 76.436 | < .001 |
| Therapeutic modalities (RTH only vs. CCRT vs. CCRT and adjuvant TMZ) | 13.797 | .002 | 14.357 | .004 |
| OS |
PFS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | p-value | HR | 95 % CI for Exp (B) | B | SE | p-value | HR | 95 % CI for Exp (B) | |
| Ki LI | 0.125 | 0.468 | .790 | 1.133 | 0.453–2.833 | –0.001 | 0.435 | .998 | 0.099 | 0.426–2.344 |
| ALK-1 protein expression | 0.357 | 0.347 | .304 | 0.429 | 0.724–2.821 | 0.505 | 0.306 | .099 | 1.657 | 0.909–3.010 |
| h-TERT protein expression | –0.368 | 0.411 | .371 | 0.692 | 0.310–1.549 | –0.352 | 0.356 | .322 | 0.703 | 0.350–1.411 |
| ALK gene alterations | 2.017 | 0.421 | < .001 | 7.514 | 3.292–17.155 | 1.550 | 0.338 | < .001 | 4.711 | 2.429–9.136 |
| Therapeutic modalities | 0.050 | 0.311 | .872 | 1.052 | 0.571–1.935 | –0.007 | 0.295 | .982 | 0.993 | 0.558–1.769 |
CC, corpus callosum; FP, fronto-parietal; PO, parieto-occipital; TO, tempero-occipital; PS, parasagittal; TP, tempero-parietal; GTR, gross total resection; STR, subtotal resection; Ki LI, Ki labeling index; RTH, radiotherapy; CCRT, concurrent chemoradiotherapy; TMZ, temozolomide.
Significant at p < .05. ALK-1, anaplastic lymphoma kinase 1; h-TERT, human telomerase reverse transcriptase; IHC, immunohistochemistry; CC, corpus callosum; FP, fronto-parietal; PO, parieto-occipital; TO, tempero-occipital; PS, parasagittal; TP, tempero-parietal; GTR, gross total resection; STR, subtotal resection; Ki LI, Ki labeling index; RTH, radiotherapy; CCRT, concurrent chemoradiotherapy; TMZ, temozolomide. Fisher exact test was used in this table except when ≤20% of the cells have expected count less than 5 chi-square test was used instead.
Significant at p < .05. ALK-1, anaplastic lymphoma kinase 1; h-TERT, human telomerase reverse transcriptase; CC, corpus callosum; FP, fronto-parietal; PO, parieto-occipital; TO, tempero-occipital; PS, parasagittal; TP, tempero-parietal; GTR, gross total resection; STR, subtotal resection; Ki LI, Ki labeling index; IHC, immunohistochemistry; RTH, radiotherapy; CCRT, concurrent chemoradiotherapy; TMZ, temozolomide.
ALK-1, anaplastic lymphoma kinase 1; h-TERT, human telomerase reverse transcriptase; IHC, immunohistochemistry. Significant; correlation is significant at the 0.01 level (2-tailed).
Significant at p < 0.5. OS, overall survival; PFS, progression-free survival; CC, corpus callosum; FP, fronto-parietal; PO, parieto-occipital; TO, tempro-occipital; PS, parasagittal; TP, tempro-parietal; GTR, gross total resection; STR, subtotal resection; Ki LI, Ki labeling index; ALK-1, anaplastic lymphoma kinase 1; IHC, immunohistochemistry; h-TERT, human telomerase reverse transcriptase; RTH, radiotherapy; CCRT, concurrent chemoradiotherapy; TMZ, temozolomide.
Significant at p < 0.5. OS, overall survival; PFS, progression-free survival; HR, hazards ratio; CI, confident interval; Ki LI, Ki labeling index; ALK-1, anaplastic lymphoma kinase 1; h-TERT, human telomerase reverse transcriptase.

 E-submission
E-submission