Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(3); 2023 > Article
-
Original Article
Clinicopathologic characterization of cervical metastasis from an unknown primary tumor: a multicenter study in Korea -
Miseon Lee1
 , Uiree Jo2
, Uiree Jo2 , Joon Seon Song2
, Joon Seon Song2 , Youn Soo Lee1
, Youn Soo Lee1 , Chang Gok Woo3
, Chang Gok Woo3 , Dong-Hoon Kim4
, Dong-Hoon Kim4 , Jung Yeon Kim5
, Jung Yeon Kim5 , Sun Och Yoon6
, Sun Och Yoon6 , Kyung-Ja Cho2
, Kyung-Ja Cho2
-
Journal of Pathology and Translational Medicine 2023;57(3):166-177.
DOI: https://doi.org/10.4132/jptm.2023.04.12
Published online: May 10, 2023
1Department of Pathology, St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
3Department of Pathology, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
4Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
5Department of Pathology, Inje University Sanggye Paik Hospital, Inje University School of Medicine, Seoul, Korea
6Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author: Kyung-Ja Cho, MD, PhD, Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-4545, Fax: +82-2-472-7898, E-mail: kjc@amc.seoul.kr
© 2023The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Research regarding cervical metastasis from an unknown primary tumor (CUP) according to human papillomavirus (HPV) and Epstein-Barr virus (EBV) status in Korea has been sporadic and small-scale. This study aims to analyze and understand the characteristics of CUP in Korea according to viral and p16 and p53 status through a multicenter study.
-
Methods
- Ninety-five cases of CUP retrieved from six hospitals in Korea between January 2006 and December 2016 were subjected to high-risk HPV detection (DNA in situ hybridization [ISH] or real-time polymerase chain reaction), EBV detection (ISH), and immunohistochemistry for p16 and p53.
-
Results
- CUP was HPV-related in 37 cases (38.9%), EBV-related in five cases (5.3%), and unrelated to HPV or EBV in 46 cases (48.4%). HPV-related CUP cases had the best overall survival (OS) (p = .004). According to the multivariate analysis, virus-unrelated disease (p = .023) and longer smoking duration (p < .005) were prognostic factors for poor OS. Cystic change (p = .016) and basaloid pattern (p < .001) were more frequent in HPV-related cases, and lymphoepithelial lesion was frequent in EBV-related cases (p = .010). There was no significant association between viral status and p53 positivity (p = .341), smoking status (p = .728), or smoking duration (p = .187). Korean data differ from Western data in the absence of an association among HPV, p53 positivity, and smoking history.
-
Conclusions
- Virus-unrelated CUP in Korea had the highest frequency among all CUP cases. HPV-related CUP is similar to HPV-mediated oropharyngeal cancer and EBVrelated CUP is similar to nasopharyngeal cancer in terms of characteristics, respectively.
- Patient selection and patient inclusion as CUP
- Between January 2006 and December 2016, 159 patients diagnosed with metastatic carcinoma in cervical lymph nodes from the unknown primary site were analyzed across six hospitals (Asan Medical Center, Seoul St. Mary’s Hospital, Sanggye Paik Hospital, Kangbuk Samsung Hospital, Chungbuk National University Hospital, and Severance Hospital, Yonsei University College of Medicine) in South Korea.
- Among 159 cases, the primary sites in 64 cases were located after pathologic diagnosis of lymph node metastasis. The remaining 95 cases were categorized as CUP, wherein the primary sites were not identified at the time of study initiation.
- Hematoxylin and eosin–stained slides and formalin-fixed paraffin-embedded tissue were used for the analysis. Tissue microarrays (TMA) were constructed from representative parts of the tumor.
- The research ethics committee of each institution deliberated on this process.
- Clinicopathologic characteristics
- Clinical data were collected through medical records, including age at diagnosis, sex, smoking history, follow-up duration, and clinical outcomes. Pathologists at each hospital reviewed the hematoxylin-and-eosin–stained slides of corresponding hospital cases; confirmed the lymph node location of the metastatic tumor; and determined the size of the largest metastasis, extranodal extension, and N category. Histological findings were also analyzed, including keratinization, cystic change, basaloid pattern, and lymphoepithelial lesions.
- Immunohistochemistry
- Immunohistochemical (IHC) staining was performed on 4-μm sections of TMA using the Ventana autostainer and UltraView DAB detection kit (Ventana Medical Systems, Tucson, AZ, USA), according to the manufacturer’s instructions. The antibodies we used were p16INK4a (1:6, clone E6H4, mouse mAb, Ventana Medical Systems) and p53 (1:1,500, clone M7001, mouse mAb, Dako, Glostrup, Denmark). According to the eighth edition of the AJCC cancer staging manual, p16 immunostaining was positive when it showed greater than a +2/+3 intensity in > 75% of tumor cells. Separately, the result of p53 was positive if nuclear staining was present in > 10% of tumor cells.
- In situ hybridization
- EBV infection was evaluated by RNA in situ hybridization (ISH) (INFORM EBER, Ventana Medical Systems) and HPV infection was evaluated by DNA ISH (INFORM HPV III Family 16 Probe (B), Ventana Medical Systems). The INFORM HPV III Family 16 Probe (B) detects the following high-risk HPV types: 16, 18, 31, 33, 35, 45, 52, 56, 58, and 66. ISH was considered positive when > 70% of tumor cells showed nuclear staining.
- Real-time polymerase chain reaction
- For cases wherein HPV ISH was unavailable, real-time polymerase chain reaction (RT-PCR) was performed. Nucleic acids were extracted from 10-μm (× 5) paraffin tissue sections, and the CFX96TM RT-PCR system (Bio-Rad Laboratories, Hercules, CA, USA) and Anyplex II HPV28 Detection system (31744024, Seegene, Seoul, Korea) were used. Anyplex II HPV28 detection (A) detects the following high-risk HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
- Grouping of cervical metastases according to HPV and EBV status
- Patient cases were divided into three groups according to HPV and EBV status, as follows: HPV-related CUP, EBV-related CUP, and CUP unrelated to both HPV and EBV. HPV-related CUP was defined by results of p16 overexpression via IHC, positive high-risk HPV via HPV ISH, or positive high-risk HPV via RT-PCR analysis. EBV-related CUP was defined by EBV confirmation via EBV ISH. HPV-unrelated and EBV-unrelated CUP was defined by results negative for p16, HPV ISH, HPV RT-PCR, and EBV ISH. Cases were categorized as “not determined” when the HPV or EBV ISH test finding was unavailable.
- N category
- According to the eighth edition of the AJCC cancer staging manual, three different approaches were applied to cases with unknown primary tumors. As the primary T category is T0, the N caegory was determined by different staging systems according to EBV and HPV status, i.e., “nasopharynx” staging for EBVrelated CUP, “HPV-mediated (p16+) oropharyngeal cancer” staging for HPV-related CUP, or “cervical lymph nodes and unknown primary tumors of the head and neck” staging for EBVunrelated and HPV-unrelated CUP.
- Statistical analysis
- Fisher’s exact test analyzed the variance between the three groups, which was then compared between them. Overall survival (OS) was counted from the first diagnosis of CUP to the date of death or final follow-up. Univariate and multivariate Cox proportional hazard regression models were used to identify a significant factor in predicting OS. Kaplan-Meier assessment was used to analyze OS, and the effect of groups on OS was investigated using the log-rank test. The variance (p < .05) significantly affecting OS in the univariate analysis was further tested through multivariate analysis. p < .05 was considered to be statistically significant. In the statistical comparison among groups according to viral status, cases that were “not determined” (n = 7) were excluded.
MATERIALS AND METHODS
- Clinicopathologic and immunohistochemical factors of CUP cases
- The histologic type of all 95 CUP cases was squamous cell carcinoma. Fifty-two patients (54.8%) were aged ≥ 60 years. Among the 95 CUP cases, 77 were male (81.1%) and 18 were female (18.9%). Thirty patients were non-smokers (31.6%), 16 were past smokers (16.8%), 32 were current smokers (33.7%), and smoking status was not available for 17 patients (17.9%). Additionally, 19 patients (20.0%) had smoked for 1–20 packyears, 16 patients (16.8%) had smoked for 21–40 pack-years, 10 (10.5%) had smoked for > 40 pack-years, and smoking duration data were not available for 20 patients (21.1%). The most frequent size of the largest metastatic lymph node was ≤ 3 cm (n = 53, 55.8%). Cervical level II lymph node involvement was identified in 68 patients (71.6%), with the highest frequency. Extranodal extension was identified in 35 patients (36.8%). Stage N1 (n=39, 41.1%) was the most common stage. Keratinization was identified in 36 patients (37.9%), cystic changes were identified in 29 patients (30.5%), a basaloid pattern was identified in 37 patients (38.9%), and lymphoepithelial lesions were identified in 20 patients (21.1%). The p16 IHC finding was positive in 34 patients (35.8%) and negative in 54 patients (56.8%). The p53 IHC finding was positive in 51 patients (53.7%) and negative in 34 patients (35.8%) (Table 1).
- High-risk HPV and EBV status by DNA ISH or RT-PCR and comparison with p16 and p53 positivity
- EBV ISH was available in 86 cases and was positive in five cases (5.8%). HPV ISH or RT-PCR was available in 82 cases, and high-risk HPV was detected in 22 cases (26.8%).
- Among the five EBV-positive cases, four (80%) were p53 positive, one (20%) was p53 negative, and none of the five cases showed p16 overexpression or identified high-risk HPV in RTPCR or HPV ISH. A p16 overexpression status was significantly associated with high-risk HPV status. Among the 22 HPV-positive cases, 19 showed p16 overexpression, while 13 among the 60 HPV-negative cases showed p16 overexpression (90.5% vs. 21.7%, p < .001) (Table 2).
- There was no significant relationship between p16 overexpression and p53 positivity (p = .113) nor between high-risk HPV infection and p53 positivity (p = .203).
- Clinicopathologic comparison among three groups based on viral status
- According to the IHC and ISH results, 37 cases (38.9%) were in the HPV-related group; five (5.3%) were in the EBV-related group; and 46 (48.45%) were in the HPV- and EBV-unrelated group, which displayed the greatest frequency of cases (Table 1).
- The frequency of those < 60 years of age was high in the HPVrelated CUP group (n = 24, 64.8%). Meanwhile, the frequency of patients aged ≥ 60 years was high in the HPV- and EBV-unrelated CUP group (n = 33, 71.7%), which showed a significant difference between groups (p = .013) (Table 1).
- There was no significant difference in smoking status (p = .738) or smoking duration (p = .187) between groups divided by HPV/EBV status.
- The ≤ 3-cm cases showed the greatest frequency of the largest lymph node size across all three groups. In the EBV-related group, the largest lymph node size was ≤ 3 cm in all five cases. In the HPV- and EBV-unrelated group, the frequency of lymph nodes > 3 cm was highest (n = 22, 47.8%) among the three groups, and the frequency of cases with lymph nodes measuring 3–6 cm was particularly high (n = 20, 43.5%).
- Level II lymph node involvement was most frequently observed across all three groups. Extranodal extension was infrequent in the HPV-related group (n = 10, 27.0%), while the HPVand EBV-unrelated group (n = 20, 43.5%) and the EBV-related group (n = 4, 80%) showed a significantly greater frequency (p = .046) (Table 1).
- All five patients in the EBV-related group were stage N1, which was the most frequent stage in the HPV-related group (n = 28, 75.7%). In the HPV- and EBV-unrelated group, N3c (n = 20, 43.5%) was the most frequent stage, and the differences were statistically significant (p < .001) (Table 1).
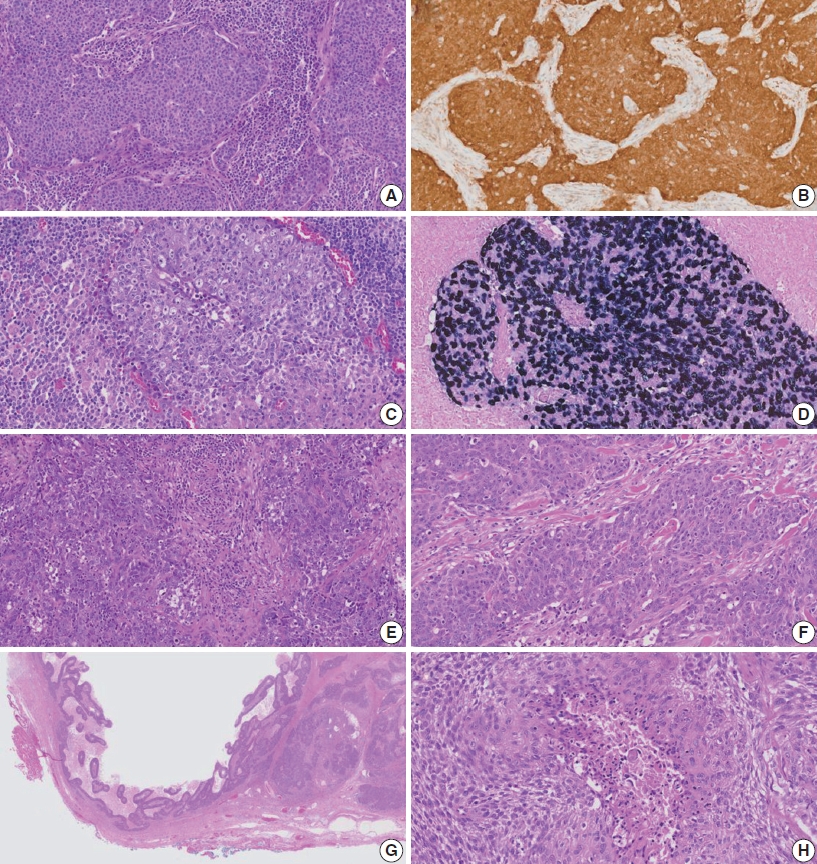
- Among histologic factors, cystic changes and the basaloid pattern were significantly frequently observed in the HPV-related group (n = 18, 48.6%, and n = 23, 62.2%, respectively). Lymphoepithelial lesions were significantly common in the EBV-related group (n = 4, 80%, p = .010). The histologic features in a representative case for the three groups are shown in Fig. 1.
- The results of the p53 IHC were not significantly different between the groups based on viral status (p = .341) (Table 1).
- Regarding the clinical outcomes, the proportion of patients with no evidence of disease (NED) was 73.0% (27/37) in the HPV-related group, representing the highest frequency among the groups. In the HPV- and EBV-unrelated group, the rate of death from disease (DOD)/death from other disease (DOC) was 37% (17/46), which was higher than that of the two virus-related groups. There was a significant difference in the clinical outcomes among the three groups (p = .011) (Table 1).
- OS estimates in CUP
- The univariate analysis revealed that ≥ 60 years of age (p < .001), current smoker (p = .024), > 40 pack-years (p = .002), presence of extranodal extension (p = .001), and HPV- and EBV-unrelated group status (p = .005) were significant factors for a poor prognosis. The presence of the basaloid pattern (p = .042) and p16 IHC positivity (p = .007) were significant prognostic factors for good outcomes. Sex, largest lymph node size, the presence of keratinization, cystic changes, lymphoepithelial lesions, and p53 IHC positivity did not significantly affect the OS in the univariate analysis (Table 3).
- In the multivariate analysis, long-term smoking (21–40 vs. ≤ 20 pack-years, p = .014; > 40 vs. ≤ 20 pack-years, p = .038) and HPV- and EBV-unrelated group vs. HPV-related group status (hazard ratio, 13.238; 95% confidence interval, 1.427 to 122.820; p = .023) were significant prognostic factors for poor outcomes (Table 3).
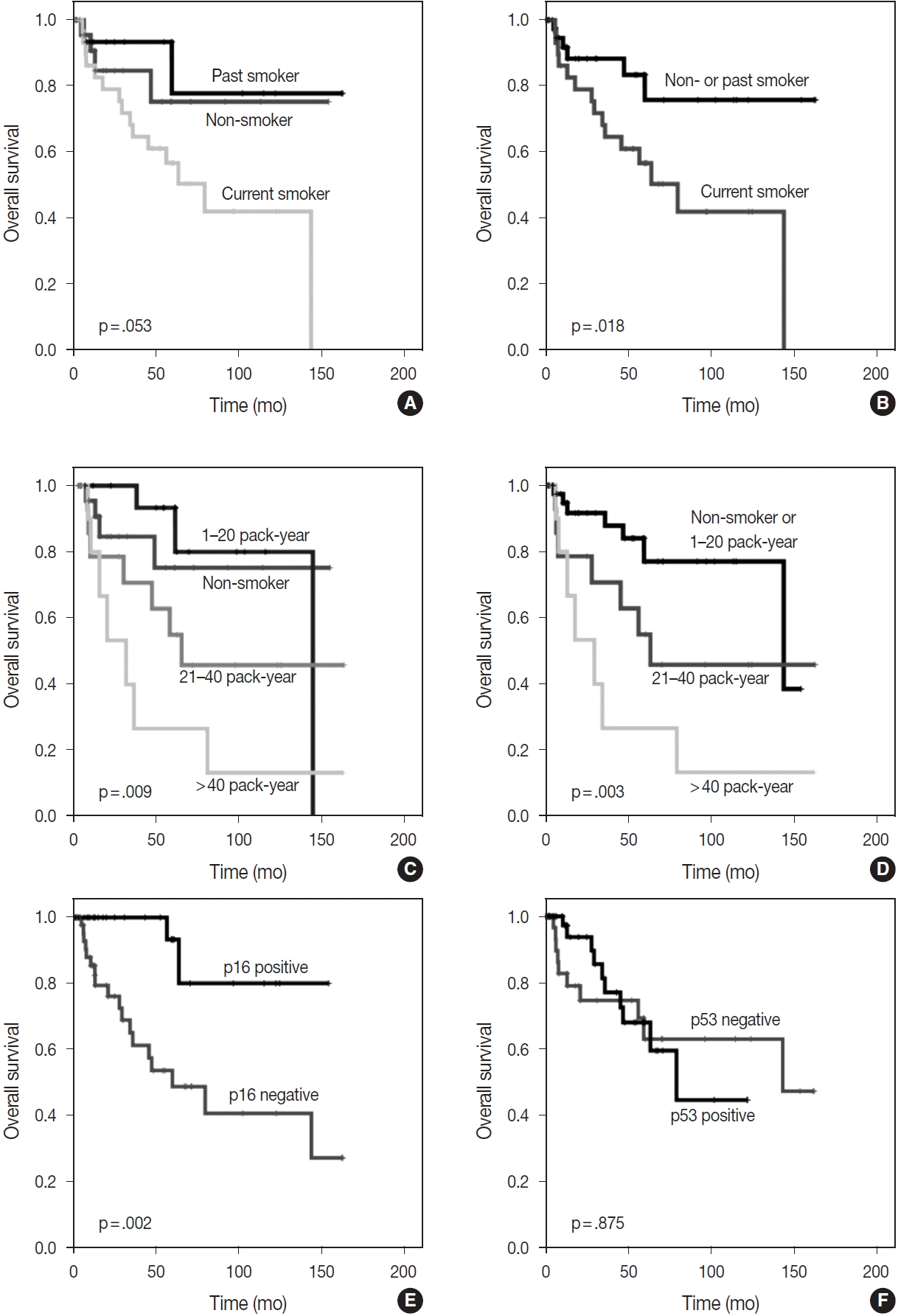
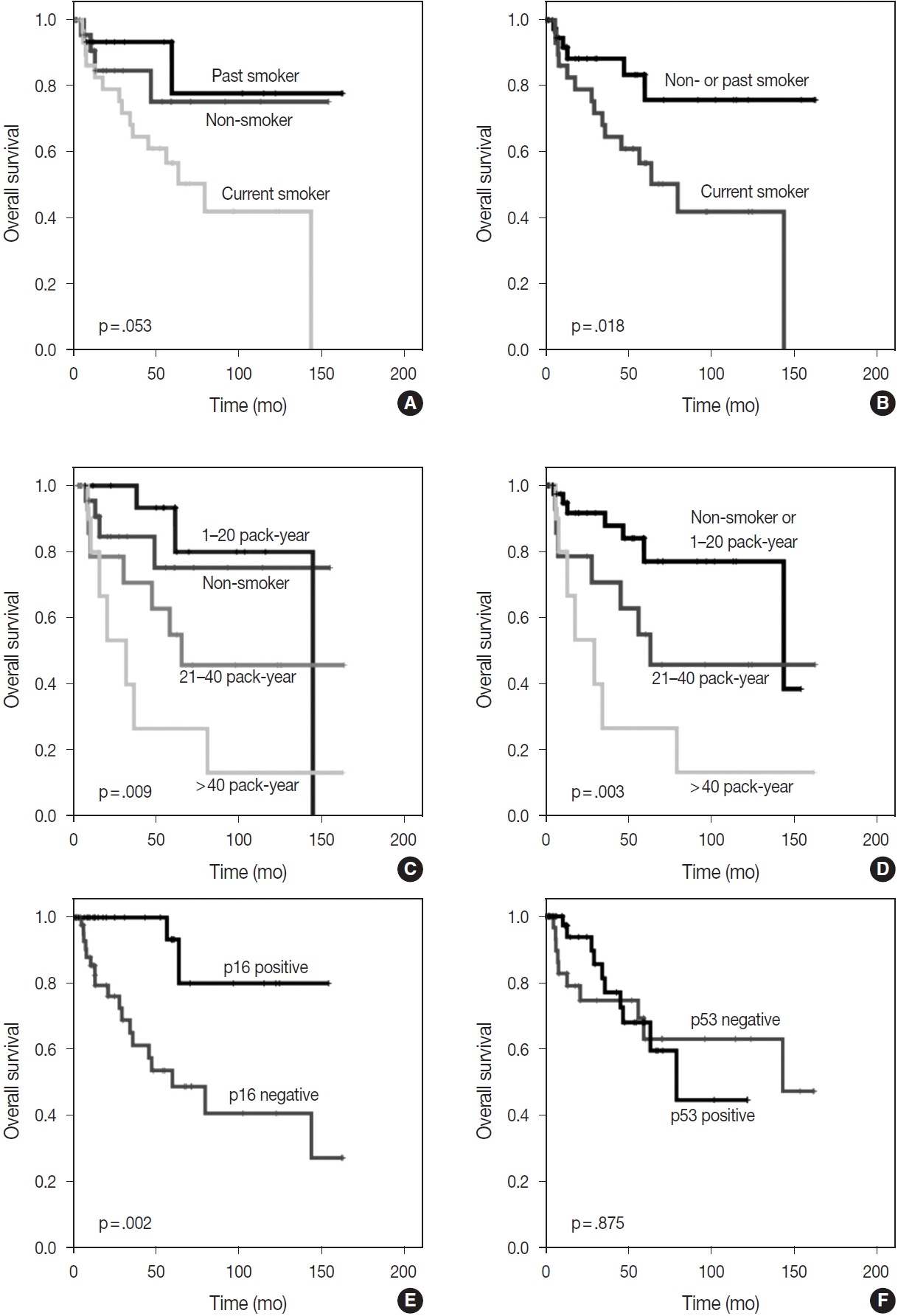
- The Kaplan-Meier survival curves also estimated that OS was significantly better in non-smokers or past smokers than in current smokers (p = .018). Furthermore, groups who had smoked for < 20 pack-years, including non-smokers, showed the best OS, followed by those who had smoked for 21–40 pack-years, while those who had smoked for > 40 pack-years showed the worst OS (p = .003) (Fig. 2). However, in the analysis of individual groups, there was no significant OS difference in HPV-related CUP according to smoking status (non-smokers or past smokers vs. current smokers, p = .160) (non-smoker or < 20 vs. 21–40 vs. > 40 pack-years, p = .340). Among HPV- and EBV-unrelated CUP cases, non-smokers or past smokers tended to show better OS times than current smokers, but there was no significant difference (p = .064). There was also no significant OS difference in smoking duration (non-smoker or < 20 vs. 21–40 vs. > 40 packyears, p = .400) among HPV- and EBV-unrelated CUP cases.
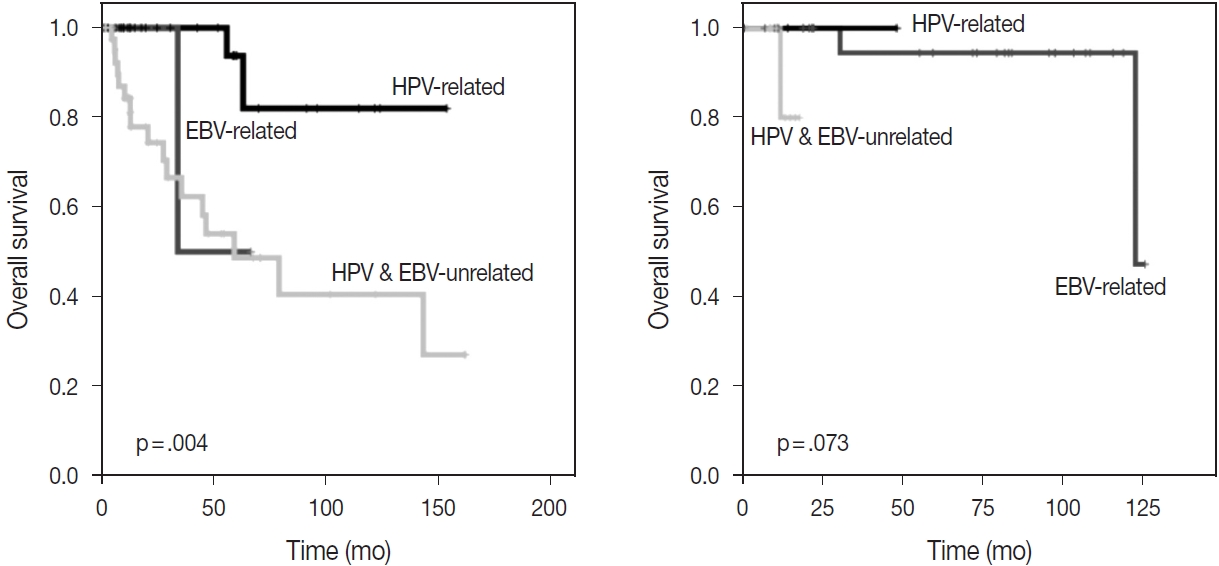
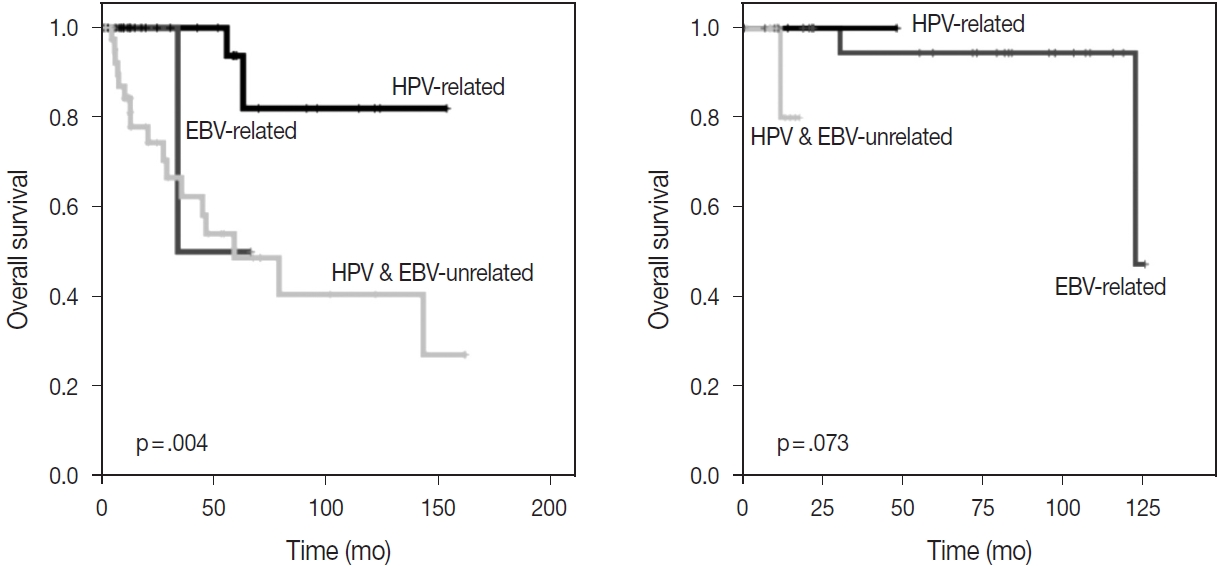
- With the exception of HPV status, p16 alone was associated with a better OS (p = .002); however, there was no significant difference in OS between p53-positive and p53-negative patients (p = .875) (Fig. 2). The HPV-related CUP cases had the longest OS, and the HPV- and EBV-unrelated CUP patients had the worst prognosis, with a significant difference among the three groups (p = .004) (Fig. 3).
- Cervical metastasis with subsequent confirmation of the primary tumors
- Among the patients initially presenting with cervical metastasis with unknown primary tumors, 64 cases were found at the primary sites. Primary tumors were most frequently found at the oropharynx (n = 25, 89.3%), followed by at the hypopharynx and nasopharynx (n = 5, 7.8%, each). There were four cases (6.3%) of the esophagus; three cases (4.7%) of the oral cavity; and one case each (1.6%) of the pharynx, not specified, retropharynx, larynx, anus, and uterine cervix. Among them, 28 cases (43.8%) were identified as HPV-related tumors through p16 IHC or HPVPCR tests. HPV-related primary tumors originated at the oropharynx, pharynx, not specified, anus, and uterine cervix. There were three cases of EBV-related tumors confirmed by EBV ISH, and the primary sites of all cases were the nasopharynx (Table 4). There was no significant difference in OS among the three groups according to viral status (p = .073) (Fig. 3). Clinical and pathologic characteristics according to viral status are presented with detailed tables in Supplementary Table S1.
RESULTS
- This is the first multicenter study in Korea on CUP and has noted several important findings. First, HPV-related cases constituted 38.9% of all CUP cases, which is approximately 10% lower than the frequency (49%) reported in a meta-analysis of 17 studies [17]. Second, HPV-related CUP in Korea showed better survival outcomes than HPV-unrelated CUP, per the studies in Western countries, including 978 cases in the United States [5] and 68 cases in the National Cancer Database [6]. Interestingly, a previous Korean study [18] reported opposing findings, a finding which might have been attributed to the small number of cases. A multicenter study is considered to have merits of case collection and reduction of bias due to the hospital size.
- Although only 5 EBV-related CUP cases were analyzed in this study, virus-related CUP had a better prognosis than the virusunrelated group, and HPV-related CUP showed the best OS (p = .004) and a high NED status frequency (73.0%). Our results were consistent with the eighth edition of the AJCC staging system that accepted the unique biologic behavior and natural history of EBV- and HPV-related tumors. In addition, our results supported a unique staging system for cervical lymphadenopathy with an unknown primary tumor to apply to oropharynx and nasopharynx staging according to HPV and EBV status.
- Characteristics of viral-related tumors were also well organized among CUP cases in Korea. HPV- and EBV-unrelated CUP cases showed the worst OS and a high DOD/DOC status frequency (37.0%, p = .011) among the three groups, with a high rate of extranodal extension (p = .046) and N staging (p = .001). HPVrelated CUP patients were younger than HPV-unrelated CUP patients (p = .013), and their lymph nodes showed a higher frequency of cystic changes (p = .016) and the basaloid pattern (p < .001) as seen in HPV-mediated oropharynx cancer. EBV-related CUP showed a high frequency of lymphoepithelial lesions (p = .010), as with nasopharynx cancer associated with EBV.
- Dixon et al. [23] found no significant differences in OS (p = .85 and p = .42) and disease-free survival (p = .87 and p = .58) in CUP through the univariate analysis of smoking duration (≤ 10 vs. > 10 pack-years) and smoking status (current smoker vs. ex-smoker vs. never-smoker). Tribius et al. [7] found that a smoking history of > 10 pack-years showed a worse prognosis than that of ≤ 10 pack-years in HPV-DNA-positive and p16-positive CUP. In addition, HPV-DNA–positive and p16-positive CUP with a smoking history of > 10 pack-years showed a similar survival curve to HPV-DNA–negative or p16-negative groups (p = .02) [7]. In our study, smoking duration was a significantly worse prognostic factor for OS in the multivariate analysis of total CUP cases. The Kaplan-Meier survival curves also showed significant differences in OS according to smoking status (non-smokers or past smokers vs. current smokers, p = .018) and smoking duration (non-smoker or < 20 vs. 21–40 vs. > 40 pack-years, p = .003). However, there was no significant difference in OS in the HPV-related CUP or virus-unrelated CUP groups according to smoking status or duration.
- As a limitation of our study, HPV-related CUP was defined by three methods of RT-PCR, DNA ISH, and p16 IHC. RT-PCR is stable and reproducible. However, as the sensitivity is high, there is a possibility of contamination by surrounding HPV-infected normal epithelium or other samples [24]. DNA ISH is widely used in research due to its low price but shows different sensitivity and specificity values according to the type of probe for the target HPV [25]. The Ventana system was used in this study, but even with Ventana, different performances were achieved [26,27] owing to the varying quality-control procedures, laboratory experience, and techniques [28]. p16 positivity in IHC is used as a surrogate marker for high-risk HPV-associated tumorigenesis because p16 can be overexpressed by the loss of inhibitory feedback of the phosphorylated Rb protein, degradated by the E7 protein of high-risk HPV [29]. However, other processes, such as inflammation, regeneration, and TP53 mutation, contribute to p16 overexpression [30,31]. The choice of one of the three methods varied across institutions and even within the same institution. In this study, 90.5% (19/21) of high-risk HPV-positive cases showed p16 overexpression, and 21.7% (13/60) of high-risk HPV-negative cases showed p16 overexpression. At Johns Hopkins Hospital, which routinely uses both the HPV DNA ISH test and p16 IHC, they found p16-positive/HPVDNA– negative cases in 18% of oropharyngeal squamous cell carcinoma [32], similar to the 16% identified in our study. They performed an additional RNA ISH assay for high-risk E6/E7 mRNA and confirmed the presence of active transcriptional active HPV in 84% of these cases. Judging from the characteristics of each method and the results of this study, the cause of the discrepancy in the cases showing p16 overexpression but DNA ISH negativity may be due to the false-negative result of DNA ISH from the background signal or due to the overexpression of p16 by another non-viral mechanism. HPV type 16 infection and disruptive TP53 mutations did not seem to overlap, so HPV infection showed an inverse relationship with TP53 mutations [21,22]. This study showed no inverse relationship between p53 and HPV infection. In this study, tumors showing nuclear staining in ≥ 10% of the tumor cells are considered positive for p53 immunostaining. The reason for the cutoff of 10% was based on the analysis of multiple studies that found significant correlations between p53 overexpression and higher tumor grade [33,34], TP53 gene mutations [35-37], or worse prognosis [38,39] when the threshold was set at 10%. However, the p53 immunostaining results in this study may not represent the entirety of the tumor due to being performed in the TMA. Different interpretations of p53 positivity among institutions may have resulted in different results in previous studies. Additionally, > 10% of p53 nuclear expression may not represent the TP53 mutation of the tumor with rarity in this study. We lacked enough follow-up data and scale because we could only secure OS data. Although this is a multicenter study, its statistical power to understand CUP remains insufficient due to the small collection of data, which is the peculiarity of the low incidence of this entity.
- In conclusion, virus-unrelated CUP in Korea had the highest frequency among CUP cases. Virus-related CUP had a better prognosis than the virus-unrelated group, and patients with HPVrelated CUP showed the best OS. HPV-related CUP was similar to HPV-mediated oropharyngeal cancer and EBV-related CUP was similar to nasopharyngeal cancer in terms of clinicopathologic characteristics. In total CUP cases, longer smoking duration and virus-unrelated CUP were significant factors for poor prognosis.
DISCUSSION
Supplementary Information
Supplementary Table S1.
Ethics Statement
This study was approved by the Institutional Review Board of Asan Medical Center (2020-1722), and patient informed consent was waived, given the retrospective nature of the study. All procedures were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author Contributions
Conceptualization: KJC. Data curation and interpretation: ML, UJ, JSS, YSL, CGW, DHK, JYK, SOY, KJC. Supervision: KJC. Writing—original draft: ML. Writing—review & editing: ML, KJC. Approval of final manuscript: all authors.
Conflicts of Interest
J.S.S., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
This study was supported by the Korean Society of Pathologists (2021).

Values are presented as number (%) unless otherwise indicated.
In the statistical comparison among groups according to viral status, cases of ‘not determined (n = 7)’ are excluded.
HPV, human papillomavirus; EBV, Epstein-Barr virus; NA, not assessed; IHC, immunohistochemistry; NED, no evidence of disease; AWD, alive with disease, DOD, death of disease, DOC, death of other cause.
|
HPV ISH or RT-PCR |
Total | |||
|---|---|---|---|---|
| Positive | Negative | ND | ||
| p16 | ||||
| Positive | 19 | 13 | 2 | 34 |
| Negative | 2 | 47 | 5 | 54 |
| ND | 1 | 0 | 6 | 7 |
| Total | 22 | 60 | 13 | 95 |
- 1. Califano J, Westra WH, Koch W, et al. Unknown primary head and neck squamous cell carcinoma: molecular identification of the site of origin. J Natl Cancer Inst 1999; 91: 599-604. ArticlePubMed
- 2. van de Wouw AJ, Jansen RL, Speel EJ, Hillen HF. The unknown biology of the unknown primary tumour: a literature review. Ann Oncol 2003; 14: 191-6. ArticlePubMed
- 3. Cheraghlou S, Cheraghlou S, Cheraghlou S, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck 2013; 35: 123-32. PubMed
- 4. Waltonen JD, Ozer E, Hall NC, Schuller DE, Agrawal A. Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg 2009; 135: 1024-9. ArticlePubMed
- 5. Cheraghlou S, Torabi SJ, Husain ZA, et al. HPV status in unknown primary head and neck cancer: prognosis and treatment outcomes. Laryngoscope 2019; 129: 684-91. ArticlePubMedPDF
- 6. Axelsson L, Nyman J, Haugen-Cange H, et al. Prognostic factors for head and neck cancer of unknown primary including the impact of human papilloma virus infection. J Otolaryngol Head Neck Surg 2017; 46: 45.ArticlePubMedPMCPDF
- 7. Tribius S, Hoffmann AS, Bastrop S, et al. HPV status in patients with head and neck of carcinoma of unknown primary site: HPV, tobacco smoking, and outcome. Oral Oncol 2012; 48: 1178-84. ArticlePubMed
- 8. Chernock RD, Lewis JS. Approach to metastatic carcinoma of unknown primary in the head and neck: squamous cell carcinoma and beyond. Head Neck Pathol 2015; 9: 6-15. ArticlePubMedPMCPDF
- 9. Abogunrin S, Di Tanna GL, Keeping S, Carroll S, Iheanacho I. Prevalence of human papillomavirus in head and neck cancers in European populations: a meta-analysis. BMC Cancer 2014; 14: 968.ArticlePubMedPMCPDF
- 10. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14: 467-75. ArticlePubMedPDF
- 11. Rickinson AB. Co-infections, inflammation and oncogenesis: future directions for EBV research. Semin Cancer Biol 2014; 26: 99-115. ArticlePubMed
- 12. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006; 15: 1765-77. ArticlePubMedPDF
- 13. Piazza C, Incandela F, Giannini L. Unknown primary of the head and neck: a new entry in the TNM staging system with old dilemmas for everyday practice. Curr Opin Otolaryngol Head Neck Surg 2019; 27: 73-9. ArticlePubMed
- 14. Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010; 11: 781-9. ArticlePubMedPMC
- 15. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010; 363: 24-35. ArticlePubMedPMC
- 16. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers: major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017; 67: 122-37. ArticlePubMedPDF
- 17. Ren J, Yang W, Su J, et al. Human papillomavirus and p16 immunostaining, prevalence and prognosis of squamous carcinoma of unknown primary in the head and neck region. Int J Cancer 2019; 145: 1465-74. ArticlePubMedPDF
- 18. Cho WK, Roh JL, Cho KJ, Choi SH, Nam SY, Kim SY. Predictors of survival and recurrence after primary surgery for cervical metastasis of unknown primary. J Cancer Res Clin Oncol 2020; 146: 925-33. ArticlePubMedPDF
- 19. Lee M, Kim SB, Lee SW, et al. Human papillomavirus prevalence and cell cycle related protein expression in tonsillar squamous cell carcinomas of Korean patients with clinicopathologic analysis. Korean J Pathol 2013; 47: 148-57. ArticlePubMedPMC
- 20. Ryu CH, Ryu J, Cho KH, et al. Human papillomavirus-related cell cycle markers can predict survival outcomes following a transoral lateral oropharyngectomy for tonsillar squamous cell carcinoma. J Surg Oncol 2014; 110: 393-9. ArticlePubMed
- 21. Maruyama H, Yasui T, Ishikawa-Fujiwara T, et al. Human papillomavirus and p53 mutations in head and neck squamous cell carcinoma among Japanese population. Cancer Sci 2014; 105: 409-17. ArticlePubMedPMCPDF
- 22. Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res 2008; 14: 366-9. ArticlePubMedPDF
- 23. Dixon PR, Au M, Hosni A, et al. Impact of p16 expression, nodal status, and smoking on oncologic outcomes of patients with head and neck unknown primary squamous cell carcinoma. Head Neck 2016; 38: 1347-53. ArticlePubMed
- 24. Boscolo-Rizzo P, Pawlita M, Holzinger D. From HPV-positive towards HPV-driven oropharyngeal squamous cell carcinomas. Cancer Treat Rev 2016; 42: 24-9. ArticlePubMed
- 25. Augustin JG, Lepine C, Morini A, et al. HPV detection in head and neck squamous cell carcinomas: what is the issue? Front Oncol 2020; 10: 1751.ArticlePubMedPMC
- 26. Schlecht NF, Brandwein-Gensler M, Nuovo GJ, et al. A comparison of clinically utilized human papillomavirus detection methods in head and neck cancer. Mod Pathol 2011; 24: 1295-305. ArticlePubMedPMCPDF
- 27. Keung ES, Souers RJ, Bridge JA, et al. Comparative performance of high-risk human papillomavirus RNA and DNA in situ hybridization on College of American Pathologists proficiency tests. Arch Pathol Lab Med 2020; 144: 344-9. ArticlePubMedPDF
- 28. Unger ER. In situ diagnosis of human papillomaviruses. Clin Lab Med 2000; 20: 289-301. ArticlePubMed
- 29. Faraji F, Zaidi M, Fakhry C, Gaykalova DA. Molecular mechanisms of human papillomavirus-related carcinogenesis in head and neck cancer. Microbes Infect 2017; 19: 464-75. ArticlePubMedPMC
- 30. Khleif SN, DeGregori J, Yee CL, et al. Inhibition of cyclin D-CDK4/CDK6 activity is associated with an E2F-mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci U S A 1996; 93: 4350-4. ArticlePubMedPMC
- 31. Rietbergen MM, Snijders PJ, Beekzada D, et al. Molecular characterization of p16-immunopositive but HPV DNA-negative oropharyngeal carcinomas. Int J Cancer 2014; 134: 2366-72. ArticlePubMed
- 32. Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 2010; 116: 2166-73. ArticlePubMed
- 33. Hashmi AA, Hussain ZF, Hashmi SK, et al. Immunohistochemical over expression of p53 in head and neck Squamous cell carcinoma: clinical and prognostic significance. BMC Res Notes 2018; 11: 433.ArticlePubMedPMCPDF
- 34. Mertens LS, Claps F, Mayr R, et al. The search for the optimal cutoff value of p53-immunohistochemistry to predict prognosis of invasive bladder cancer: a multi-center, multi-laboratory analysis. Int J Surg Pathol 2023; 31: 157-66. ArticlePubMedPDF
- 35. Tanboon J, Williams EA, Louis DN. The diagnostic use of immunohistochemical surrogates for signature molecular genetic alterations in gliomas. J Neuropathol Exp Neurol 2016; 75: 4-18. ArticlePubMed
- 36. Hwang HJ, Nam SK, Park H, et al. Prediction of TP53 mutations by p53 immunohistochemistry and their prognostic significance in gastric cancer. J Pathol Transl Med 2020; 54: 378-86. ArticlePubMedPMCPDF
- 37. Alsner J, Jensen V, Kyndi M, et al. A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol 2008; 47: 600-7. ArticlePubMed
- 38. Wang L, Yu X, Li J, Zhang Z, Hou J, Li F. Prognostic significance of p53 expression in patients with esophageal cancer: a meta-analysis. BMC Cancer 2016; 16: 373.ArticlePubMedPMCPDF
- 39. Carlos de Vicente J, Junquera Gutierrez LM, Zapatero AH, Fresno Forcelledo MF, Hernandez-Vallejo G, Lopez Arranz JS. Prognostic significance of p53 expression in oral squamous cell carcinoma without neck node metastases. Head Neck 2004; 26: 22-30. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Differenzierung von benignen und malignen Halszysten – eine diagnostische Herausforderung
Christina Sauter, Matthias Sand, Karim Plath, Michaela Maria Plath
Laryngo-Rhino-Otologie.2025; 104(05): 296. CrossRef - Unlocking the Hidden: Advancing Imaging Techniques in Diagnosing Cancers of Unknown Primary in the Head and Neck Region
Daniela Messineo, Filippo Valentini, Giovanni Francesco Niccolini, Federica Zoccali, Francesca Ripari, Enrico Marotta, Marcello Caratozzolo, Pasquale Frisina
Applied Sciences.2025; 15(4): 2194. CrossRef - Expansion of tumor-infiltrating lymphocytes from head and neck squamous cell carcinoma to assess the potential of adoptive cell therapy
Sangjoon Choi, Mofazzal Hossain, Hyun Lee, Jina Baek, Hye Seon Park, Chae-Lyul Lim, DoYeon Han, Taehyun Park, Jong Hyeok Kim, Gyungyub Gong, Mi-Na Kweon, Hee Jin Lee
Cancer Immunology, Immunotherapy.2024;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1.
Fig. 2.
Fig. 3.
| Total (n = 95) | HPV-related (n = 37, 38.9%) | EBV-related (n = 5, 5.3%) | HPV and EBV-unrelated (n = 46, 48.4%) | Not determined (n = 7, 7.4%) | p-value | ||
|---|---|---|---|---|---|---|---|
| Age (yr) | .013 | ||||||
| < 50 | 12 (12.6) | 7 (18.9) | 1 (20.0) | 4 (8.7) | 0 | ||
| 50–59 | 31 (32.6) | 17 (45.9) | 2 (40.0) | 9 (19.6) | 3 (42.9) | ||
| 60–69 | 26 (27.4) | 8 (21.6) | 2 (40.0) | 15 (32.6) | 1 (14.3) | ||
| ≥ 70 | 26 (27.4) | 5 (13.5) | 0 | 18 (39.1) | 3 (42.9) | ||
| Sex | .907 | ||||||
| Male | 77 (81.1) | 31 (83.8) | 4 (80.0) | 37 (80.4) | 5 (71.4) | ||
| Female | 18 (18.9) | 6 (16.2) | 1 (20.0) | 9 (19.6) | 2 (28.6) | ||
| Smoking status | .738 | ||||||
| Non-smoker | 30 (31.6) | 11 (29.7) | 1 (20.0) | 16 (34.8) | 2 (28.6) | ||
| Past smoker | 16 (16.8) | 7 (18.9) | 0 | 9 (19.6) | 0 | ||
| Current smoker | 32 (33.7) | 11 (29.7) | 1 (20.0) | 15 (32.6) | 5 (71.4) | ||
| NA | 17 (17.9) | 8 (21.6) | 3 (60.0) | 6 (13.0) | 0 | ||
| Smoking duration (pack-years) | .187 | ||||||
| Never-smoker | 30 (31.6) | 11 (29.7) | 1 (20.0) | 16 (34.8) | 2 (28.6) | ||
| 1–20 | 19 (20.0) | 9 (24.3) | 0 | 7 (15.2) | 2 (28.6) | ||
| 21–40 | 16 (16.8) | 8 (16.2) | 0 | 11 (19.5) | 1 (14.3) | ||
| ≥ 41 | 10 (10.5) | 1 (2.7) | 1 (20.0) | 7 (15.2) | 1 (14.3) | ||
| NA | 20 (21.1) | 10 (27.0) | 3 (60.0) | 7 (15.2) | 0 | ||
| Lymph node size (cm) | .030 | ||||||
| ≤ 3.0 | 53 (55.8) | 23 (62.2) | 5 (100) | 24 (52.2) | 1 (14.3) | ||
| > 3.0, ≤ 6.0 | 28 (29.5) | 6 (16.2) | 0 | 20 (43.5) | 2 (28.6) | ||
| > 6.0 | 7 (7.4) | 5 (13.5) | 0 | 2 (4.3) | 0 | ||
| NA | 7 (7.4) | 3 (8.1) | 0 | 0 | 4 (57.1) | ||
| Lymph node level | |||||||
| Level I | 11 (11.6) | 4 (10.8) | 1 (20.0) | 6 (13.0) | 0 | ||
| Level II | 68 (71.6) | 30 (81.1) | 4 (80.0) | 28 (60.9) | 6 (85.7) | ||
| Level III | 35 (36.8) | 12 (32.4) | 1 (20.0) | 21 (45.7) | 1 (14.3) | ||
| Level IV | 16 (16.8) | 3 (8.1) | 1 (20.0) | 12 (26.1) | 0 | ||
| Level V | 9 (9.5) | 1 (2.7) | 2 (40.0) | 5 (10.9) | 1 (14.3) | ||
| Level VI | 0 | 0 | 0 | 0 (0) | 0 | ||
| Retropharyngeal | 2 (2.1) | 0 | 1 (20.0) | 1 (2.2) | 0 | ||
| Axillary | 1 (1.1) | 0 | 0 | 1 (2.2) | 0 | ||
| Supraclavicular | 3 (3.2) | 1 (2.7) | 0 | 2 (4.3) | 0 | ||
| Extranodal extension | .046 | ||||||
| Positive | 35 (36.8) | 10 (27.0) | 3 (60.0) | 20 (43.5) | 2 (28.6) | ||
| Negative | 44 (46.3) | 25 (67.6) | 2 (40.0) | 15 (32.6) | 2 (28.6) | ||
| NA | 16 (16.8) | 2 (5.4) | 0 | 11 (23.9) | 3 (42.9) | ||
| N category | .001 | ||||||
| 1 | 39 (41.1) | 28 (75.7) | 5 (100) | 5 (10.9) | 1 (14.3) | ||
| 2 | 1 (1.1) | 1 (2.7) | 0 | 0 | 0 | ||
| 2a | 6 (6.3) | 0 | 0 | 6 (13.0) | 0 | ||
| 2b | 5 (5.3) | 0 | 0 | 5 (10.9) | 0 | ||
| 2c | 2 (2.1) | 0 | 0 | 2 (4.3) | 0 | ||
| 3 | 5 (5.3) | 5 (13.5) | 0 | 0 | 0 | ||
| 3a | 0 | 0 | 0 | 0 | 0 | ||
| 3c | 22 (23.2) | 0 | 0 | 20 (43.5) | 2 (28.6) | ||
| NA | 15 (15.8) | 3 (8.1) | 0 | 8 (17.4) | 4 (57.1) | ||
| Keratinization | .023 | ||||||
| Present | 36 (37.9) | 11 (29.7) | 0 | 24 (52.2) | 1 (14.3) | ||
| Absent | 59 (62.1) | 26 (70.3) | 5 (100) | 22 (47.8) | 6 (85.7) | ||
| Cystic change | .016 | ||||||
| Present | 29 (30.5) | 18 (48.6) | 0 | 11 (23.9) | 0 | ||
| Absent | 66 (69.5) | 19 (51.4) | 5 (100) | 35 (76.1) | 7 (100) | ||
| Basaloid pattern | < .001 | ||||||
| Present | 37 (38.9) | 23 (62.2) | 2 (40.0) | 11 (23.9) | 1 (14.3) | ||
| Absent | 58 (61.1) | 14 (37.8) | 3 (60.0) | 35 (76.1) | 6 (85.7) | ||
| Lymphoepithelial lesion | .010 | ||||||
| Present | 20 (21.1) | 8 (21.6) | 4 (80.0) | 7 (15.2) | 1 (14.3) | ||
| Absent | 75 (78.9) | 29 (78.4) | 1 (20.0) | 39 (84.8) | 6 (85.7) | ||
| p16 IHC | < .001 | ||||||
| Positive | 34 (35.8) | 34 (91.9) | 0 | 0 | 0 | ||
| Negative | 54 (56.8) | 2 (5.4) | 5 (100) | 46 (100) | 1 (14.3) | ||
| NA | 7 (7.4) | 1 (2.7) | 0 | 0 | 6 (85.7) | ||
| p53 IHC | .341 | ||||||
| Positive | 51 (53.7) | 18 (48.6) | 4 (80.0) | 29 (63.0) | 0 | ||
| Negative | 34 (35.8) | 17 (45.9) | 1 (20.0) | 16 (34.8) | 0 | ||
| NA | 10 (10.5) | 2 (5.4) | 0 | 1 (2.2) | 7 (100) | ||
| Follow-up duration (mo) | |||||||
| Median | 23.0 | 47.63 | 6.0 | 16.7 | 52.8 | ||
| Range | 0.0–163.0 | 0–154 | 1–67 | 0–163 | 4–113 | ||
| Clinical outcome | |||||||
| NED | 56 (58.9) | 27 (73.0) | 2 (40.0) | 22 (47.8) | 5 (71.4) | .011 | |
| AWD | 16 (16.8) | 7 (18.9) | 2 (40.0) | 7 (15.2) | 0 | ||
| DOD/DOC | 23 (24.3) | 3 (8.1) | 1 (20.0) | 17 (37.0) | 2 (28.6) | ||
| HPV ISH or RT-PCR |
Total | |||
|---|---|---|---|---|
| Positive | Negative | ND | ||
| p16 | ||||
| Positive | 19 | 13 | 2 | 34 |
| Negative | 2 | 47 | 5 | 54 |
| ND | 1 | 0 | 6 | 7 |
| Total | 22 | 60 | 13 | 95 |
| Variable | Univariate |
Multivariate |
|||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (≥ 60 yr vs. < 60 yr) | 13.532 (3.137–58.374) | < .001 | 0.000 (0.000–5.590e128) | .929 | |
| Sex (male vs. female) | 0.878 (0.256–2.960) | .825 | |||
| Smoking (current smoker vs. non- or past smoker) | 2.990 (1.152–7.760) | .024 | 1.552 (0.209–11.545) | .668 | |
| Smoking | |||||
| Non-smoker, or 1–20 pack-years | 1 (reference) | ||||
| 21–40 pack-years | 2.558 (0.894–7.315) | .080 | 11.893 (1.638–86.333) | .014 | |
| > 40 pack-years | 5.362 (1.852–15.525) | .002 | 9.742 (1.131–83.944) | .038 | |
| Lymph node size (cm) | 1 (reference) | ||||
| ≤ 3.0 | |||||
| > 3.0, ≤ 6.0 | 1.471 (0.585–3.703) | .412 | |||
| > 6.0 | 0.457 (0.060–3.506) | .451 | |||
| Keratinization (present vs. absent) | 1.529 (0.634–3.683) | .344 | |||
| Cystic change (present vs. absent) | 0.438 (0.169–1.138) | .090 | |||
| Basaloid pattern (present vs. absent) | 0.375 (0.146–0.965) | .042 | 3.130 (0.482–20.328) | .898 | |
| Lymphoepithelial lesion (present vs. absent) | 0.512 (0.172–1.527) | .230 | |||
| Extranodal extension (present vs. absent) | 9.017 (2.509–32.412) | .001 | 0.440 (0.055–3.488) | .470 | |
| Group | |||||
| HPV-related | 1 (reference) | ||||
| EBV-related | 6.608 (0.596–73.322) | .124 | 1.213e7 (0.000–1.018e142) | .918 | |
| HPV and EBV-unrelated | 8.078 (1.859–35.106) | .005 | 13.238 (1.427–122.820) | .023 | |
| p16 IHC (positive vs. negative) | 0.135 (0.031–0.585) | .007 | - | ||
| p53 IHC (positive vs. negative) | 0.930 (0.375–2.304) | .875 | |||
| Primary site (n = 64) | HPV-related (n = 28, 43.8%) | EBV-related (n = 3, 4.7%) | HPV & EBV-unrelated (n = 10, 15.6%) | Not determined (n = 23, 35.9%) |
|---|---|---|---|---|
| Oropharynx (n = 41, 64.1%) | 25 (89.3) | 0 | 1 (10) | 15 (65.2) |
| Hypopharynx (n = 5, 7.8%) | 0 | 0 | 1 (10) | 4 (17.4) |
| Nasopharynx (n = 5, 7.8%) | 0 | 3 (100) | 1 (10) | 1 (4.3) |
| Oral cavity (n = 3, 4.7%) | 0 | 0 | 2 (20) | 1 (4.3) |
| Pharynx, not specific (n = 2, 3.1%) | 1 (3.6) | 0 | 0 | 1 (4.3) |
| Retropharynx (n = 1, 1.6%) | 0 | 0 | 0 | 1 (4.3) |
| Esophagus (n = 4, 6.3%) | 0 | 0 | 4 (40) | 0 |
| Larynx (n = 1, 1.6%) | 0 | 0 | 1 (10) | 0 |
| Anus (n = 1, 1.6%) | 1 (3.6) | 0 | 0 | 0 |
| Uterine cervix (n = 1, 1.6%) | 1 (3.6) | 0 | 0 | 0 |
Values are presented as number (%) unless otherwise indicated. In the statistical comparison among groups according to viral status, cases of ‘not determined (n = 7)’ are excluded. HPV, human papillomavirus; EBV, Epstein-Barr virus; NA, not assessed; IHC, immunohistochemistry; NED, no evidence of disease; AWD, alive with disease, DOD, death of disease, DOC, death of other cause.
HPV, human papillomavirus; ISH, in situ hybridization; RT-PCR, real-timepolymerase chain reaction; ND, not determined.
HR, hazard ratio; CI, confidence interval; HPV, human papillomavirus; EBV, Epstein-Barr virus; IHC, immunohistochemistry.
Values are presented as number (%). HPV, human papillomavirus; EBV, Epstein-Barr virus.

 E-submission
E-submission