Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(2); 2024 > Article
-
Newsletter
What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system -
Andrey Bychkov1
 , Chan Kwon Jung2
, Chan Kwon Jung2
-
Journal of Pathology and Translational Medicine 2024;58(2):98-101.
DOI: https://doi.org/10.4132/jptm.2024.03.06
Published online: March 13, 2024
1Department of Pathology, Kameda Medical Center, Kamogawa, Japan
2Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea
- Corresponding Author: Andrey Bychkov, MD, PhD, FRCPath Department of Pathology, Kameda Medical Center, Kamogawa, Japan E-mail: bychkov.andrey@kameda.jp
- This article has been published jointly, with consent, in both Journal of Pathology and Translational Medicine and PathologyOutlines.com.
• Received: January 13, 2024 • Accepted: March 6, 2024
© 2024The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- In line with the release of the 5th edition WHO Classification of Tumors of Endocrine Organs (2022) and the 3rd edition of the Bethesda System for Reporting Thyroid Cytopathology (2023), the field of thyroid pathology and cytopathology has witnessed key transformations. This digest brings to the fore the refined terminologies, newly introduced categories, and contentious methodological considerations pivotal to the updated classification.
- Changes in terminology and volume structure
- • Adopted in all WHO 5th edition volumes (2019–2023; the iteration cycle of the Blue Books is every 5 years)
- ° The term “variant” has been replaced by “subtype” to avoid confusion with genetic variants; the former was historically widely used in thyroid pathology
- ° Gene fusion notation has been revised according to the HUGO Gene Nomenclature Committee recommendations, replacing the hyphen (-) or forward-slash (/) with a double colon (::)
- ° All key entities are supplied with a list of essential and desirable diagnostic criteria
- ° Nonneoplastic (tumor-like) lesions are included as a part of the classification, for differential diagnosis purposes and convenience of readers
- ° Tumors not specific to particular organs/systems (e.g., mesenchymal, hematolymphoid, metastasis) are combined in separate chapters, representing such entities from the entire volume (i.e., endocrine)
- ° Size and area
- - Tumor size is reported in mm, not cm
- - For mitotic count, tumor area is measured in mm2 and not in high-power fields (10 HPF are approximated as 2 mm2; detailed conversion tables are available); this is aimed for standardization and reflects adoption of digital pathology tools
- • Thyroid tumors
- ° The new WHO classification divides follicular cell-derived neoplasms into benign, low-risk, and malignant
- ° Invasive encapsulated follicular variant of papillary thyroid carcinoma (IEFVPTC) is now a distinct entity and no longer a subtype of papillary thyroid carcinoma (PTC)
- - IEFVPTC has a RAS-like mutational and transcriptomic profile similar to that of follicular adenoma (FA) and follicular thyroid carcinoma (FTC)
- - Classic PTC and the infiltrative follicular subtype of PTC are BRAF-like tumors
- ° A grading concept (Table 1) is introduced for differentiated and medullary thyroid carcinomas (MTC)
- - The new tumor type, high-grade follicular cell-derived non-anaplastic thyroid carcinoma, has 2 histologic subtypes: traditional poorly differentiated thyroid carcinoma (PDTC) based on Turin criteria and a new subtype, differentiated high-grade thyroid carcinoma (DHGTC)
- ° The new WHO thyroid classification has been effective since its online release in March 2022 and should be adopted by practicing pathologists
- - Pathologists are responsible for educating clinicians about relevant changes and new terminology
- - Detailed and illustrated reviews are available in subscription [1] and open access [2-4] journals
- Benign and low-risk neoplasms
- • Thyroid follicular nodular disease (FND) was introduced to describe a multifocal benign proliferation with nodular hyperplasia
- ° This terminology reflects the complex blend of nonclonal/hyperplastic and clonal/neoplastic proliferations
- ° Clinically fits to multinodular goiter; also known as adenomatous nodules/hyperplasia
- • Follicular adenoma with papillary architecture
- ° Typically an autonomous hyperfunctioning nodule with intrafollicular papillary growth
- ° Differentiated from conventional follicular adenoma by its specific gene (EZH1, TSHR or GNAS) mutations
- • A new group of low-risk follicular cell-derived neoplasia, coded borderline by ICD-O (/1)
- ° Includes noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), tumors of uncertain malignant potential (FT-UMP and WDTUMP), and hyalinizing trabecular tumor
- ° Extremely low risk of metastatic spread or recurrence and excellent prognosis, but not categorized as benign
- • NIFTP
- ° Diagnostic criteria not changed, including <1% papillae cutoff
- ° Newly added subtypes: oncocytic NIFTP, subcentimeter NIFTP
- Oncocytic tumors
- • The term “Hürthle cell” has been replaced with “oncocytic cell,” updating the names of the related adenoma (OA) and carcinoma (OCA)
- ° Hürthle cell is a historical misnomer, a term that originally described not oncocytic cells but C cells in dogs
- ° Oncocytic terminology is consistently used across other locations (e.g., kidney, salivary)
- ° Nevertheless, “Hürthle cell carcinoma” remains an acceptable term because of wide use by clinicians
- • Not a standalone group like in the previous edition but separate entities in benign (adenoma) and malignant (carcinoma) tumors. Nonetheless, both OA and OCA share similar molecular mechanisms, such as alterations in mitochondrial genes.
- Encapsulated follicular cell-derived carcinomas
- • FTC and IEFVPTC, despite their identical genomic profiles (RAS-like) and clinical behaviors, remain separate, but the future edition may combine them into a single diagnostic category, the follicular-patterned differentiated thyroid carcinoma
- • FTC, IEFVPTC, and OCA are categorized into 3 subtypes: minimally invasive (capsular invasion only), encapsulated angioinvasive, and widely invasive (entirely obliterated or with focally intact tumor capsule and/or gross invasion through the gland)
- ° Foci of vascular invasion in encapsulated angioinvasive carcinomas should be counted, with <4 and ≥4 foci continuing to be designated as limited and extensive angioinvasion, respectively
- ° Widely invasive FTCs are rare and require ruling out PDTC and DHGTC
- Papillary thyroid carcinoma
- • PTC diagnosis requires a set of distinctive nuclear features, plus either papillary or solid/trabecular architecture or infiltrative growth in follicular-patterned tumors
- ° Unlike in the previous editions, the importance of PTC nuclear features is now overshadowed by the molecular signature
- ° This category is reserved only for the BRAF-like neoplasms
- - IEFVPTC is moved out, being a RAS-like tumor
- - The tumor formerly known as cribriform-morular PTC no longer belongs to PTC and is now listed among thyroid tumors of uncertain histogenesis (see below)
- • 13 histologic subtypes exist based on predominant pattern or other morphologic features
- • PTC with follicular pattern ° The macrofollicular variant is no longer a subtype of PTC
- ° The only PTC subtype with a follicular pattern is now infiltrative follicular PTC
- - Advanced infiltrative follicular PTC may have morphological overlap with widely invasive IEFVPTC when the capsule of the latter is almost completely lost, but the genetic signature is different (BRAF-like versus RAS-like)
- • Papillary microcarcinoma
- ° No longer considered a histopathological subtype
- ° Now required to be subtyped by the pattern (e.g., classic PTC, 6 mm)
- • Solid/trabecular PTC, which is more common in children than in adults, is diagnosed when >50% of the tumor has a solid, trabecular or nested growth pattern (unlike the “all or nearly all” cutoff in the previous edition)
- • Aggressive histologic subtypes of PTC: (1) tall cell, (2) hobnail, (3) columnar cell
- ° Tall cell PTC
- - Tall cell is defined as being at least 3 times taller than their width (unlike 2–3 to 1 in the previous edition) as well as having dense eosinophilic cytoplasm and distinct cell membranes
- - At least 30% of the tumor should be composed of tall cells
- ° Hobnail PTC
- - Cutoff of hobnail cells is not explicitly stated; supposed to remain ≥30%
- - True hobnail cells should not be confused with hobnail-like degenerative atypia frequently seen in cystic PTC
- ° Diffuse-sclerosing subtype is no longer considered aggressive PTC
- ° Aggressive PTCs are typically seen in older patients, show angiovascular invasion, advanced pathological stage and increased mitotic activity
- - In contrast, aggressive (by histologic pattern) PTCs with an indolent clinical course occur at a younger age, are usually encapsulated or well circumscribed and show low proliferation rate
- ° Included in the ATA clinical risk stratification system
- Differentiated high-grade thyroid carcinoma (DHGTC)
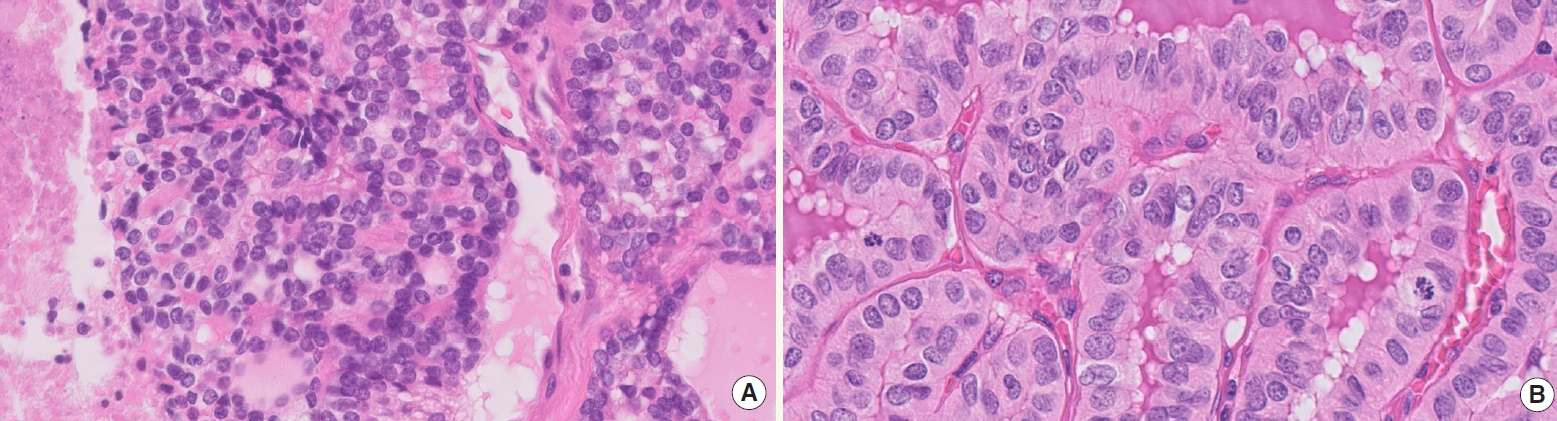
- • This new category includes follicular cell-derived carcinomas with high-grade features, emphasizing the significance of mitoses and necrosis in differentiated thyroid carcinomas (Fig. 1) and the lack of anaplastic foci
- ° Necrosis may extend from focal and comedo-like to large areas; it should be distinguished from degenerative changes (e.g., cystic and oncocytic tumors)
- ° Mitosis is counted in hot spots, per 2 mm2 area
- ° Unlike in MTC (see below), Ki67 is not used for grading
- • If an FTC or PTC has a mitotic count ≥ 5 per 2 mm2 and/or necrosis, it is classified as DHGTC
- ° Most DHGTC develop from PTC, usually aggressive subtypes
- ° Recorded in diagnosis as “high-grade PTC (FTC/OCA)”, followed by subtype
- ° If an FTC with areas of solid or trabecular growth has a mitotic count of ≥3 per 2 mm2 and/or necrosis, it is diagnosed as PDTC
- Anaplastic thyroid carcinoma (ATC)
- • By definition, ATC is composed of undifferentiated cells but may have focal features of thyroid follicular differentiation and/or a pre-existing differentiated thyroid carcinoma
- • Primary squamous cell carcinoma of the thyroid is now considered a histologic pattern of ATC and not a standalone entity
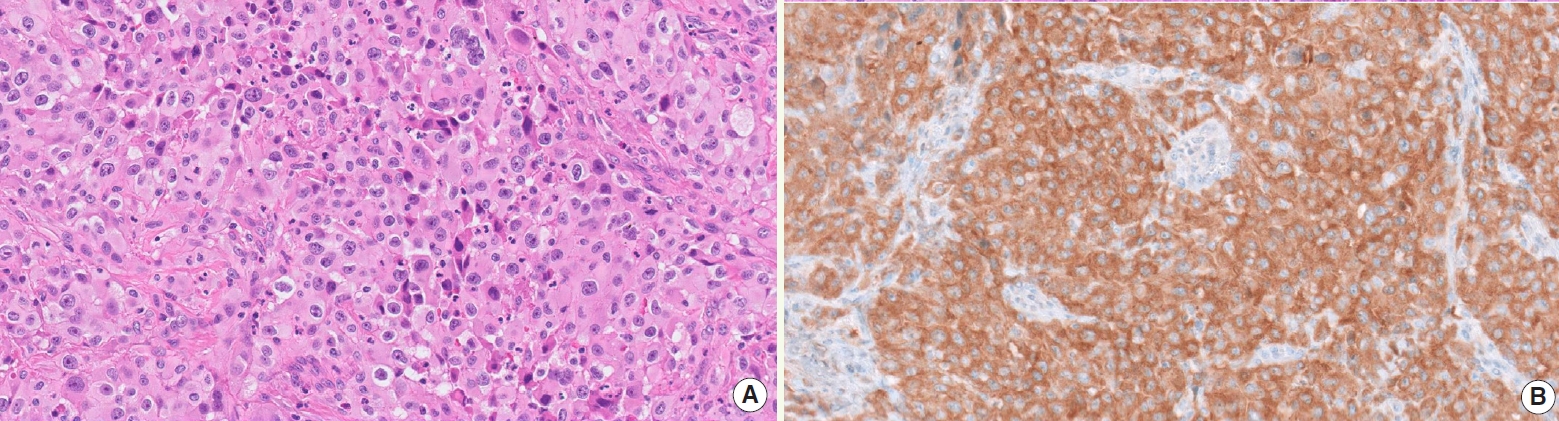
- • BRAF V600E detection by immunostaining (Fig. 2) and/or genotyping should be performed due to the availability of targeted therapy
- Thyroid C cell-derived carcinoma
- • MTC remains the archetypal neuroendocrine neoplasm of the thyroid
- ° Driven predominantly by RET and RAS alterations
- ° No known benign counterpart
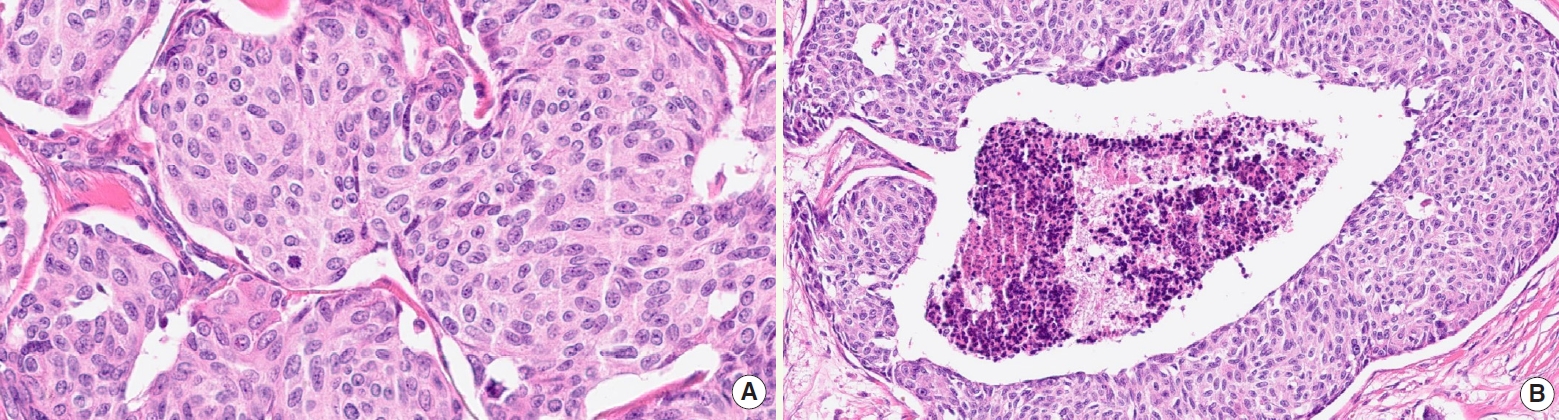
- • Grading (high versus low): based on mitotic count, Ki67, and tumor necrosis
- ° High-grade MTC (Fig. 3) must display at least one of the following features: mitotic count ≥5 per 2 mm2, tumor necrosis, Ki67 proliferation index ≥5%
- ° High-grade MTCs are associated with lower disease-specific survival and recurrence-free survival rates; they constitute 10–20% of all cases
- ° Ki67 proliferation index should not rely solely on eye-balling (approximate count); instead, formal manual count or automated image analysis is recommended
- ° Grading of MTC is reserved for surgical specimens
- Salivary gland-type carcinomas of the thyroid
- • Salivary gland-type carcinomas of the thyroid consist of mucoepidermoid carcinoma and secretory carcinoma, previously known as mammary analogue secretory carcinoma (MASC)
- • Secretory carcinoma and mucoepidermoid carcinoma have specific gene fusions, ETV6:: NTRK3 fusion and CRTC1::MAML2 fusion, respectively
- Thyroid tumors of uncertain histogenesis
- • A new category, thyroid tumors of uncertain histogenesis, includes sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) and cribriform-morular thyroid carcinoma (CMTC); additional studies are needed to further refine these neoplasms
- • The previous classification, dated 2017, considered SMECE a subtype of salivary gland-type carcinomas of the thyroid gland
- ° SMECE is presumed to arise from solid cell nests (ultimobranchial body remnants) and has genetic alterations, such as MET hyperploidy and mutations in APC, NTRK3, and NF1
- • CMTC, previously classified as a distinct variant/subtype of PTC, is now listed as a cancer type among thyroid tumors of uncertain histogenesis
- ° CMTC features genetic alterations involved in the Wnt/beta-catenin pathway, such as mutations in APC and CTNNB1; no cases of CMTC with BRAF V600E have been reported
- - Nuclear expression of beta-catenin, estrogen receptor, and progesterone receptor
- - No colloid formation; often negative for markers of thyroid follicular cell differentiation (thyroglobulin and PAX8)
- - Cribriform component is TTF1 positive, but morulae are negative
- Other tumors
- ° The term “thyroblastoma” was introduced for malignant teratomas or carcinosarcomas with DICER1 mutations
- ° Extranodal marginal zone lymphoma (E-MZL) is a new preferred terminology for MALT lymphoma
- Molecular immunohistochemistry
- • The role of immunohistochemistry is emphasized, necessitating robust methodology with proper reagents, stringent validation protocols, and the use of positive controls
- • Important biomarkers: surrogate immunostains (VE1, pan-TRK, SP174/RAS, ALK, PTEN, beta-catenin), theranostic markers (PDL1) [5]
2022 WHO CLASSIFICATION
- • A 3rd edition of the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) was released in July 2023 and contains only minor updates [6]
- • TBSRTC assigns a single, distinct name for each of its 6 diagnostic categories: (I) nondiagnostic, (II) benign, (III) atypia of undetermined significance (AUS), (IV) follicular neoplasm, (V) suspicious for malignancy, (VI) malignant
- ° Alternate names for 3 of the diagnostic categories (I/unsatisfactory, III/follicular lesion of undetermined significance, and IV/suspicious for a follicular neoplasm) have been eliminated to avoid confusion
- ° TBSRTC 2023 continues to advise using the category names and optionally, their corresponding category numbers, e.g. benign (Bethesda II), follicular neoplasm (Bethesda IV)
- • Each category is associated with an updated risk of malignancy (ROM) based on new data since the 2017 edition; clinical management algorithms for each category have also been revised
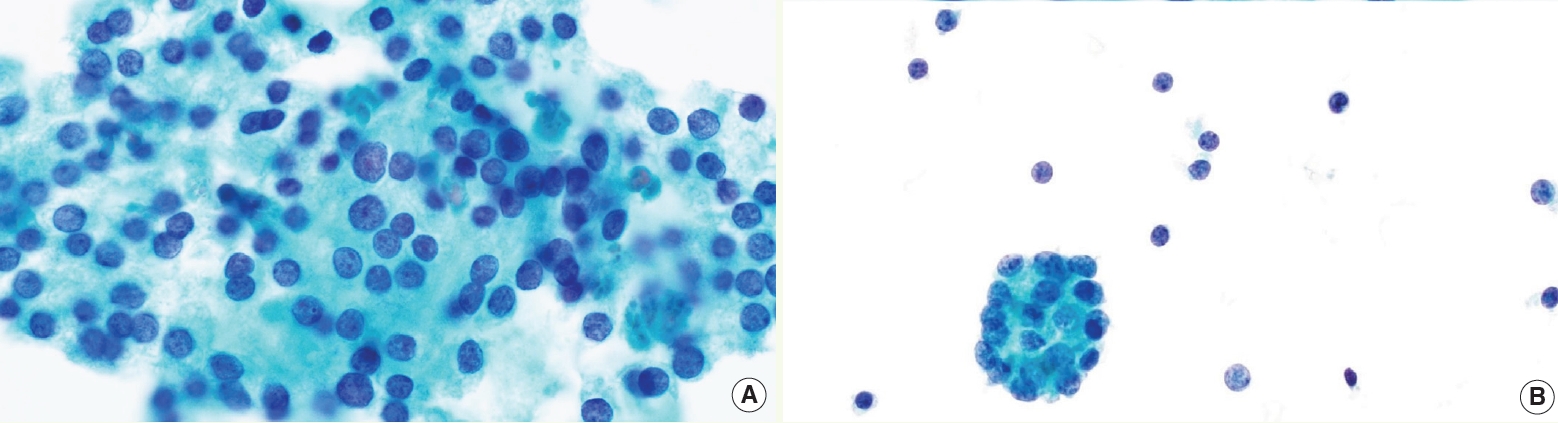
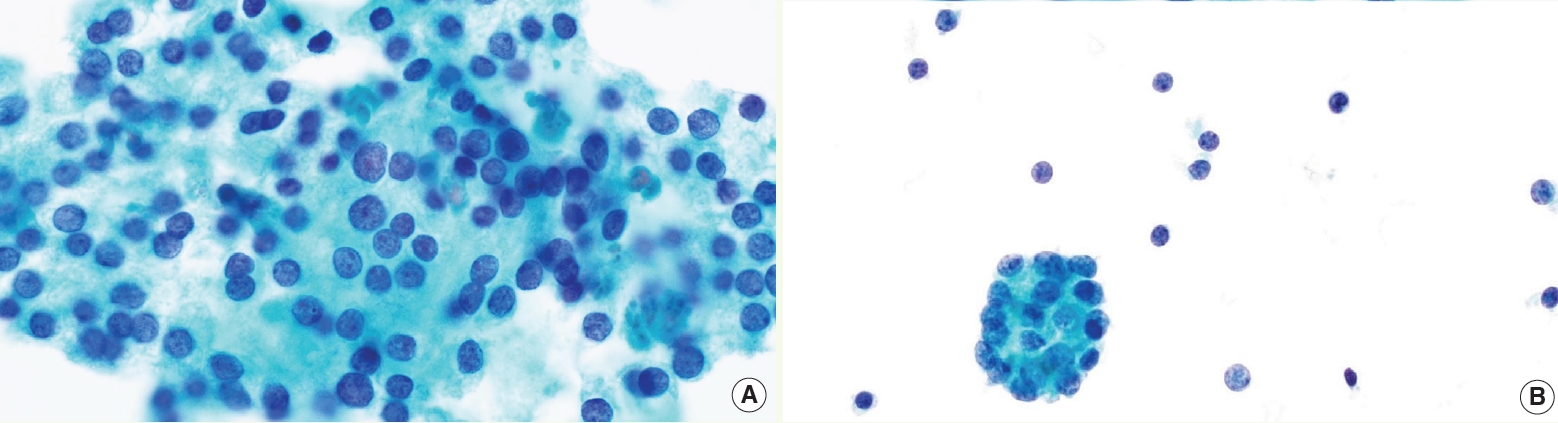
- • AUS category is now divided into 2 subgroups (Fig. 4): AUS-nuclear atypia and AUS-other, with different implied ROM (higher in AUS-nuclear) and molecular profile
- ° The term “nuclear atypia” (previously known as cytologic atypia) refers to mild nuclear alterations, such as slight enlargement, pale chromatin, and irregular contours
- ° AUS-other includes architectural atypia; oncocytic atypia; atypia, NOS; atypical lymphoid cells, rule out lymphoma
- • Follicular neoplasm (FN) is defined in 2 scenarios: “typical” FN without nuclear atypia and “potential NIFTP/EFVPTC” with mild or focal nuclear atypia
- • New discussions on pediatric thyroid disease have been included, supplied with pediatric ROMs and management algorithms
- • Revised terminology in accordance with the WHO 5th edition
- • Two new chapters have been added, acknowledging the importance of clinical perspectives, imaging findings, and the expanding role of molecular testing in thyroid disease
2023 BETHESDA SYSTEM
- Dr. Bychkov has been an author for PathologyOutlines. com since 2015, a part of the editorial board since 2016, and the subspecialty section editor for Thyroid in 2017-2022. He is currently the Director of Digital Pathology at Kameda Medical Center, Japan. His research interests are thyroid histopathology and digital pathology, and he was a contributor to the 2022 WHO classification of endocrine tumors.
- Dr. Chan Kwon Jung is a Professor of Pathology at The Catholic University of Korea (Seoul). He is a practicing endocrine pathologist and cytopathologist, and was a contributor to the 2022 WHO classification of thyroid tumors.
Meet the Authors
Fig. 1.Differentiated high-grade thyroid carcinoma. (A) High-grade FTC with necrosis (left) and mitosis (upper right). (B) High-grade PTC with mitotic activity.
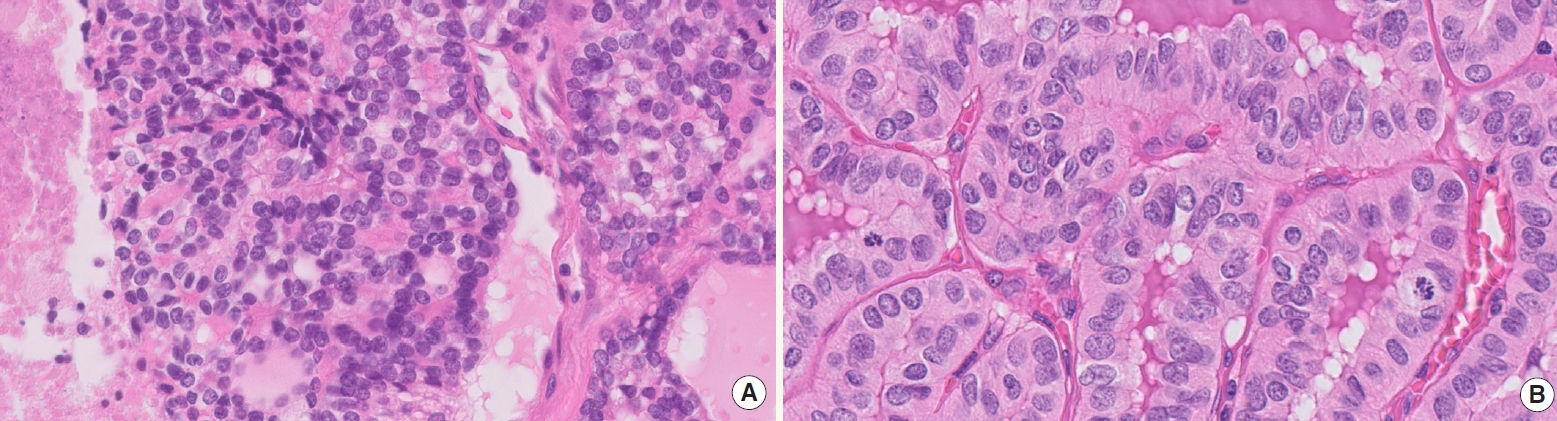

Fig. 3.High-grade MTC is identified by combination of mitotic activity (A, center) and tumor necrosis (B).
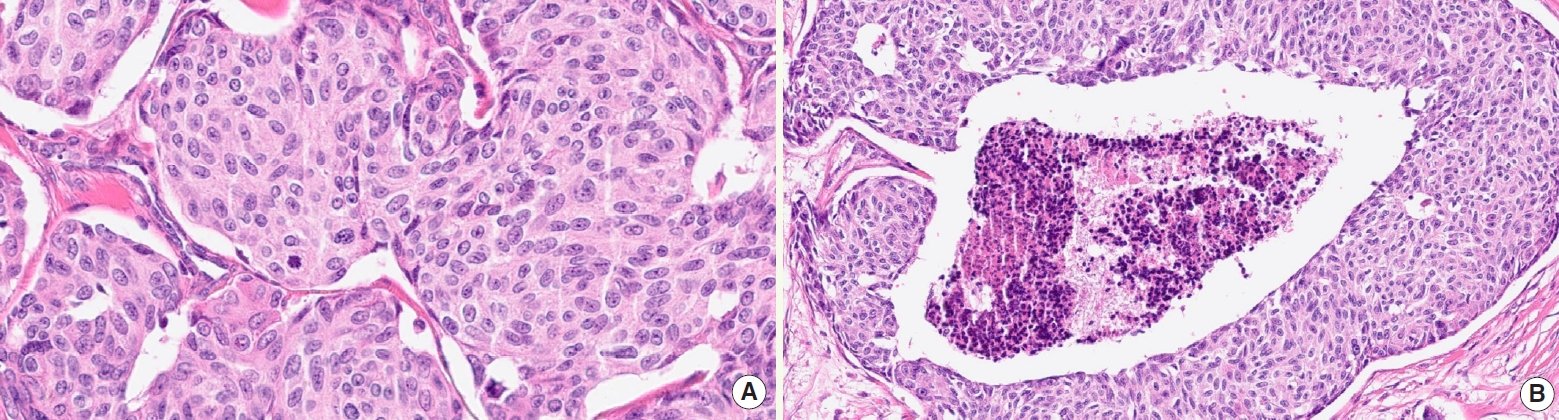

Table 1.Histopathological grading scheme
| Tumor type | Tumor necrosis | Mitoses per 2 mm2 | Ki67 index |
|---|---|---|---|
| PDTC | Present | ≥3 | Not required |
| DHGTC | Present | ≥5 | Not required |
| MTC | |||
| High-grade | Present | ≥5 | ≥5% |
| Low-grade | Absent | <5 | <5% |
- 1. Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocrine Pathology 2022; 33: 27-63. ArticlePubMedPDF
- 2. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinology and Metabolism (Seoul, Korea) 2022; 37: 703-18. ArticlePubMedPMCPDF
- 3. Basolo F, Macerola E, Poma AM, Torregrossa L. The 5th edition of WHO classification of tumors of endocrine organs: changes in the diagnosis of follicular-derived thyroid carcinoma. Endocrine 2023; 80: 470-6. ArticlePubMedPMCPDF
- 4. Juhlin C, Mete O, Baloch ZW. The 2022 WHO classification of thyroid tumors: novel concepts in nomenclature and grading. Endocrine-related Cancer 2023; 30: e220293.PubMed
- 5. Agarwal S, Bychkov A, Jung CK. Emerging Biomarkers in Thyroid Practice and Research. Cancers 2021; 14: 204.ArticlePubMedPMC
- 6. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023; 33: 1039-44. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Diagnosis and management of thyroid nodule
Suganya Sekar, Deepak Thomas Abraham
Current Opinion in Endocrinology, Diabetes & Obesity.2025; 32(5): 167. CrossRef - Impact of thyroid Bethesda category IV (follicular neoplasm) terminology unification on atypia of undetermined significance reporting patterns in thyroid fine-needle aspiration
Shirin Abbasi, Lorena Marcano-Bonilla, Syed Z. Ali
Journal of the American Society of Cytopathology.2025;[Epub] CrossRef - Diagnostic Challenges, Prognostic Assessment, and Treatment Strategies in High-Grade Differentiated Thyroid Carcinoma
Chan Kwon Jung, Agnes Stephanie Harahap
Endocrinology and Metabolism.2025; 40(6): 830. CrossRef - Cytologic and Clinicopathologic Features of Papillary Thyroid Carcinoma with Prominent Hobnail Features on FNAC
Deepali Saxena, Ravi Hari Phulware, Prashant Durgapal, Arvind Kumar, Amit Kumar Tyagi
Indian Journal of Otolaryngology and Head & Neck Surgery.2024; 76(5): 4885. CrossRef - FHL1: A novel diagnostic marker for papillary thyroid carcinoma
Yeting Zeng, Dehua Zeng, Xingfeng Qi, Hanxi Wang, Xuzhou Wang, Xiaodong Dai, Lijuan Qu
Pathology International.2024; 74(9): 520. CrossRef - Nouveautés en pathologie thyroïdienne : classification OMS 2022, système Bethesda 2023, biologie moléculaire et testing moléculaire
Mohamed Amine Bani, Sophie Moog, Voichita Suciu, Livia Lamartina, Abir Al Ghuzlan
Bulletin du Cancer.2024; 111(10): 10S5. CrossRef - Cytologic hallmarks and differential diagnosis of papillary thyroid carcinoma subtypes
Agnes Stephanie Harahap, Chan Kwon Jung
Journal of Pathology and Translational Medicine.2024; 58(6): 265. CrossRef - Surgical and Pathological Challenges in Thyroidectomy after Thermal Ablation of Thyroid Nodules
Ting-Chun Kuo, Kuen-Yuan Chen, Hsiang-Wei Hu, Jie-Yang Jhuang, Ming-Tsan Lin, Chin-Hao Chang, Ming-Hsun Wu
Thyroid®.2024; 34(12): 1503. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
- Related articles
-
- What’s new in hematopathology 2025: myeloid neoplasms in the WHO 5th edition and ICC
- What’s new in neuropathology 2024: CNS WHO 5th edition updates
- What’s new in adrenal gland pathology: WHO 5th edition for adrenal cortex
- What’s new in genitourinary pathology 2023: WHO 5th edition updates for urinary tract, prostate, testis, and penis
- What’s new in hematopathology 2023: updates on mature T-cell neoplasms in the 5th edition of the WHO classification
What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system




Fig. 1. Differentiated high-grade thyroid carcinoma. (A) High-grade FTC with necrosis (left) and mitosis (upper right). (B) High-grade PTC with mitotic activity.
Fig. 2. BRAF-mutant ATC (A) detected by VE1 immunostaining (B).
Fig. 3. High-grade MTC is identified by combination of mitotic activity (A, center) and tumor necrosis (B).
Fig. 4. AUS subtypes. AUS-nuclear atypia with mild nuclear changes (A) versus AUS-other (B).
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
What’s new in thyroid pathology 2024: updates from the new WHO classification and Bethesda system
| Tumor type | Tumor necrosis | Mitoses per 2 mm2 | Ki67 index |
|---|---|---|---|
| PDTC | Present | ≥3 | Not required |
| DHGTC | Present | ≥5 | Not required |
| MTC | |||
| High-grade | Present | ≥5 | ≥5% |
| Low-grade | Absent | <5 | <5% |
Table 1. Histopathological grading scheme

 E-submission
E-submission