Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 57(5); 2023 > Article
-
Case Study
Hepatic small vessel neoplasm: not totally benign, not yet malignant -
Madison Miranda
 , David Howell
, David Howell , Tony El Jabbour
, Tony El Jabbour
-
Journal of Pathology and Translational Medicine 2023;57(5):273-277.
DOI: https://doi.org/10.4132/jptm.2023.06.19
Published online: August 24, 2023
Department of Pathology, Anatomy, and Laboratory Medicine, West Virginia University, Morgantown, WV, USA
-
Corresponding Author: Tony El Jabbour, MD, Department of Pathology, Anatomy, and Laboratory Medicine, West Virginia University, 64 Medical Center Drive, PO Box 9203, Morgantown, WV 26506-9203, USA Tel: +1-304-293-3212, Fax: +1-304-293-1627, E-mail: tony.eljabbour@hsc.wvu.edu
Corresponding Author: Madison Miranda, MD, Department of Pathology, Anatomy, and Laboratory Medicine, West Virginia University, 64 Medical Center Drive, PO Box 9203, Morgantown, WV 26506-9203, USA Tel: +1-304-293-3212, Fax: +1-304-293-1627, E-mail: madison.miranda@hsc.wvu.edu
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Hepatic small vessel neoplasm (HSVN) is a rare vascular tumor with few reports in the literature. While imaging findings may show characteristic enhancement patterns, limited available literature may not reveal the full potential for image-based diagnosis. Histologically, HSVN mimics other entities, though certain morphologic and immunohistochemical findings provide clues for diagnosis. However, HSVN still provides diagnostic challenges, especially on core biopsies with limited material for morphologic and molecular evaluation. While current recommendations are surgical resection and close observation, the long-term course of the tumor is unknown. We report a case of HSVN in a liver with additional feature of organized lymphoid aggregates necessitating additional hematopathology consultation and workup to rule out concurrent entities.
- A 63-year-old asymptomatic male with a medical history of hypertension and occasional alcohol use presented for his annual exam. Routine labs demonstrated mildly elevated liver enzymes to include alanine aminotransferase 110 U/L and aspartate aminotransferase 63 U/L. Alkaline phosphatase was normal (50 U/L).
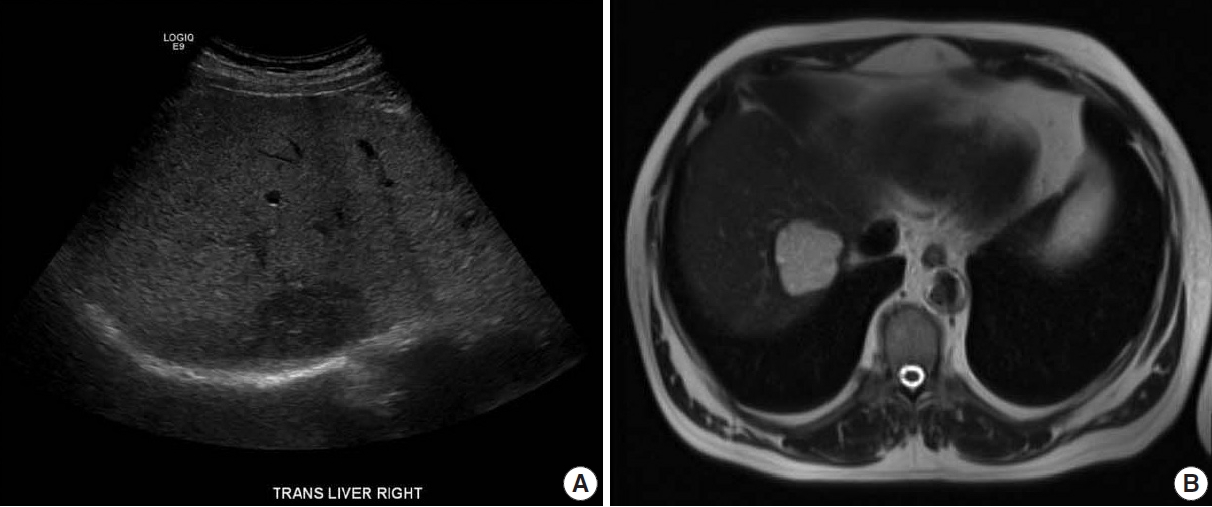
- Radiologic features
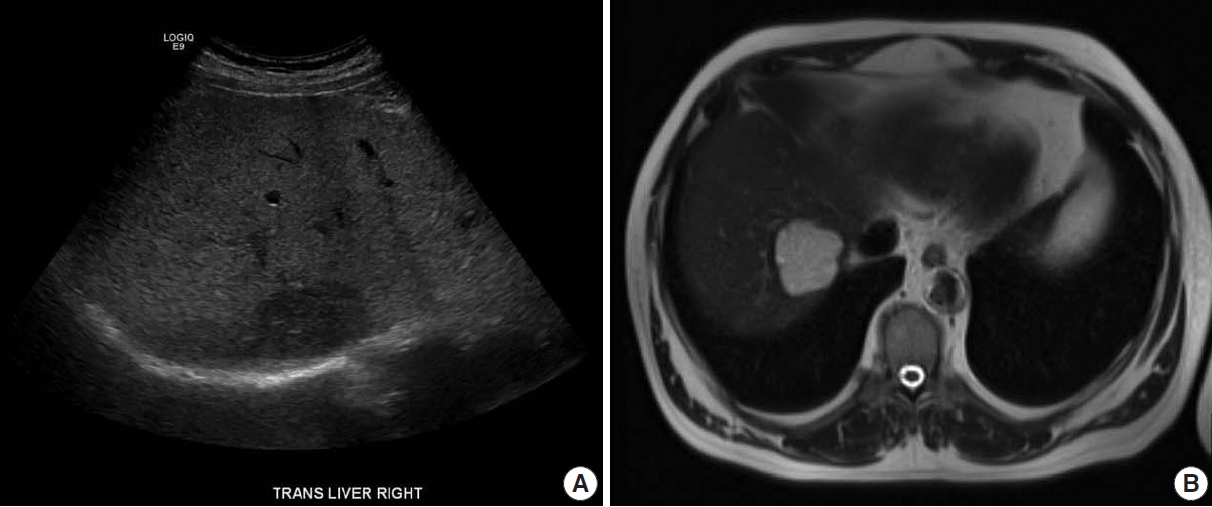
- An ultrasound demonstrated a 4.7 cm hypoechoic lesion in the right lobe of the liver. Liver protocol magnetic resonance imaging (MRI) with intravenous gadolinium-based contrast revealed a well-circumscribed 4.65 cm lesion that was hyperintense on T2 with prompt heterogenous contrast enhancement without washout (Fig. 1). The sonographic appearance and MRI enhancement pattern were not typical for benign lesions, so the patient subsequently underwent image-guided biopsy.
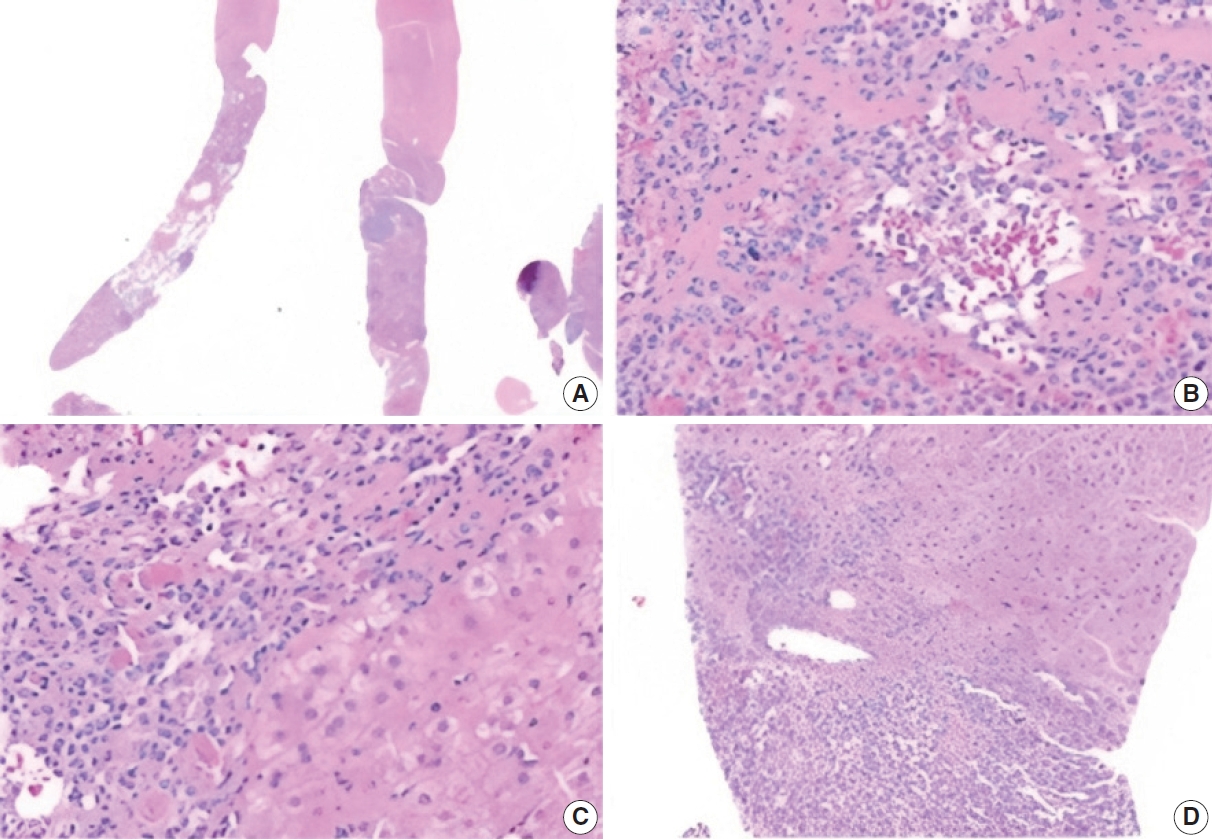
- Morphologic features
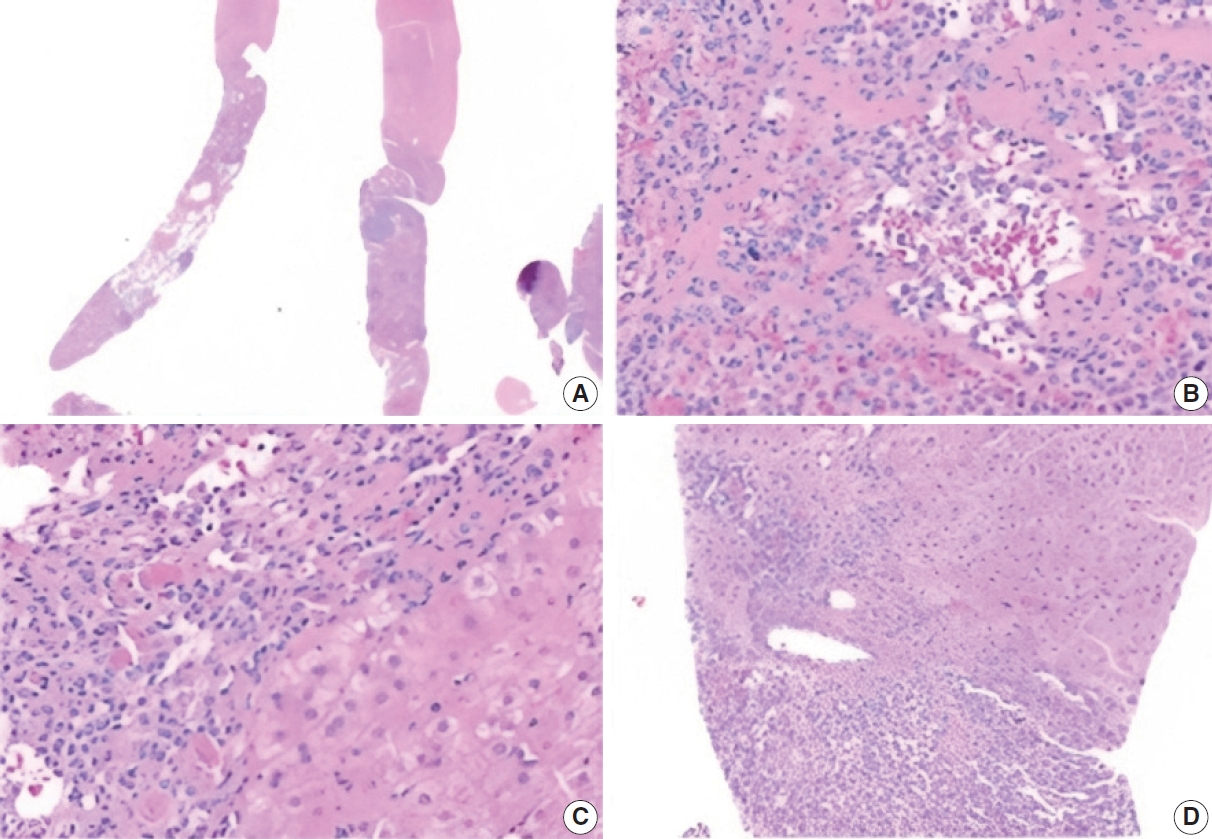
- The biopsy showed a neoplasm comprised of closely packed, small, thin-walled anastomosing vascular channels lined by bland endothelial cells. The lesion was well-demarcated from adjacent parenchyma, though focally infiltrated into a portal tract. The cells had no significant nuclear atypia or mitotic activity. There was no hobnailing, papillary formation, complex architecture, or necrosis. Background liver showed mild steatosis with no other significant abnormalities. Additionally, there were various-sized aggregates of small lymphoid cells (Fig. 2).
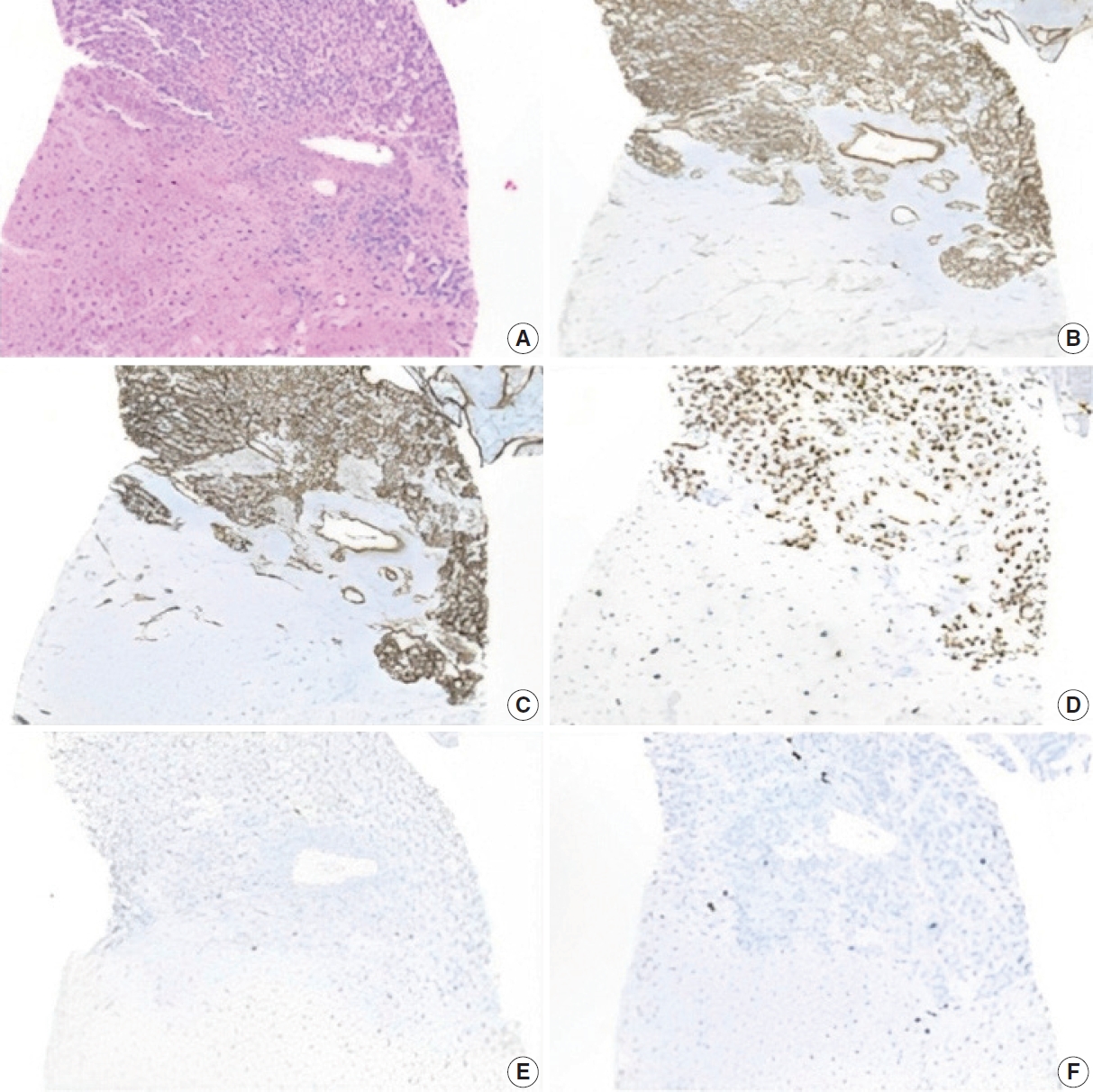
- Immunohistochemistry
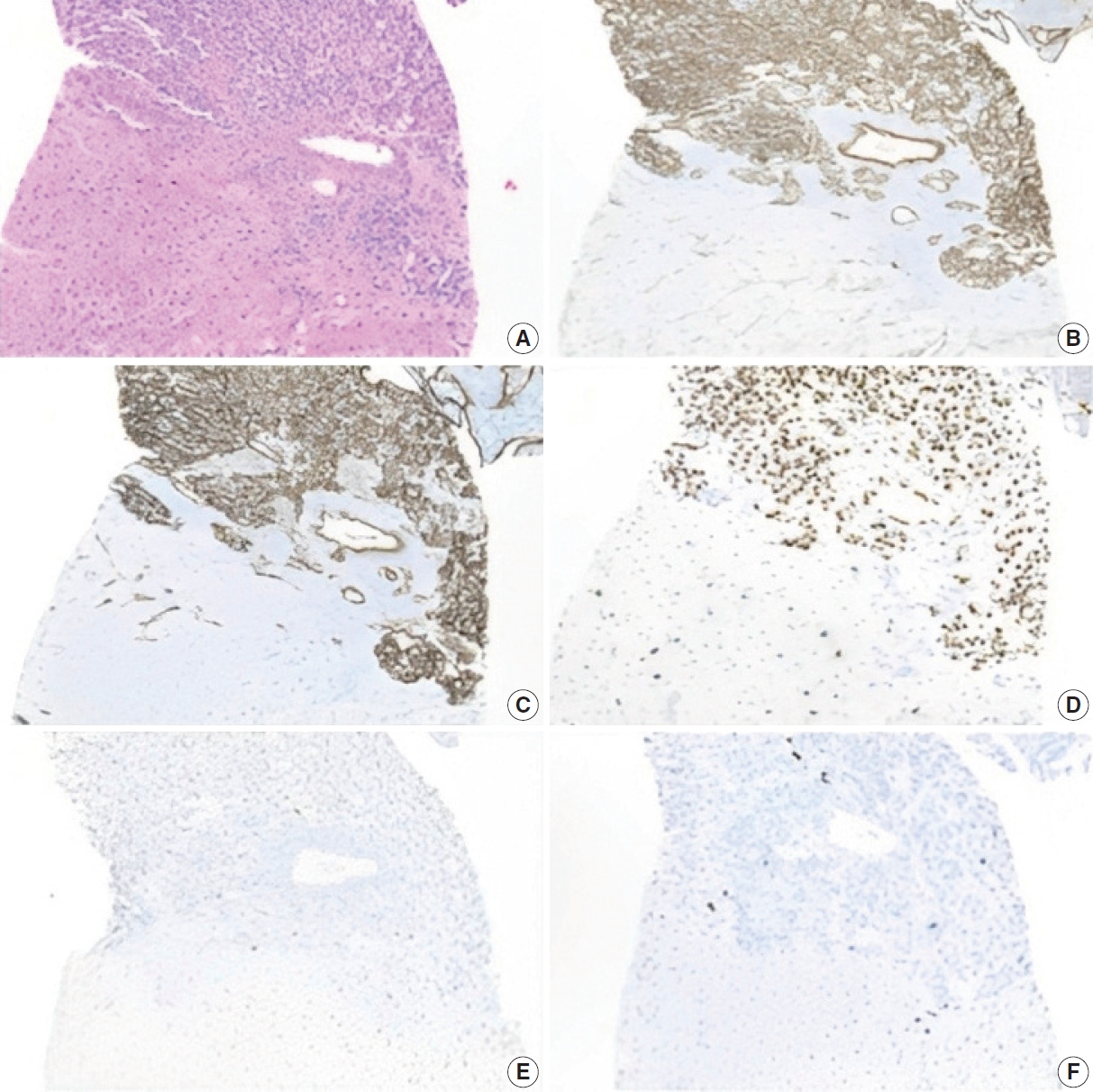
- The lesion displayed diffuse positivity for vascular markers (CD34, CD31, and ERG). p53 was patchy/weak (wild type). Neoplastic cells showed Ki-67 proliferation index of 5%–10% (Fig. 3). Iron, Periodic Acid-Schiff with diastase, and copper stains were negative in the background liver.
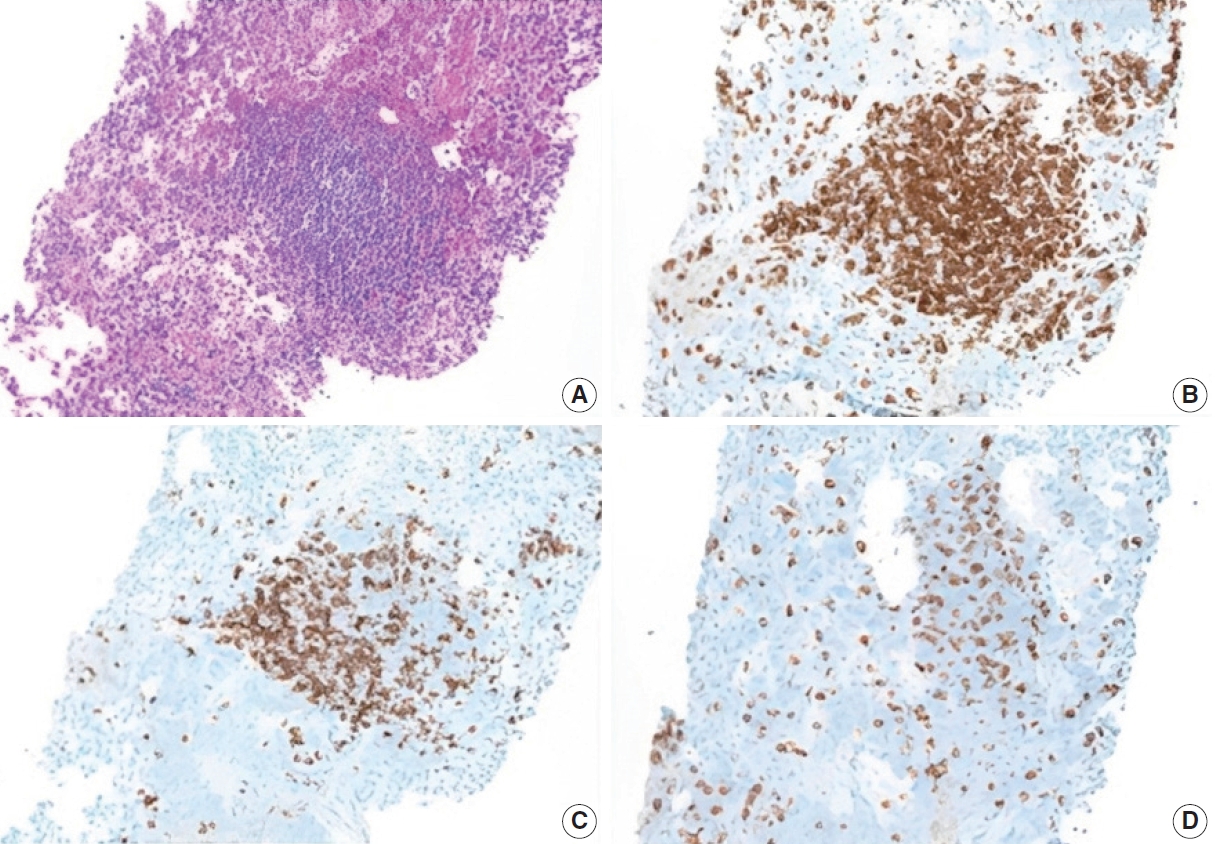
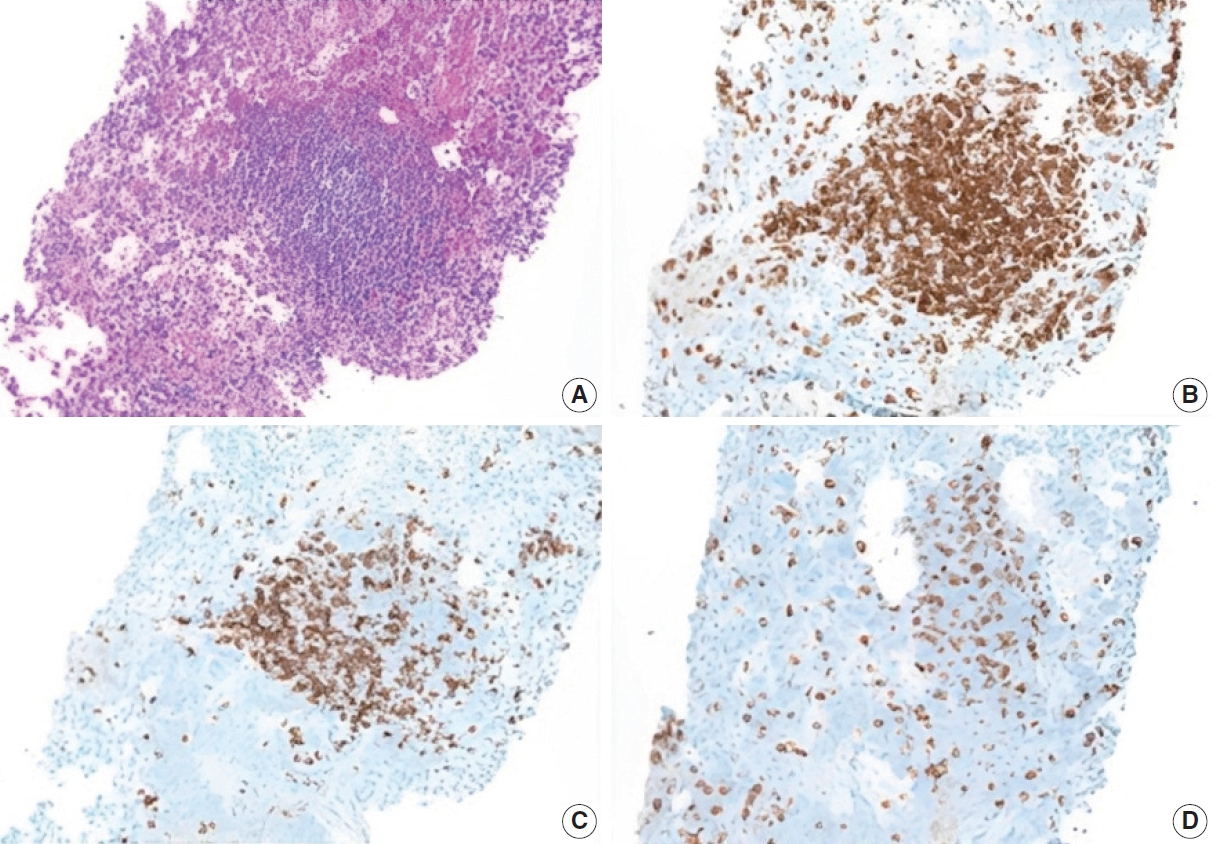
- Additional work-up was completed to exclude ectopic spleen and/or lymphoma. CD3 and CD20 immunostains highlight mixtures of mature T cells and B cells, respectively, with a predominance of the former. CD8 stains a subset of the T cells, and no splenic sinusoidal endothelial cells are present. These findings exclude ectopic splenosis and are not compatible with a B-cell lymphoma (Fig. 4).
- Morphologic and immunohistochemical findings were consistent with HSVN. Though most of the biopsy specimen showed a well-demarcated neoplasm, focal infiltration into a portal tract favored this diagnosis. The case was sent to an expert with special interest in HSVN, who agreed with our assessment [1].
- Follow-up
- The patient was seen by the hepatobiliary surgical team. Treatment options were discussed, including surgical resection, yttrium radioembolization, or observation with serial imaging. The patient opted for observation.
CASE REPORT
- HSVN is a rare vascular neoplasm of the liver with morphologic features that make diagnosis challenging. HSVN is usually incidentally found, and ranges in size from 0.2 to 16 cm [1-3]. It is characterized by strong expression of vascular markers (CD31, CD34, FLI-1, and ERG) and is pancytokeratin negative. All cases have a low Ki-67 proliferation index (<10%) [4]. Next-generation sequencing has shown activating hotspot mutations in the alpha subfamily of G-proteins (GNAQ, GNA11, and GNA14) [5]. In one particular case, foci of high-grade transformation were noticed microscopically while RAF1 and GNA11 concurrent mutations were detected at the molecular level. A link between these unique morphology and molecular profile was hypothesized [6].
- Anastomosing hemangioma (AH), another rare vascular neoplasm, was at the top of our differential diagnosis list. It is characterized by anastomosing vascular spaces lined by bland, round-to-oval endothelial cells with scattered hobnail morphology. While cases have been reported in many organs, the kidney is most frequently affected [7]. AH is immunoreactive for CD34 and CD31 and negative for keratin [8]. AH shares many morphologic features as HSVN including the characteristic mutations in the G-protein subfamily [5]. However, AH is well-demarcated with no infiltrative growth.
- Hepatic angiosarcoma (HAS) is the most common malignant mesenchymal liver tumor and is highly aggressive, with survival less than 6 months at time of diagnosis. Histologically, HAS displays infiltrating anastomosing vascular channels with tumor cells alongside dilated sinusoids. Endothelial cells may be round or spindle-shaped and exhibit severe nuclear atypia with frequent mitotic activity [9]. CD31 and CD34 are positive. Ki-67 levels are higher with a mean of 42.8% [3,4]. Thus, the Ki-67 proliferation index can be a useful distinction in differentiating these two neoplasms. Furthermore, the G-protein mutations seen in HSVN and AH are not found in HAS, which often has TP53 or KDR mutations [6].
- Different imaging modalities have been used in the workup of HSVN. Paisant et al. [2] reported possible characteristic imaging findings in a case series involving four patients who underwent contrast-enhanced ultrasound (CEUS), computed tomography, and MRI. All modalities demonstrated similar enhancement patterns on arterial phase imaging with early, irregularly thick peripheral and continuous enhancement. The appearance at arterial phase on larger lesions was described as “flower petal shaped” due to early septa enhancement. Imaging-specific characteristics include persistent thick rim enhancement with mild central washout on CEUS and heterogenous hyperintensity on T2- weighted imaging [2]. However, there has been a case with innumerable enhancing lesions that were only visualized on the arterial phase [6].
- While these radiological features are not totally diagnostic, they may be used to distinguish HSVN from other vascular hepatic lesions. For example, in classic hemangioma, there is peripheral, globular discontinuous enhancement as opposed to the thick rim, continuous enhancement seen in HSVN [2]. Hemangiomas also frequently display homogenous hyperintense T2 signal. AH shows nonspecific heterogenous, nodular enhancement [10]. HAS commonly shows heterogenous centripetal enhancement. It is also more likely to be poorly-circumscribed with a higher likelihood of intratumoral hemorrhage [11].
- Unique to our case was the concern for hepatic splenosis or lymphoma. Spenlosis is one type of ectopic spleen tissue, that typically occurs following abdominal trauma or splenectomy. Viable splenic tissue is autotransplanted in the abdominal cavity or more rarely in the thoracic cavity or brain. Hepatic splenosis is exceedingly rare and often asymptomatic with nonspecific imaging that can mimic other hepatic lesions [12]. CD8 stains a subset of T cells, and is useful for identifying splenic tissue because the endothelial cells lining the splenic sinuses are positive for CD8, a finding that is unique to those endothelial cells (littoral cells). In addition, there are case reports of contiguous primary marginal zone B-cell lymphoma and hemangioma [13]. The presumed T-cell predominant lymphoid aggregate in our HSVN case is not consistent with a marginal zone B-cell lymphoma and are more typical of reactive lymphoid aggregates.
- Currently there are no treatment guidelines. While HSVN is considered a benign lesion, its full course is not understood, and the current recommendation is complete resection and close observation. The reported follow-up period is variable, with a mean of seven months. Both those with complete resection and those with positive margins showed no evidence of disease recurrence [14]. Long-term data is still needed to understand the disease course and guide definitive management.
DISCUSSION
Ethics Statement
Institutional Review Board approval was waived due to the use of retrospective, de-identified data. This study adhered to the guidelines enacted by the Office of Human Research Protection that is supported by the U.S. Department of Health & Human Services. All information included in this report is de-identified and no personal details that may be used to identify the patient are included in this report. Written informed consent for the publication of this report was obtained from the patient.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author contributions
Conceptualization: TEJ. Investigation: MM. Supervision: TEJ. Writing—original draft: MM. Writing—review & editing: MM, DH, TEJ. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.
- 1. Gill RM, Buelow B, Mather C, et al. Hepatic small vessel neoplasm, a rare infiltrative vascular neoplasm of uncertain malignant potential. Hum Pathol 2016; 54: 143-51. ArticlePubMedPMC
- 2. Paisant A, Bellal S, Lebigot J, Canivet CM, Michalak S, Aube C. Imaging features of hepatic small vessel neoplasm: case series. Hepatology 2021; 74: 2894-6. ArticlePubMedPDF
- 3. Walcott-Sapp S, Tang E, Kakar S, Shen J, Hansen P. Resection of the largest reported hepatic small vessel neoplasm. Hum Pathol 2018; 78: 159-62. ArticlePubMed
- 4. Cicala CM, Monaca F, Giustiniani MC, Di Salvatore M. Multifocal hepatic small vessel neoplasm with spleen dissemination. BMJ Case Rep 2022; 15: e248785.ArticlePubMedPMC
- 5. Joseph NM, Brunt EM, Marginean C, et al. Frequent GNAQ and GNA14 mutations in hepatic small vessel neoplasm. Am J Surg Pathol 2018; 42: 1201-7. ArticlePubMed
- 6. Akanuma N, Joseph NM, Stachler M, Behr S, Takahashi T, Gill RM. Malignant hepatic vascular neoplasm with novel RAF1 and GNA11 mutations: risk stratification considerations for hepatic small vessel neoplasm (HSVN). Hum Pathol Rep 2022; 29: 300671.Article
- 7. Lappa E, Drakos E. Anastomosing hemangioma: short review of a benign mimicker of angiosarcoma. Arch Pathol Lab Med 2020; 144: 240-4. ArticlePubMedPDF
- 8. Lin J, Bigge J, Ulbright TM, Montgomery E. Anastomosing hemangioma of the liver and gastrointestinal tract: an unusual variant histologically mimicking angiosarcoma. Am J Surg Pathol 2013; 37: 1761-5. PubMed
- 9. Bioulac-Sage P, Laumonier H, Laurent C, Blanc JF, Balabaud C. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis 2008; 28: 302-14. ArticlePubMed
- 10. O’Neill AC, Craig JW, Silverman SG, Alencar RO. Anastomosing hemangiomas: locations of occurrence, imaging features, and diagnosis with percutaneous biopsy. Abdom Radiol (NY) 2016; 41: 1325-32. ArticlePubMedPDF
- 11. Liu Z, Yi L, Chen J, et al. Comparison of the clinical and MRI features of patients with hepatic hemangioma, epithelioid hemangioendothelioma, or angiosarcoma. BMC Med Imaging 2020; 20: 71.ArticlePubMedPMCPDF
- 12. Kampa KC, Guerra JA, Percicotte AP, Zaparolli M, Raeder Costa MA, Ramos EJ. Hepatic splenosis: a report of a very rare case. Gastroenterol Hepatol Open Access 2019; 10: 88-91. Article
- 13. Shiozawa K, Watanabe M, Ikehara T, et al. A case of contiguous primary hepatic marginal zone B-cell lymphoma and hemangioma ultimately diagnosed using contrast-enhanced ultrasonography. Case Rep Oncol 2015; 8: 50-6. ArticlePubMedPMCPDF
- 14. Goh IY, Mulholland P, Sokolova A, Liu C, Siriwardhane M. Hepatic small vessel neoplasm: a systematic review. Ann Med Surg (Lond) 2021; 72: 103004.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Mesenchymal Tumors of the Liver: An Update Review
Joon Hyuk Choi, Swan N. Thung
Biomedicines.2025; 13(2): 479. CrossRef - Revisiting Hepatic Small Vessel Neoplasms and Anastomosing Hemangiomas as a Unique Capillary Liver Neoplasm: A 25-Case Series With Pathomolecular Correlations
Aurélie Beaufrère, Astrid Laurent-Bellue, Antoinette Lemoine, Agnès Bourillon, Nathalie Sturm, Pierre Gosset, Virginie Cahn, Valérie Vilgrain, Maité Lewin, Valérie Paradis, Catherine Guettier
Modern Pathology.2025; 38(10): 100835. CrossRef - Imaging features of recently identified low-grade vascular neoplasia of the liver: hepatic small vessel neoplasm and anastomosing hemangioma
Maïté Lewin, Rauda Aldhaheri, Aurélie Beaufrère, Christophe Desterke, Anita Paisant, Ivan Bricault, Paul Borde, Gabriel Simon, Mickaël Lesurtel, Daniel Cherqui, Clara Prud’Homme, Valérie Vilgrain, Astrid Laurent-Bellue
European Radiology.2025;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure





 E-submission
E-submission