Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 56(4); 2022 > Article
-
Original Article
Correlation between myoferlin expression and lymph node metastasis in papillary thyroid carcinoma -
Ji Min Na1
 , Dong Chul Kim1,2,3
, Dong Chul Kim1,2,3 , Dae Hyun Song2,3,4
, Dae Hyun Song2,3,4 , Hyo Jung An4
, Hyo Jung An4 , Hyun Min Koh5
, Hyun Min Koh5 , Jeong-Hee Lee1,2,3
, Jeong-Hee Lee1,2,3 , Jong Sil Lee1,2,3
, Jong Sil Lee1,2,3 , Jung Wook Yang1,2,3
, Jung Wook Yang1,2,3 , Min Hye Kim1
, Min Hye Kim1
-
Journal of Pathology and Translational Medicine 2022;56(4):199-204.
DOI: https://doi.org/10.4132/jptm.2022.03.19
Published online: May 11, 2022
1Department of Pathology, Gyeongsang National University Hospital, Jinju, Korea
2Department of Pathology, Gyeongsang National University School of Medicine, Jinju, Korea
3Gyeongsang Institute of Health Science, Jinju, Korea
4Department of Pathology, Changwon Gyeongsang National University Hospital, Changwon, Korea
5Department of Pathology, Jeju National University Hospital, Jeju, Korea
- Corresponding Author: Dong Chul Kim, MD, PhD, Department of Pathology, Gyeongsang National University School of Medicine, 15 Jinju-daero 816beon-gil, Jinju 52727, Korea, Tel: +82-55-772-8060, Fax: +82-55-759-7952, E-mail: kdcjes@gnu.ac.kr
© 2022 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,079 Views
- 180 Download
Abstract
-
Background
- Myoferlin is a multifunctional protein expressed in various normal and cancer cells, with novel oncogenic roles being newly discovered. Recently, correlations have been found between myoferlin expression and unfavorable prognosis in various carcinomas. This study investigated the prognostic role of myoferlin expression in papillary thyroid carcinoma (PTC), specifically that associated with nodal metastasis.
-
Methods
- We collected clinicopathological data and PTC tissues from 116 patients who had been admitted to Gyeongsang National University Hospital in 2010. Immunohistochemical analysis was performed on surgical specimen-derived tissue microarray blocks. Myoferlin expression was graded, and the relationship between expression level and pathological features of tumors based on the American Joint Committee on Cancer staging system was evaluated.
-
Results
- Of the 116 patient samples, 100 cases exhibited positive myoferlin expression. Higher grade of myoferlin expression was correlated with lower T category group (p = .010). Presence of lymph node metastasis was determined to be significantly correlated with low-grade myoferlin expression (p = .019), with no significant difference between pN1a and pN1b tumors.
-
Conclusions
- Our study revealed an adverse correlation between myoferlin expression and pathological features of PTC, evidence of the potential prognostic role of myoferlin in PTC lymph node metastasis.
- Case selection
- We collected clinicopathological data from the electronic medical records of PTC patients who underwent either total thyroidectomy or lobectomy in 2010 at Gyeongsang National University Hospital, Jinju, Korea. Cases with morphologic variants other than conventional (classic) PTC were excluded. The TNM stages of the tumors were assessed according to the American Joint Committee on Cancer staging manual, 8th edition [2]. All gross photographs and hematoxylin and eosin-stained slides of the surgical specimens were reviewed by two pathologists.
- Tissue microarray
- Tumor specimens from the selected cases were fixed overnight in buffered neutral formalin (10%) and embedded in paraffin blocks. Representative cores 2 mm in diameter were selected and obtained from each paraffin block after microscopic examination. They were then arranged onto new recipient tissue microarray (TMA) blocks.
- Immunohistochemical analysis
- To investigate protein expression, immunohistochemical staining was performed on 4 μm sections from the TMA blocks. The sections were deparaffinized, rehydrated, and incubated in 3% hydrogen peroxide. After heating in 10 mM citrate buffer (pH 6.0), the slides were incubated with a mouse monoclonal primary myoferlin antibody (1:100, 7D2, ab76746, Abcam, Cambridge, UK). 3,3'-Diaminobenzidine staining and counterstaining of hematoxylin were carried out for visualization. The expression of myoferlin in the tumor cells was graded from negative to weak (+1), moderate (+2), and strong (+3) positivity by two experienced pathologists. Normal thyroid follicular cells were used as the internal control. If tumor cells exhibited equivalent intensity of myoferlin expression compared to normal follicular cells, they were graded as moderate positive. Similarly, tumor cells that exhibited stronger or weaker expression were graded as strong and weak positive, respectively.
- Statistical analysis
- Pearson’s chi square test and Fisher’s exact test were used to evaluate correlations between myoferlin expression level and a range of clinicopathological features including age and sex of patients and the size, extension, lymph node metastasis, and pathologic T category and N category in AJCC staging of the tumors. For all statistical analyses, differences were considered statistically significant at p < .05. IBM SPSS Statistics for Windows, ver. 21.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis.
MATERIALS AND METHODS
- Clinicopathological features
- Table 1 summarizes the clinical data and pathological features of the patients enrolled in this study. Of the 116 PTC patients enrolled, the mean age was 49.5 years (range, 25 to 88 years), with 20 male (17.2%) and 96 female (82.8%) patients. The largest diameter tumor sizes ranged from 0.1–6.0 cm (mean, 1.12 cm). Notably, in 11 (9.5%) of these cases, gross tumor extension beyond the thyroid gland was observed. Most patients (96/116, 82.7%) had pT1 carcinomas, whereas seven (6%), eight (6.9%), and five (4.3%) were observed for pT2, pT3, and pT4 tumors, respectively. Lymph node dissection was performed in 51 (44.0%) patients, of whom 24 (20.7%) were found to have lymph node PTC metastasis. There were 17 (14.7%) pN1a tumors and seven (6.0%) pN1b tumors. No distant metastasis was observed in any of the cases.
- Myoferlin expression in PTC
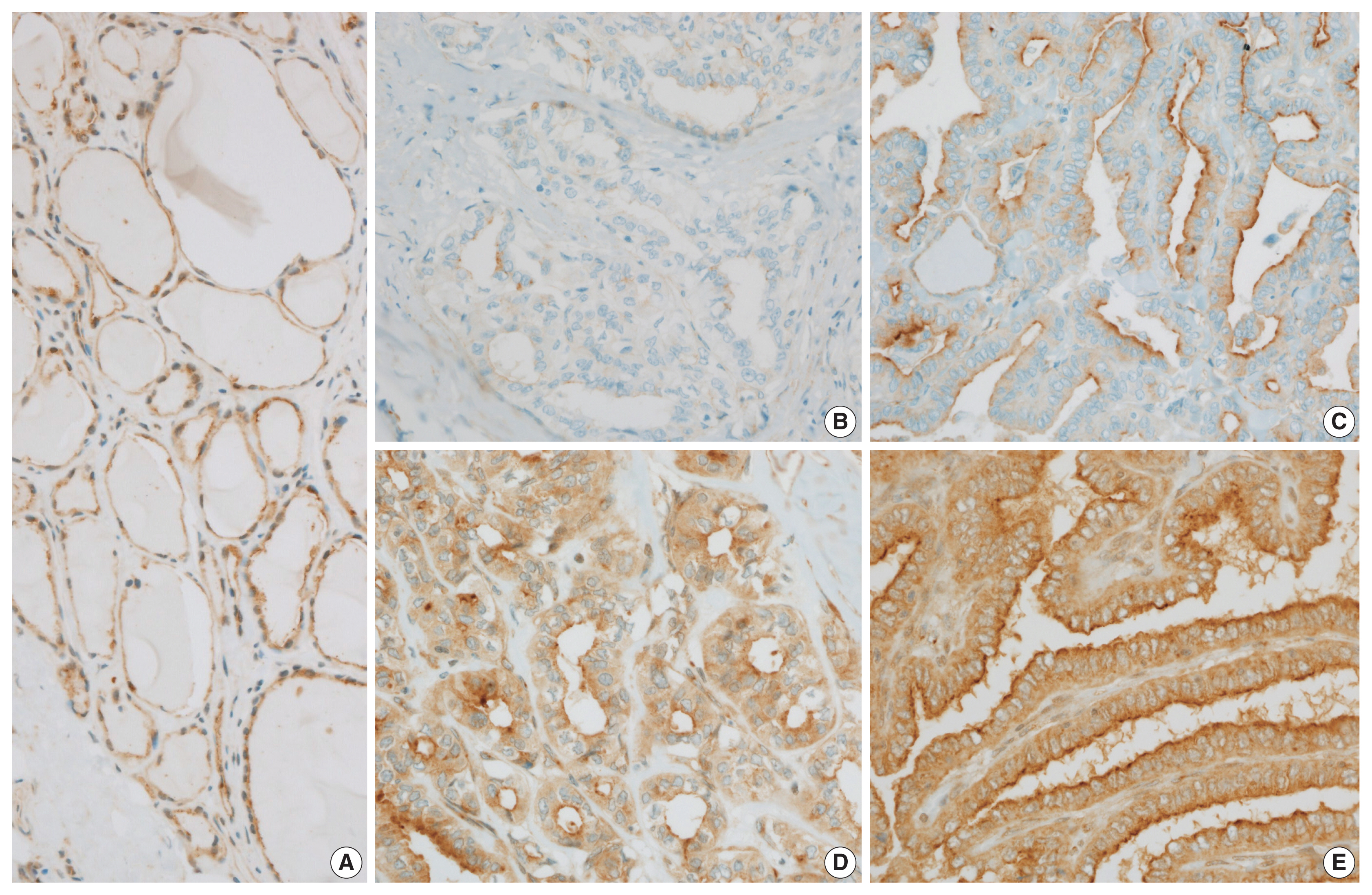
- Normal thyroid follicular cells exhibited myoferlin expression in the cell cytoplasm and membrane (Fig. 1A). Myoferlin expression in PTC cells was observed in 100 cases (86.2%), showing a even expression pattern involving the cytoplasm and membrane (Fig. 1B–E). Myoferlin expression level was graded as weak (+1) in 35 cases (30.2%), moderate (+2) in 53 cases (45.75%), and strong (+3) in 12 cases (10.3%) (Table 1). To facilitate reproducibility, these grading categories were further simplified into two categories: low (negative or only weakly positive, +1) and high (moderate, +2 to strong, +3) expression levels. Of all patients, 51 (44.0%) exhibited negative or weak myoferlin expression level, whereas 65 (56.0%) showed moderate to strong expression.
- Correlation of myoferlin expression with T category and N category of PTC
- Table 2 summarizes the associations between myoferlin expression level and the clinicopathological features of patients. Patient age and sex, tumor size, and presence of gross extrathyroidal extensions did not demonstrate a significant correlation with myoferlin expression of tumors. In pT1 tumors, a larger number exhibited a high (moderate or strong) level of myoferlin expression (n = 60, 51.7%) compared to low (negative or weak) expression (n = 36, 31.0%). In pT4 tumors, it was the opposite, as the number of tumors with a low level of expression (n = 4, 3.4%) outnumbered those with a high level of expression (n = 1, 0.9%). Overall, the T category and myoferlin expression level of tumors exhibited the following tendency: tumors of the higher T category group showed a correlation with a lower level of myoferlin expression (p = .010).
- Among the cases that underwent lymph node dissection, in lymph node metastasis-free (pN0) tumors, six (5.2%) exhibited low myoferlin expression level, whereas high levels were observed in 21 (18.1%). Among lymph node metastatic tumors (pN1), 13 (11.2%) indicated low myoferlin expression levels, whereas 11 (9.5%) cases revealed high levels. Statistically, pN1 tumors with lymph node metastasis were significantly correlated with low myoferlin expression levels (p = .019). Whether the tumor of the pN1 category was pN1a or pN1b showed no significant association with myoferlin expression (p = .386).
RESULTS
- This study confirmed that myoferlin is expressed in PTC cells. In previous studies, myoferlin overexpression was indicative of poor disease survival in various types of carcinomas including non-small cell lung carcinoma [16], pancreatic adenocarcinoma [17], and clear cell renal cell carcinoma [18]. However, our retrospective study data showed a statistically significant correlation of loss of myoferlin expression with a higher T category of tumor and lymph node PTC metastasis. With both features being risk factors for poor survival and disease recurrence, this correlation counters the majority of recent research observations that have reported an evident correlation between higher myoferlin level and unfavorable prognosis.
- In a study from our institution on endometrioid carcinoma, loss of myoferlin expression level correlated with high histologic grade [20]. This result also counters the previously described correlation of high myoferlin expression with poor prognosis in some carcinomas. The continuous cyclic regeneration of normal endometrial tissue was suggested as a possible cause of this correlation. Myoferlin is a multifunctional protein in normal cells, although its role in various cancer cells is unknown. Research findings suggest that myoferlin expression has different tendencies due to its various roles in different types of carcinomas.
- No previous myoferlin studies were performed on endocrine cell–derived tumors. Myoferlin is expressed in normal thyroid follicular cells, but its specific roles in normal cells or oncogenic roles have yet to be studied. Therefore, one plausible hypothesis is that the endocrine activity of thyroid follicular cells influences the distinctive pattern of myoferlin expression in the thyroid. The difference in key functions of myoferlin could possibly be the cause of its expression tendency differing in PTC from lung and pancreatic epithelial tumors. Further molecular studies on the role of myoferlin in different types of tumor cells are needed to clarify the specific function of this protein.
- Of the 116 patients enrolled, there were only four cases with recurrent disease and no patient death during follow-up. With regard to these findings, owing to the limited sample size in the present study, an analysis of a larger cohort is necessary to confirm correlations with survival rates.
- In differentiated papillary carcinoma, therapeutic neck dissection is strongly recommended and routinely performed in clinically palpable or biopsy-proven nodal disease, as it is well established that nodal metastasis is associated with disease recurrence [6]. However, the use of prophylactic neck dissection for clinically node-negative PTC is controversial. If prediction of occult lymph node metastatic status is determinable by pre-surgical cytology or biopsy specimens, a justification could be made for more individualized disease management, which could include determining whether to perform neck dissection or to closely follow-up on the case. The correlation proposed in the present study points to a potential prognostic role of myoferlin in lymph node PTC metastasis.
- Conclusively, our study demonstrated correlation of myoferlin expression with pathological features in papillary thyroid carcinoma. Loss of expression of myoferlin was correlated with a higher T category of tumor and the presence of lymph node metastasis in PTC. This result shows the potential prognostic role of myoferlin in PTC as a helpful biomarker in therapeutic management.
DISCUSSION
Ethics Statement
This study was approved by the Institutional Review Board of Gyeongsang National University Hospital, and the need for informed consent was waived (GNUH-10-026).
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: DCK, DHS. Data curation: JMN, DCK, JHL, JSL, JWY, HJA, MHK. Investigation: DCK, DHS, JHL, JSL, JWY, MHK. Methodology: JMN, DCK. Project administration: DCK, DHS. Resources: DHS. Supervision: DHS, MHK, JWY. Validation: DCK, HJA, HMK. Visualization: JMN. Writing—original draft: JMN, DCK. Writing—review & editing: JMN, DCK, JWY. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This work was supported by the fund of research promotion program, Gyeongsang National University, 2015.
- 1. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see commetns]. Cancer 1998; 83: 2638-48. ArticlePubMed
- 2. Amin MB, Edge S, Greene FL, et al. AJCC cancer staging manual. 8th ed. New York: Spinger, 2017; 873-90.
- 3. Harwood J, Clark OH, Dunphy JE. Significance of lymph node metastasis in differentiated thyroid cancer. Am J Surg 1978; 136: 107-12. ArticlePubMed
- 4. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery 2008; 144: 1070-7. ArticlePubMed
- 5. Nixon IJ, Wang LY, Palmer FL, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery 2014; 156: 137-46. ArticlePubMed
- 6. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26: 1-133. PubMedPMC
- 7. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 2004; 135: 139-48. ArticlePubMed
- 8. Wang LY, Palmer FL, Nixon IJ, et al. Central lymph node characteristics predictive of outcome in patients with differentiated thyroid cancer. Thyroid 2014; 24: 1790-5. ArticlePubMed
- 9. Jeon MJ, Yoon JH, Han JM, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol 2013; 168: 219-25. ArticlePubMed
- 10. Lee J, Song Y, Soh EY. Prognostic significance of the number of metastatic lymph nodes to stratify the risk of recurrence. World J Surg 2014; 38: 858-62. ArticlePubMedPDF
- 11. Doherty KR, Cave A, Davis DB, et al. Normal myoblast fusion requires myoferlin. Development 2005; 132: 5565-75. ArticlePubMedPMCPDF
- 12. Demonbreun AR, Posey AD, Heretis K, et al. Myoferlin is required for insulin-like growth factor response and muscle growth. FASEB J 2010; 24: 1284-95. ArticlePubMedPMCPDF
- 13. Leung C, Yu C, Lin MI, Tognon C, Bernatchez P. Expression of myoferlin in human and murine carcinoma tumors: role in membrane repair, cell proliferation, and tumorigenesis. Am J Pathol 2013; 182: 1900-9. PubMed
- 14. Volakis LI, Li R, Ackerman WEt, et al. Loss of myoferlin redirects breast cancer cell motility towards collective migration. PLoS One 2014; 9: e86110.ArticlePubMedPMC
- 15. Li R, Ackerman WEt, Mihai C, Volakis LI, Ghadiali S, Kniss DA. Myoferlin depletion in breast cancer cells promotes mesenchymal to epithelial shape change and stalls invasion. PLoS One 2012; 7: e39766.ArticlePubMedPMC
- 16. Song DH, Ko GH, Lee JH, et al. Myoferlin expression in non-small cell lung cancer: Prognostic role and correlation with VEGFR-2 expression. Oncol Lett 2016; 11: 998-1006. ArticlePubMedPMC
- 17. Wang WS, Liu XH, Liu LX, et al. iTRAQ-based quantitative proteomics reveals myoferlin as a novel prognostic predictor in pancreatic adenocarcinoma. J Proteomics 2013; 91: 453-65. ArticlePubMed
- 18. Song DH, Ko GH, Lee JH, et al. Prognostic role of myoferlin expression in patients with clear cell renal cell carcinoma. Oncotarget 2017; 8: 89033-9. ArticlePubMedPMC
- 19. Hermanns C, Hampl V, Holzer K, et al. The novel MKL target gene myoferlin modulates expansion and senescence of hepatocellular carcinoma. Oncogene 2017; 36: 3464-76. ArticlePubMedPDF
- 20. Kim MH, Song DH, Ko GH, et al. Myoferlin expression and its correlation with FIGO histologic grading in early-stage endometrioid carcinoma. J Pathol Transl Med 2018; 52: 93-7. ArticlePubMedPMCPDF
- 21. Uhlen M, Fagerberg L, Hallstrom BM, et al. Proteomics: tissue-based map of the human proteome. Science 2015; 347: 1260419.PubMed
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

Fig. 1
| Variable | No. of patients (%) |
|---|---|
| Age (yr), mean (range) | 49.5 (25–88) |
| Sex | |
| Male | 20 (17.2) |
| Female | 96 (82.8) |
| Size (cm), mean (range) | 1.1 (0.1–6.0) |
| Gross extrathyroidal extension | |
| Absent | 105 (90.5) |
| Present | 11 (9.5) |
| T category | |
| T1a | 73 (62.9) |
| T1b | 23 (19.8) |
| T2 | 7 (6.0) |
| T3a | 2 (1.7) |
| T3b | 6 (5.2) |
| T4a | 5 (4.3) |
| N category | |
| NX | 65 (56.0) |
| N0 | 27 (23.3) |
| N1 | 24 (20.7) |
| N1a | 17 (14.7) |
| N1b | 7 (6.0) |
| Myoferlin | |
| Negative | 16 (13.8) |
| Weak (+1) | 35 (30.2) |
| Moderate (+2) | 53 (45.7) |
| Strong (+3) | 12 (10.3) |
| Myoferlin expression | Total (n = 116) | p-value | ||
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Age (yr) | 50.8 ± 13.3 | 48.5 ± 12.1 | .343 | |
| Sex | ||||
| Male | 11 (9.5) | 9 (7.8) | 20 (17.2) | .274 |
| Female | 40 (34.5) | 56 (48.3) | 96 (82.8) | |
| Size (cm) | 1.25 ± 1.12 | 1.02 ± 0.65 | .194 | |
| Gross extrathyroidal extension | ||||
| Absent | 43 (37.0) | 62 (53.4) | 105 (90.5) | .057 |
| Present | 8 (6.9) | 3 (2.6) | 11 (9.5) | |
| T category | ||||
| 1 | 36 (31.0) | 60 (51.7) | 96 (82.8) | .010 |
| 2 | 6 (5.2) | 1 (0.9) | 7 (6.0) | |
| 3 | 5 (4.3) | 3 (2.6) | 8 (6.9) | |
| 4 | 4 (3.4) | 1 (0.9) | 5 (4.3) | |
| N category | ||||
| X | 32 (27.6) | 33 (28.4) | 65 (56.0) | |
| 0 | 6 (5.2) | 21 (18.1) | 27 (23.3) | .019 |
| 1 | 13 (11.2) | 11 (9.5) | 24 (20.7) | |
| 1a | 8 (6.9) | 9 (7.8) | .386 | |
| 1b | 5 (4.3) | 2 (1.7) | ||
| Total | 51 (44.0) | 65 (56.0) | 116 | |
Values are presented as mean ± SD or number (%). SD, standard deviation.

 E-submission
E-submission