Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(3); 2023 > Article
-
Review
Trouble-makers in cytologic interpretation of the uterine cervix -
Eunah Shin
 , Jaeeun Yu
, Jaeeun Yu , Soon Won Hong
, Soon Won Hong
-
Journal of Pathology and Translational Medicine 2023;57(3):139-146.
DOI: https://doi.org/10.4132/jptm.2023.04.25
Published online: May 15, 2023
Department of Pathology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- Corresponding Author: Soon Won Hong, MD, PhD, Department of Pathology, Yongin Severance Hospital, Yonsei University College of Medicine, 363 Dongbaekjukjeon-daero, Giheung-gu, Yongin 16995, Korea Tel: +82-31-5189-8250, Fax: +82-31-5189-8248, E-mail: soonwonh@yuhs.ac
© 2023The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- The development and standardization of cytologic screening of the uterine cervix has dramatically decreased the prevalence of squamous cell carcinoma of the uterine cervix. Advances in the understanding of biology of human papillomavirus have contributed to upgrading the histologic diagnosis of the uterine cervix; however, cytologic screening that should triage those that need further management still poses several difficulties in interpretation. Cytologic features of high grade intraepithelial squamous lesion (HSIL) mimics including atrophy, immature metaplasia, and transitional metaplasia, and glandular lesion masquerades including tubal metaplasia and HSIL with glandular involvement are described with accentuation mainly on the differential points. When the cytologic features lie in a gray zone between the differentials, the most important key to the more accurate interpretation is sticking to the very basics of cytology; screening the background and cellular architecture, and then scrutinizing the nuclear and cytoplasmic details.
- Cytologic features that are diagnostic of HSIL can be described in two aspects; cytologic features of discrete, singly scattered individual cells and those of tissue fragments. Cells diagnostic of HSIL are usually small and round, but with high nuclear:cytoplasmic (N:C) ratio. The nuclear features include rounded but sharp nuclear membrane, coarsely granular chromatin, and longitudinal nuclear groove with lack of nucleoli. The cytoplasm is scant in amount or almost indiscernible. Syncytial tissue fragments are a common architectural presentation of HSIL, and the above diagnostic nuclear details can be found within these syncytial tissue fragments by changing the plane of focus. However, not all HSIL cases consistently show these cytologic features clearcut and not all the diagnostic features are exclusively seen in HSIL. Discrete, singly scattered cells that mimic HSIL can be found in immature squamous metaplasia, intrauterine device associated changes, atrophic cervicitis, and endometrial stromal cells. Tissue fragments that mimic HSIL can be found in squamous metaplasia, tubal metaplasia, endocervical adenocarcinoma in situ (AIS), endometrial adenocarcinoma, and atrophic cervicitis. Acs et al. [1] have reported that when 130 cases with cervical cytologic diagnosis of HSIL or atypical squamous cells of uncertain significance - cannot exclude HSIL (ASC-H) that had corresponding biopsy were reviewed, 13 cases were histologically diagnosed as low grade squamous intraepithelial lesion (LSIL) or reactive. Re-examination of the cervical cytology slides of these 13 cases revealed repair changes, atypical squamous metaplasia, atrophy, tubal metaplasia, lower uterine sampling, and histiocytes and lymphocytes [1]. Therefore, many conditions work as confounding factors in diagnosing HSIL, and these so-called HSIL mimics, namely atrophic cervicitis, immature or atypical squamous metaplasia, and transitional metaplasia are described more in detail.
- Atrophic cervicitis (atrophy)
- Abnormal results are reported more frequently in postmenopausal women than in pre-menopausal women, and Kaminski et al. [2] attributed this to the strong correlation between estrogen deficiency and squamous atypia, and concluded that estrogen therapy can revert this squamous atypia to normal cytology. Since atrophic cervicovaginal smears can exhibit various atypical patterns, they can easily mislead to overdiagnosis of squamous atypia or even carcinoma. On the contrary, due to the reduced number of exfoliated cells after menopause, a high false negative rate on cytologic screening is also problematic in postmenopausal women. Additional confounding factors are frequent drying artifacts and inflammation, resulting in low cellularity, nuclear hyperchromasia and poor nuclear preservation [3]. Therefore, it is very critical that cervicovaginal smears in postmenopausal women be examined with extra care to avoid both overdiagnosis and underestimation.
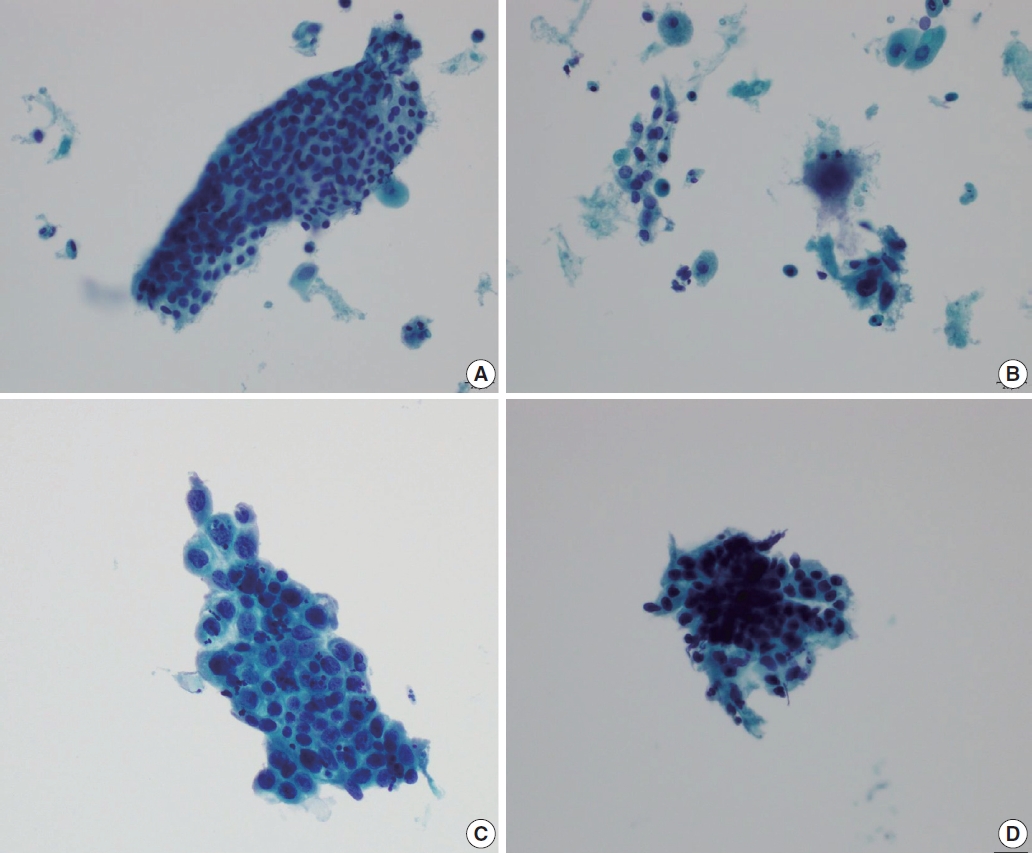
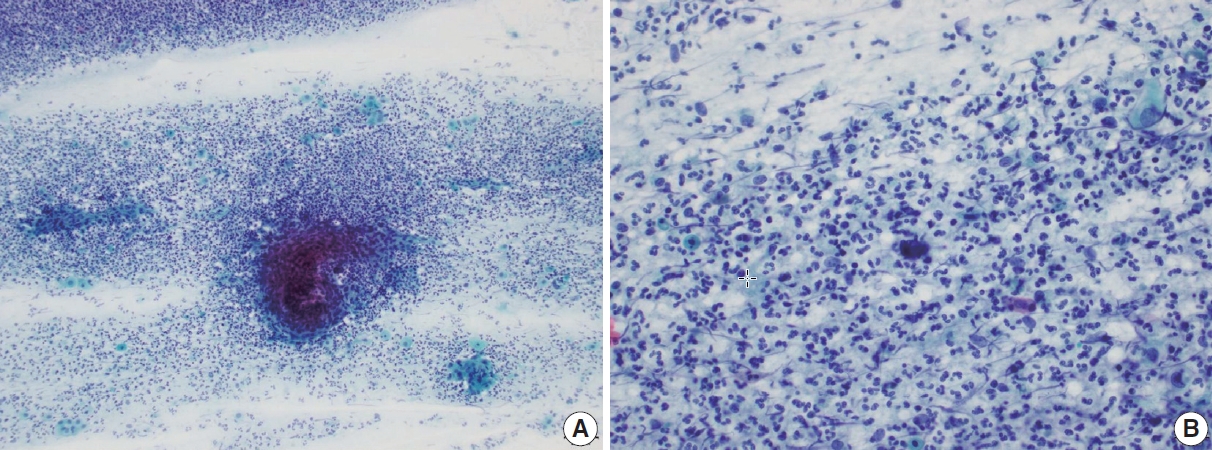
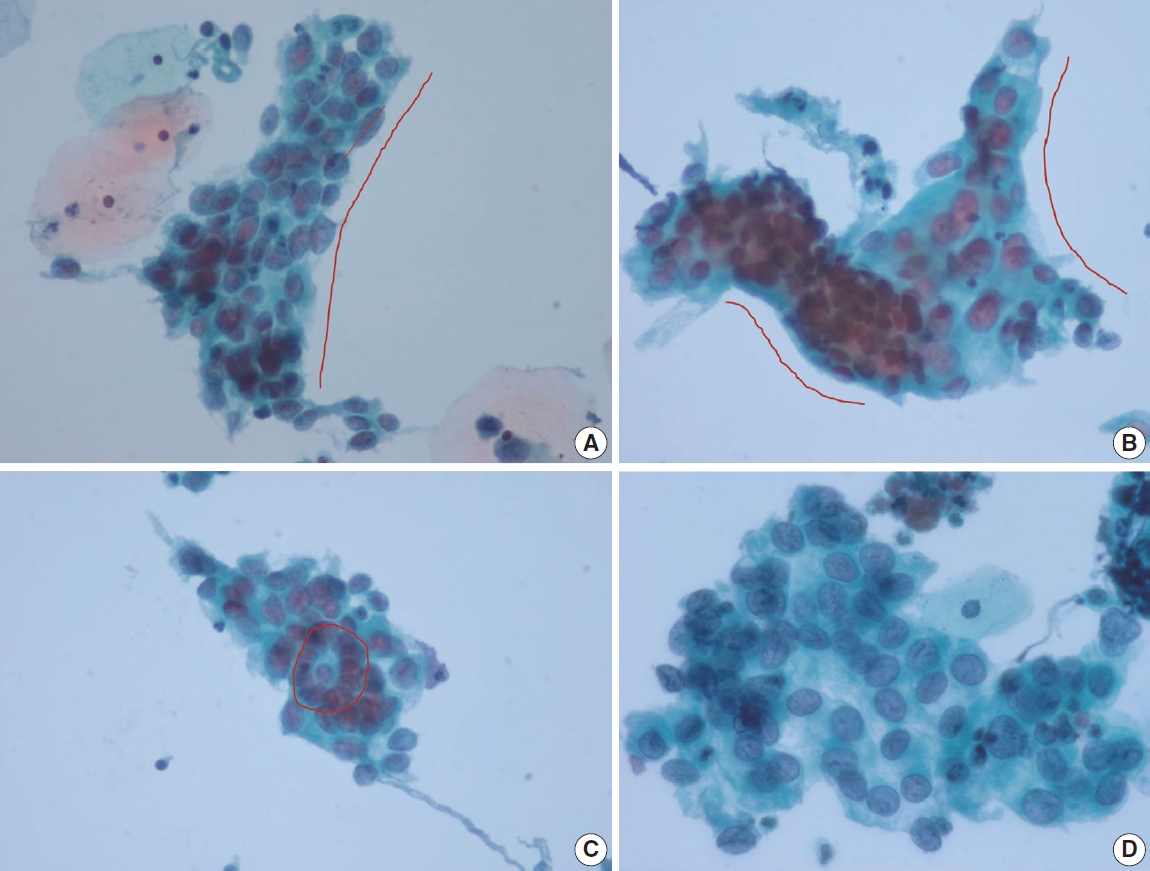
- Cervical smears of atrophic cervicitis show tissue fragments composed of uniform cell population with a streaming pattern in the background of cellular and inflammatory debris. These tissue fragments are not true syncytial tissue fragments and they often show folding of the edges. The uniform cell population comprising the tissue fragments are mostly parabasal cells with nuclear enlargement or miniature squamous cells with orangeophilic cytoplasm and pyknotic nuclei. The nuclear details of the atrophic parabasal cells comprising tissue fragments can be highlighted by changing the plane of focus. The entire cell population in the tissue fragments is uniform and very similar to the squamous cells present in the background. The tissue fragments show more crowding of smaller nuclei that are densely stained and have scant cytoplasm with high N:C ratios, often misleading to the diagnosis of HSIL. Moreover, atrophic fragments are not truly syncytial as in HSIL fragments. One of the other helpful diagnostic features would be the similarity between the background cells, which lack the nuclear characteristics of HSIL cells. The singly scattered cells around these tissue fragments are often pleomorphic in shape, but when carefully examined, the nuclei are either smudged or too hyperchromatic (Fig. 1). In a study by Chivukula and Shidham [4], they subcategorized reactive cytomorphologic patterns into microglandular hyperplasia-like, repair-like and atrophy-like patterns, and they described atrophy-like pattern as either single cells or hyperchromatic crowded groups of parabasal cells. In the single cell pattern, the individual cells had abundant blue cytoplasm and open chromatin with or without nucleoli. In the parabasal pattern, the cells showed small dark nuclei and variable cytoplasm, usually with a low N:C ratio. In constrast, they described HSIL-like pattern as single cells with nuclear hyperchromasia and coarse chromatin. In a study by Mokhtar et al. [5], the authors described a coarse chromatin pattern as a distinct predictive feature of HSIL in follow-up biopsies. An increase in nuclear size of at least twice that of an intermediate cell nucleus with significant hyperchromasia, irregular nuclear contours or chromatin distribution and marked cellular pleomorphism are features that can qualify for atypical squamous cells of uncertain significance in postmenopausal women. Additional feature is a variation in nuclear size [6]. In a study by Acs et al. [1], unequivocal cytologic features of those diagnosed in cytology as atypical squamous cells and later histologically confirmed as squamous intraepithelial lesion (SIL) were atypical parakeratotic/dyskeratotic cells showing irregular and hyperchromatic nuclei, orangeophilic cytoplasm with anisocytosis, and increased N:C ratio. Pseudokoilocytosis characterized by perinuclear halo with slightly increased size of nuclei can also be seen in atrophic smear and mislead to an overdiagnosis of LSIL, however, the nuclear enlargement is mild (1.5–2.5 intermediate cell nuclei), nuclear contours are mostly smooth, and chromatin pattern is evenly distributed and smooth [7]. Nuclear hyperchromasia and irregular nuclear contours are the most reliable cytomorphological criteria [8,9]. The nuclear membrane in HSIL is thickened twice that of non-HSIL cells in the background, and it appears as if the nuclear membrane has been drawn over. It should be noted that degenerated and dried small parabasal cells often appear orangeophilic in postmenopausal smears and it may be difficult to distinguish them from atypical parakeratosis or dyskeratotic cells of HSIL [10]. Nuovo et al. [11], has reported that parakeratosis was the only feature noted at a higher rate in the human papillomavirus–positive cases from postmenopausal women.
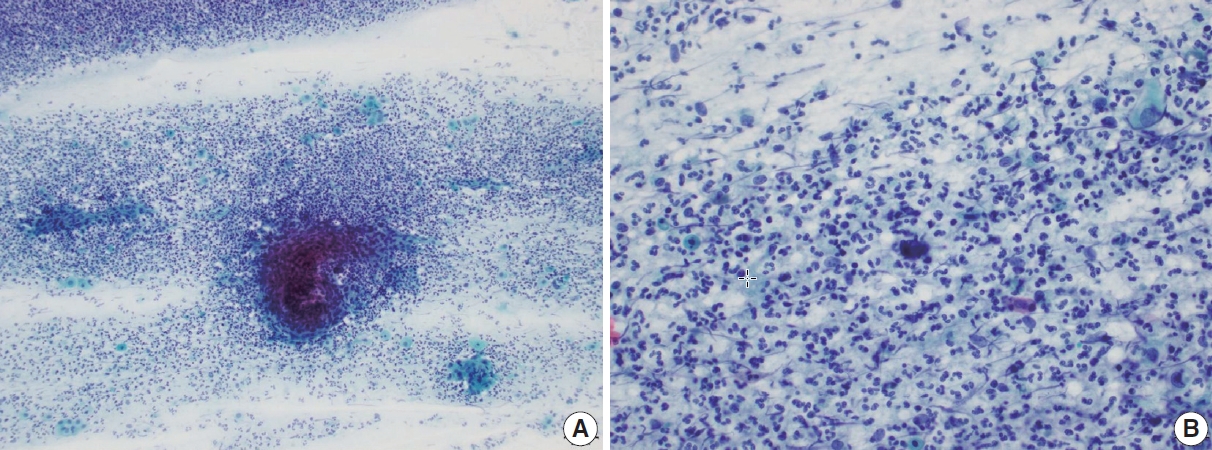
- Another pseudo-alarming cytologic feature in atrophic smear is the blue blobs, which are round to oval, smudged, and densely cyanophilic bodies with ill-defined borders, mostly seen in postmenopausal smears (Fig. 2) [12]. They have been once thought to be inspissated mucus, but Abdulla et al. [13] have demonstrated positive immunoreactivity to cytokeratin and epithelial membrane antigen and cellular skeletons with residual tonofilaments on transmission electron microscopy, concluding that they are degenerated parabasal cells. Blue blobs appear in atrophic smear in postmenopausal women due to the lack of mucus and stagnation of exfoliated cells, which are then further degenerated. Disintegration of chromatin results in the characteristic dense cyanophilic appearance of blue blobs and they eventually become granular background material. Blue blobs should be differentiated rightly because they can be mistaken for naked dyskeratotic or malignant cell nuclei. Lack of chromatin and lack of background inflammatory reaction or diathesis should help identify blue blobs from true dyskeratotic cells. The differential diagnostic points of atrophy from HSIL are summarized in Table 1.
- Immature squamous metaplasia
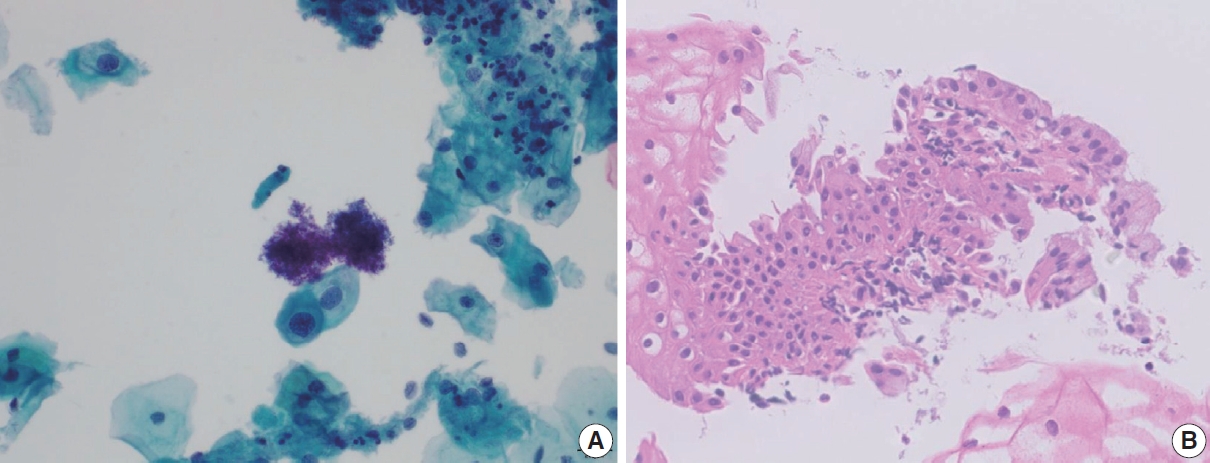
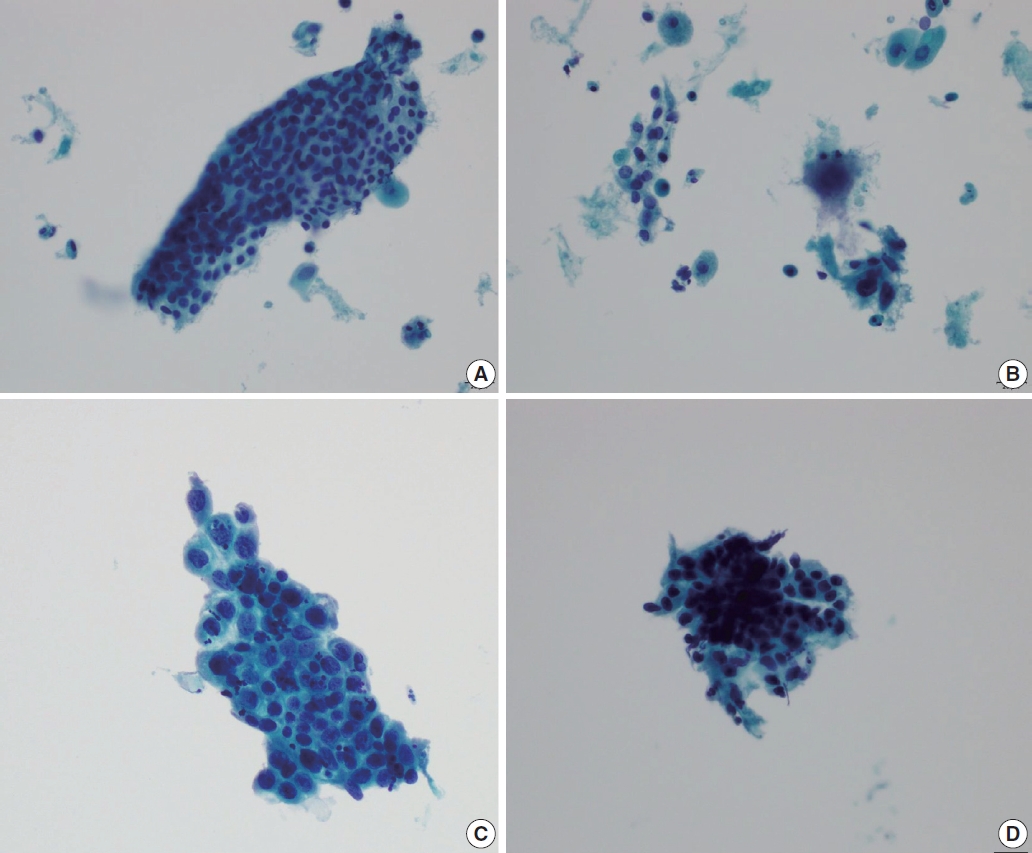
- Immature metaplastic cells with slight nuclear enlargement are often difficult to differentiate from SIL. A common cytologic presentation of HSIL is syncytial tissue fragments as in immature squamous metaplasia. However, tissue fragments in immature squamous metaplasia is basically monolayered, composed of metaplastic cells characterized by well-defined cell borders, appreciable cytoplasm, uniform nuclei with minimal nuclear enlargement. The cells show low N:C ratio with evenly dispersed chromatin. The singly scattered metaplastic cells also show distinct cytoplasmic border, smooth nuclear membrane and evenly distributed finely granular chromatin pattern. The nucleoli are often present, which is one of the important differential points from SIL. Cytoplasmic processes can also be present. Immature squamous metaplasia is associated with a fine chromatin pattern and dense cytoplasmic differentiation [14]. A fine chromatin pattern along with dense cytoplasmic differentiation is a significant diagnostic feature of immature squamous metaplasia, and the presence of prominent nucleoli is highly predictive of reactive cell change (Fig. 3) [5], and nuclear crowding, overlapping and pleomorphism are not distinctive. The differential diagnostic points between HSIL and immature squamous metaplasia are summarized in Table 2.
- Transitional metaplasia
- Transitional metaplasia can be mistaken as HSIL because they appear as hyperchromatic crowded groups comprised of small hyperchromatic cells with increased N:C ratio. Differential points include no distinct cytoplasmic border, longitudinal nuclear grooves, and relatively bland chromatin pattern. HSIL cells can show longitudinal nuclear grooves, which can be one of the differential points distinguishing HSIL from glandular abnormality. Therefore, when differentiating tubal metaplasia from HSIL, longitudinal nuclear grooves should be taken into account with other nuclear features, such as presence of nucleoli, but with bland and even chromatin pattern, nearly inconspicuous nuclear membrane, and the presence of relatively ample cytoplasm. Characteristically, the cell groups show a monolayered streaming pattern of ovoid to spindle shaped nuclei [15]. Whirling and streaming pattern of the cells within the cellular cluster can be an additionally helpful point.
HIGH GRADE INTRAEPITHELIAL SQUAMOUS LESION MIMICS
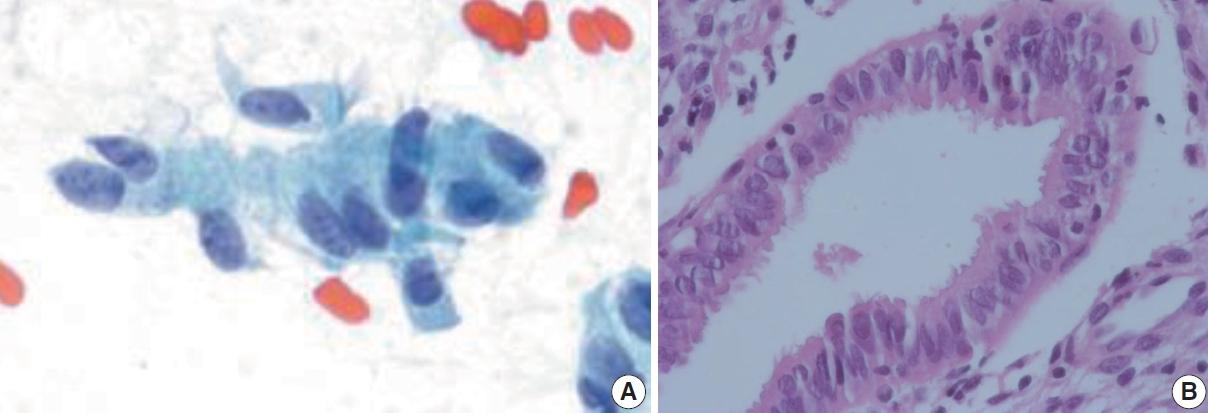
- Tubal metaplasia
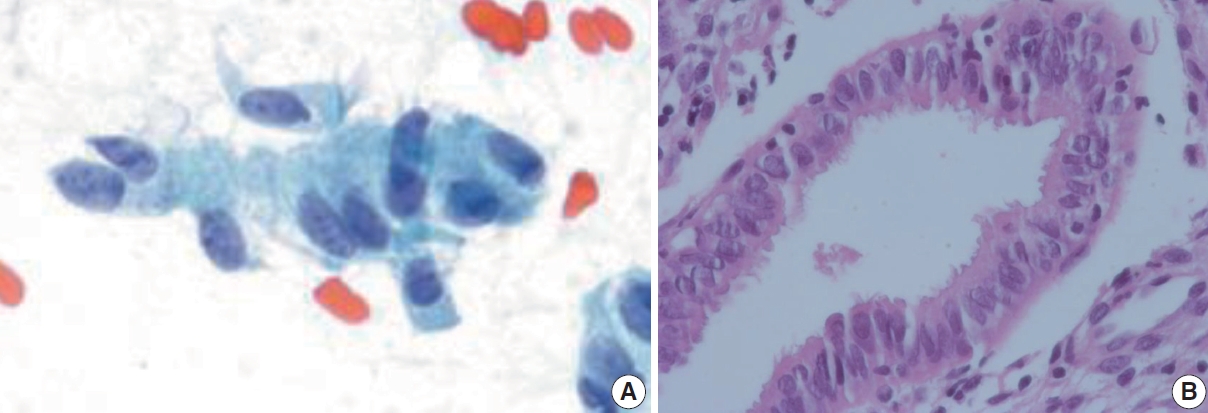
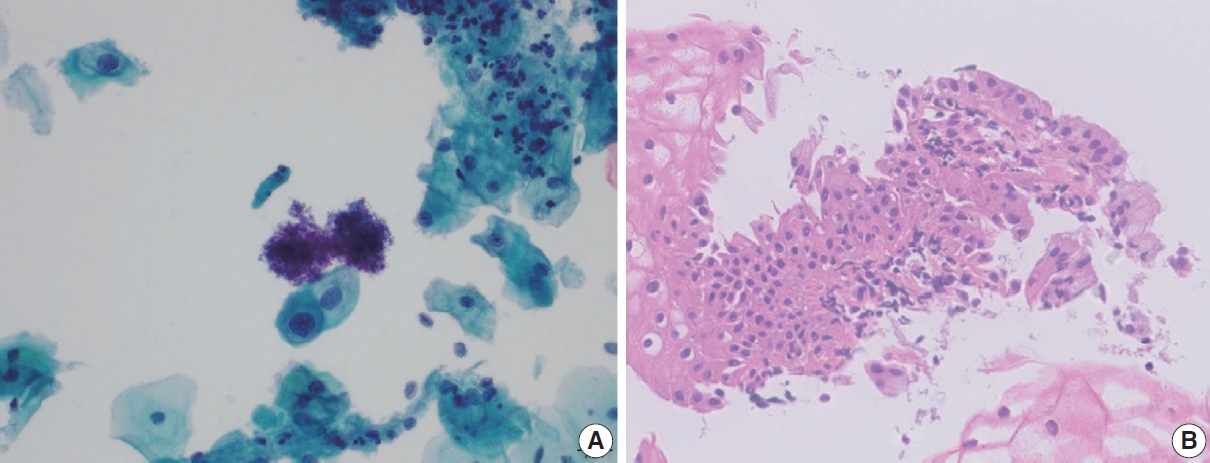
- Tubal metaplasia indicates endocervical glandular epithelium replaced by cells resembling the lining epithelial cells of the fallopian tubes. It is a common diagnostic pitfall, especially in differential diagnosis of endocervical AIS, because the cells are alarming at first glance. Usually, they appear as flat monolayered sheets, pseudostratified strips, or cohesive clusters of cuboidal or columnar cells with basally oriented nuclei and cilia in the other end of the cytoplasm when the cells are arranged parallel to each other (Fig. 4) [16]. However, they can appear hyperchromatic with loss of polarity, and increased N:C ratio, especially when the cells are arranged en face on the slide and cilia cannot be ascertained. The nuclei show finely granular chromatin and nucleoli are usually not seen. Therefore, in order to overcome this diagnostic pitfall and avoid overdiagnosis, an extra care should be taken to locate other cellular clusters that reveal slender columnar cells with tapering in one end and cilia in the other end. In addition, cellular sheets of tubal metaplasia reveal rare or no mitotic figures, no apoptotic bodies, and clean background [16,17].
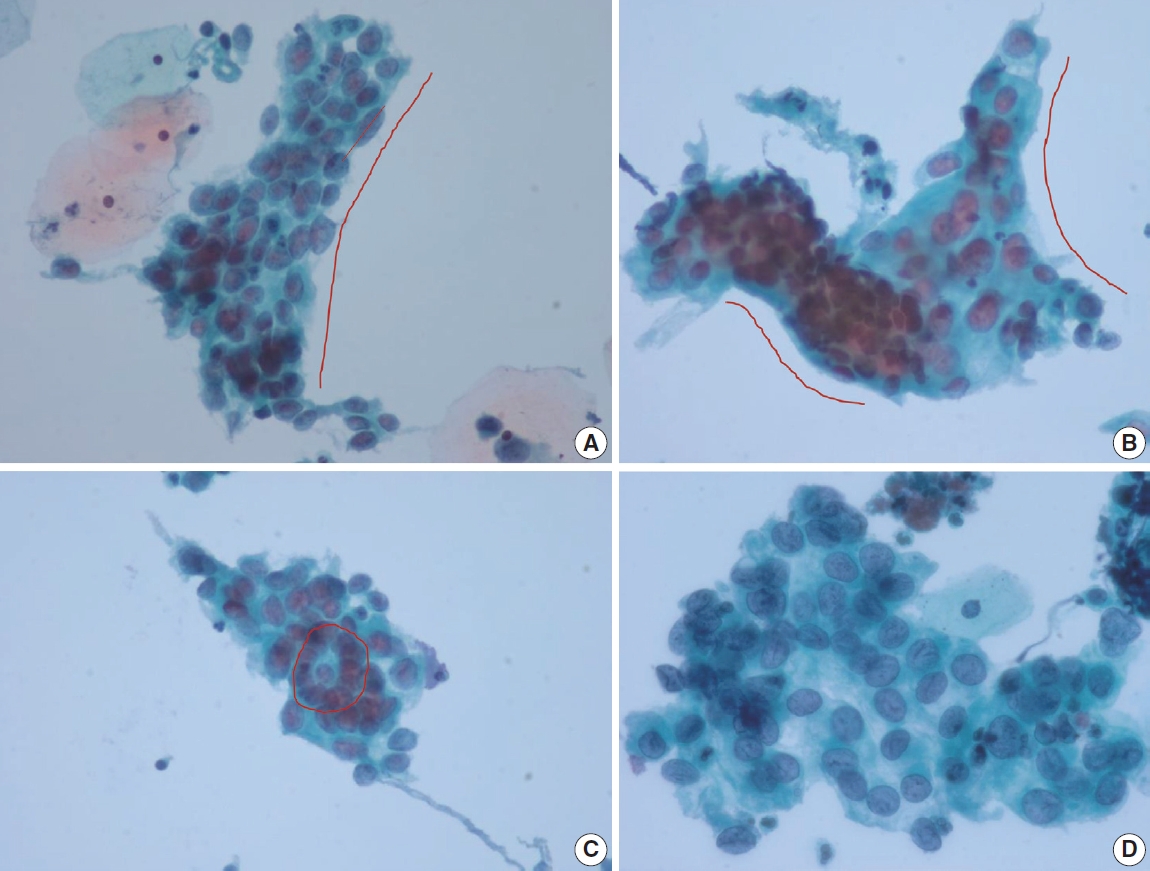
- HSIL with glandular involvement
- Benign mimics of glandular lesion are two dimensional with honeycomb pattern, showing cytoplasmic streaming, nuclear polarity, and cytoplasmic vacuoles. Architectural features that help to distinguish benign mimics from neoplastic glandular lesions are sheets with pseudostratification, maintenance of polarity, prominent nucleoli, and less hyperchromasia.
- Pre-neoplastic or neoplastic glandular cells show poorly oriented architecture, irregular nuclear membranes, decreased cytoplasm, loss of honeycomb pattern, nuclear crowding and overlap, loss of nuclear polarity, atypical single cells, and coarsely granular chromatin. Tight clusters of stretched cells with a high N:C ratio, hyperchromatic nuclei with fine nuclear chromatin and a thin rim of finely vacuolated cytoplasm upon changing plane of focus point towards a glandular origin. Moreover, nuclear enlargement with scant cytoplasm resulting in high N:C ratio, nuclear size variation, cellular crowding and stratification, nuclear hyperchromasia with coarse chromatin pattern and small or absent nucleoli in clean background are diagnostic features of endocervical AIS [18]. Apoptosis and mitoses can also be frequently seen in AIS, but the most helpful diagnostic clue would be ‘feathering’ at the periphery of the abnormal cell clusters.
- Glandular lesion of the uterine cervix accounts for 0.1%–0.8% of the cervical smears, and 17%–80% of the cytologic diagnoses have significant follow-up results, 40%–50% of which coexist with squamous lesion. Clinically, the diagnosis of atypical glandular cells (AGC) warrants direct colposcopic biopsy with endocervical sampling and additional endometrial sampling for women older than 45 years. It is reported that the most common histologic diagnosis following cytologic diagnosis of AGC is SIL. Upon histologic correlation of AGC, about 15% have been reported to be associated with SIL, 1% with AIS, and 0.5% with adenocarcinoma [19]. This is attributed to the fact that it is most difficult to differentiate glandular abnormality from SIL with glandular involvement [20].
- The most frequent and helpful cytological features of HSIL with glandular extension that are indicative of squamous origin are as follows: Syncytial clusters, sheets with loss of central polarity, oval nuclei, inconspicuous nucleoli, peripheral nuclear flattening, and nuclear grooves. The chromocenters in HSIL nuclei can often be misinterpreted as nucleoli, which may mislead to AGC [19]. The longitudinal axis of the peripheral nuclei are parallel to the long axis of the cellular cluster with one sided flattening (Fig. 5). Occasionally, the cellular clusters show whirling of the cells in the center so that the cells may appear to be piling up (Table 3) [17]. Also, singly scattered dysplastic squamous cells should be searched for in the background when HSIL should be differentiated from glandular lesion.
- The most frequent reasons for misclassification of SIL as AGC would be few dysplastic squamous cells masked by abundant benign or AGC and sheets of dysplastic squamous cells peripherally lined by glandular cells, which should rightly be interpreted as glandular involvement of squamous lesion rather than AGC per se.
GLANDULAR LESION MASQUERADES
- Interpretation of uterine cervical cytology can pose diagnostic difficulties because the transformation zone of the uterine cervix is vulnerable to many indigenous and exogenous factors. When evaluating cervical smears, it is important to spot tissue fragments and singly scattered cells, and careful examination of both components is essential. In evaluation of tissue fragments, polarity of the composing cells should be noted first, followed by the examination of homogeneity of the cells in size and shape. Finally, comparing whether the cells comprising the tissue fragments and the cells scattered in the background are the same cells should follow. Upon completion of examining the tissue fragments, single cells scattered in the background of the tissue fragments should be evaluated, including the cytoplasmic border, the amount of cytoplasm, N:C ratio, nuclear shape, nuclear membrane, and chromatin pattern. All these aspects should be considered comprehensively to reach the cytologic diagnosis, avoiding potential pitfalls. Additionally, it should always be kept in mind that when distinct nucleoli are seen, then the cytologic diagnosis of SIL is less likely. Rather, cells showing distinct nucleoli are either reactive, including various types of metaplasia, or overtly malignant cancer, either squamous or glandular in origin. Hyperchromatic crowded groups of cells showing nucleoli can be alarming at first, but careful evaluation of the architecture, nuclear membrane, and chromatin pattern can help avoid overdiagnosis. In summation, the most important key facilitating more accurate interpretation in spite of various diagnostic pitfalls would be sticking to the very basics of cytologic criteria of epithelial abnormality. Nuclear details such as chromatin pattern (fine or coarse), presence or absence of a prominent nucleolus, N:C ratio, and cytoplasmic differentiation (pale or dense) are important diagnostic features that may help distinguish those that are more likely in need of further management.
CONCLUSION
Ethics Statement
Not applicable.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author Contributions
Conceptualization: ES, SWH. Data curation: JY. Methodology: ES. Supervision: SWH. Writing—original draft: ES. Writing—review & editing: JY. Approval of final manuscript: all authors.
Conflicts of Interest
E.S., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding to declare.
- 1. Acs G, Gupta PK, Baloch ZW. Glandular and squamous atypia and intraepithelial lesions in atrophic cervicovaginal smears: one institution’s experience. Acta Cytol 2000; 44: 611-7. PubMed
- 2. Kaminski PF, Sorosky JI, Wheelock JB, Stevens CW Jr. The significance of atypical cervical cytology in an older population. Obstet Gynecol 1989; 73: 13-5. PubMed
- 3. Waddell CA. The influence of the cervix on smear quality. I: Atrophy. An audit of cervical smears taken post-colposcopic management of intraepithelial neoplasia. Cytopathology 1997; 8: 274-81. ArticlePubMedPDF
- 4. Chivukula M, Shidham VB. ASC-H in Pap test: definitive categorization of cytomorphological spectrum. Cytojournal 2006; 3: 14.ArticlePubMedPMC
- 5. Mokhtar GA, Delatour NL, Assiri AH, Gilliatt MA, Senterman M, Islam S. Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion: cytohistologic correlation study with diagnostic pitfalls. Acta Cytol 2008; 52: 169-77. PubMed
- 6. Rader AE, Rose PG, Rodriguez M, Mansbacher S, Pitlik D, Abdul-Karim FW. Atypical squamous cells of undetermined significance in women over 55: comparison with the general population and implications for management. Acta Cytol 1999; 43: 357-62. PubMed
- 7. McHugh KE, Reynolds JP, Suarez AA. Postmenopausal squamous atypia: cytologic features, hybrid capture 2 tests and contribution to the ASCUS pool. Acta Cytol 2018; 62: 418-22. ArticlePubMedPDF
- 8. Ejersbo D, Jensen HA, Holund B. Efficacy of Ki-67 antigen staining in Papanicolaou (Pap) smears in post-menopausal women with atypia: an audit. Cytopathology 1999; 10: 369-74. ArticlePubMedPDF
- 9. Abati A, Jaffurs W, Wilder AM. Squamous atypia in the atrophic cervical vaginal smear: a new look at an old problem. Cancer 1998; 84: 218-25. ArticlePubMed
- 10. Voytek TM, Kannan V, Kline TS. Atypical parakeratosis: a marker of dysplasia? Diagn Cytopathol 1996; 15: 288-91. ArticlePubMed
- 11. Nuovo GJ, Cottral S, Richart RM. Occult human papillomavirus infection of the uterine cervix in postmenopausal women. Am J Obstet Gynecol 1989; 160: 340-4. ArticlePubMed
- 12. Yakoushina TV, Medina IM, Hoda RS. “String of pearls” appearance of blue blobs in postmenopausal atrophy on ThinPrep Pap test. Diagn Cytopathol 2009; 37: 738-9. ArticlePubMed
- 13. Abdulla M, Hombal S, Kanbour A, et al. Characterizing “blue blobs”. Immunohistochemical staining and ultrastructural study. Acta Cytol 2000; 44: 547-50. PubMed
- 14. Sheils LA, Wilbur DC. Atypical squamous cells of undetermined significance: stratification of the risk of association with, or progression to, squamous intraepithelial lesions based on morphologic subcategorization. Acta Cytol 1997; 41: 1065-72. PubMed
- 15. Duggan MA. Cytologic and histologic diagnosis and significance of controversial squamous lesions of the uterine cervix. Mod Pathol 2000; 13: 252-60. ArticlePubMedPDF
- 16. Selvaggi SM, Haefner HK. Microglandular endocervical hyperplasia and tubal metaplasia: pitfalls in the diagnosis of adenocarcinoma on cervical smears. Diagn Cytopathol 1997; 16: 168-73. ArticlePubMed
- 17. Torous VF, Pitman MB. Interpretation pitfalls and malignant mimics in cervical cytology. J Am Soc Cytopathol 2021; 10: 115-27. ArticlePubMed
- 18. Chaump M, Pirog EC, Panico VJ, d Meritens AB, Holcomb K, Hoda R. Detection of in situ and invasive endocervical adenocarcinoma on ThinPrep Pap Test: morphologic analysis of false negative cases. Cytojournal 2016; 13: 28.ArticlePubMedPMC
- 19. Khan MY, Bandyopadhyay S, Alrajjal A, Choudhury MS, Ali-Fehmi R, Shidham VB. Atypical glandular cells (AGC): cytology of glandular lesions of the uterine cervix. Cytojournal 2022; 19: 31.ArticlePubMedPMC
- 20. Lee KR, Manna EA, St John T. Atypical endocervical glandular cells: accuracy of cytologic diagnosis. Diagn Cytopathol 1995; 13: 202-8. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Risk of cervical stenosis after cervical excision in postmenopausal patients
Eva Hauge, Line Winther Gustafson, Mette Tranberg, Pinar Bor
European Journal of Obstetrics & Gynecology and Reproductive Biology.2025; 308: 208. CrossRef - Pitfalls in Gynecological Cytology: Review of the Common and Less Frequent Entities in Pap Test
Danijela Vrdoljak-Mozetič, Snježana Štemberger-Papić, Damjana Verša Ostojić, Roberta Rubeša, Marko Klarić, Senija Eminović
Acta Cytologica.2024; 68(3): 281. CrossRef - Cytological features of human papillomavirus‐infected immature squamous metaplastic cells from cervical intraepithelial neoplasia grade 2
Mitsuaki Okodo, Kaori Okayama, Koji Teruya, Ruku Shinohara, Shuichi Mizuno, Rei Settsu, Yasuyoshi Ishii, Masahiko Fujii, Hirokazu Kimura, Mizue Oda
Journal of Medical Virology.2023;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure





Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
| Cytologic features | Atrophy | HSIL |
|---|---|---|
| Cell border | Poorly defined | Distinct |
| Nuclear:cytoplasmic ratio | Low | High |
| Nucleoli | Often present | Absent |
| Cell population | Uniform | Heterogeneous |
| Polarity | Streaming | Haphazard loss of polarity |
| Background | Same cells | Singly scattered HSIL cells |
| Cytologic features | Immature squamous metaplasia | HSIL |
|---|---|---|
| Nuclear features | ||
| Chromatin pattern | Evenly distributed finely granular | Clumped coarsely granular |
| Nuclear membrane | Smooth | Sharp, undulating, grooving |
| Nucleoli | Often present | Absent |
| Cytoplasmic features | ||
| Cytoplasmic processes | Present | Absent |
| Amount | Appreciable | Scanty, often indiscernible |
| N:C ratio | Low | High |
| Cytologic feature | HSIL with glandular involvement | Glandular lesion |
|---|---|---|
| Architecture | ||
| Syncytial clusters | Loss of honeycomb pattern | |
| Peripheral nuclear flattening | Loss of nuclear polarity | |
| Whirling in the center | Nuclear crowding with overlapping | |
| Nuclear features | ||
| Chromatin pattern | Coarse | Fine |
| Nuclear grooves | Often present | Absent |
| Nucleoli | Absent | Often present |
| Cytoplasmic features | ||
| Cytoplasmic processes | Present | Absent |
| Vacuolation | Absent | Present |
HSIL, high grade intraepithelial squamous lesion.
HSIL, high grade intraepithelial squamous lesion; N:C, nuclear:cytoplasmic.
HSIL, high grade intraepithelial squamous lesion.

 E-submission
E-submission