Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 58(6); 2024 > Article
-
Review
Cervical intraepithelial neoplasia and cervical cytology in pregnancy -
Ji-Young Kim
 , Jeong Yun Shim
, Jeong Yun Shim
-
Journal of Pathology and Translational Medicine 2024;58(6):283-290.
DOI: https://doi.org/10.4132/jptm.2024.10.17
Published online: November 7, 2024
Department of Pathology, CHA University School of Medicine, CHA Gangnam Medical Center, Seoul, Korea
- Corresponding Author: Jeong Yun Shim, MD, PhD, Department of Pathology, CHA University School of Medicine, CHA Gangnam Medical Center, 566 Nonhyeon-ro, Gangnam-gu, Seoul 06135, Korea Tel: +82-2-3468-2612, Fax: +82-2-3468-2619, E-mail: jyshim@cha.ac.kr
© 2024 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- Abstract
- PAPANICOLAOU SCREENING AS ROUTINE ANTENATAL CARE
- CYTOLOGIC CHANGES DURING PREGNANCY AND ISSUES IN CYTOLOGICAL INTERPRETATION
- NATURAL HISTORY OF CERVICAL INTRAEPITHELIAL NEOPLASIA AND RATE OF REGRESSION DURING PREGNANCY
- POSSIBLE MECHANISMS OF REGRESSION AND PREDICTING FACTORS
- MANAGEMENT OF ABNORMAL CYTOLOGY DURING PREGNANCY
- CONCLUSION
- NOTES
- REFERENCES
Abstract
- Cervical cancer screening during pregnancy presents unique challenges for cytologic interpretation. This review focuses on pregnancy-associated cytomorphological changes and their impact on diagnosis of cervical intraepithelial neoplasia (CIN) and cervical cancer. Pregnancy-induced alterations include navicular cells, hyperplastic endocervical cells, immature metaplastic cells, and occasional decidual cells or trophoblasts. These changes can mimic abnormalities such as koilocytosis, adenocarcinoma in situ, and high-grade squamous intraepithelial lesions, potentially leading to misdiagnosis. Careful attention to nuclear features and awareness of pregnancy-related changes are crucial for correct interpretation. The natural history of CIN during pregnancy shows higher regression rates, particularly for CIN 2, with minimal risk of progression. Management of abnormal cytology follows modified risk-based guidelines to avoid invasive procedures, with treatment typically deferred until postpartum. The findings reported in this review emphasize the importance of considering pregnancy status in cytological interpretation, highlight potential problems, and provide guidance on differentiating benign pregnancy-related changes from true abnormalities. Understanding these nuances is essential for accurate diagnosis and proper management of cervical abnormalities in pregnant women.
- Although cervical cancer remains a very rare condition, the peak incidence of CIN, the direct precursor of cervical cancer, usually occurs in child-bearing age. The increasing trend of delayed childbearing has led to a significant increase in the incidence of cervical cancer and CIN during pregnancy in recent decades [6,7]. Consequently, Papanicolaou (Pap) screening has been established as a standard procedure in routine antenatal care [5]. Notably, pregnancy can provide an opportunity to screen for cervical cancer in women who otherwise would not be tested. Abnormal Pap results are reported in approximately 3.3%–5% of pregnant women, comparable with non-pregnant women [8,9].
- Human papillomavirus (HPV) DNA testing has recently been implemented in cervical cancer screening as either a standalone screening modality or as a co-test with cytology [10-12]. HPV DNA testing has also been utilized for cervical cancer screening in pregnant women with satisfactory results [13]. However, HPV DNA testing is considered less specific, particularly in young women, due to a higher prevalence of HPV infection in women under 30 years of age. In a recent literature review over a 10-year period, Pap testing was an important first modality for cervical cancer screening in pregnant women [14].
PAPANICOLAOU SCREENING AS ROUTINE ANTENATAL CARE
- Pregnancy incurs profound physiological changes in the cervical and endocervical mucosa causing various morphologic alterations on cytology. Knowing the pregnancy status of a patient is pertinent to avoid misinterpretation. In pregnancy, the squamous epithelium of cervical mucosa reamins less mature due to lack of estrogen-driven differentiation. The cervix also shows transformation zone eversion, increased vascularity, endocervical glandular hyperplasia, and increased mucus production. On rare occasions, a small number of degraded cells from decidua or trophoblasts may be shed and released. All these changes may cause cytologic alterations and can cause potential issues in interpretation.
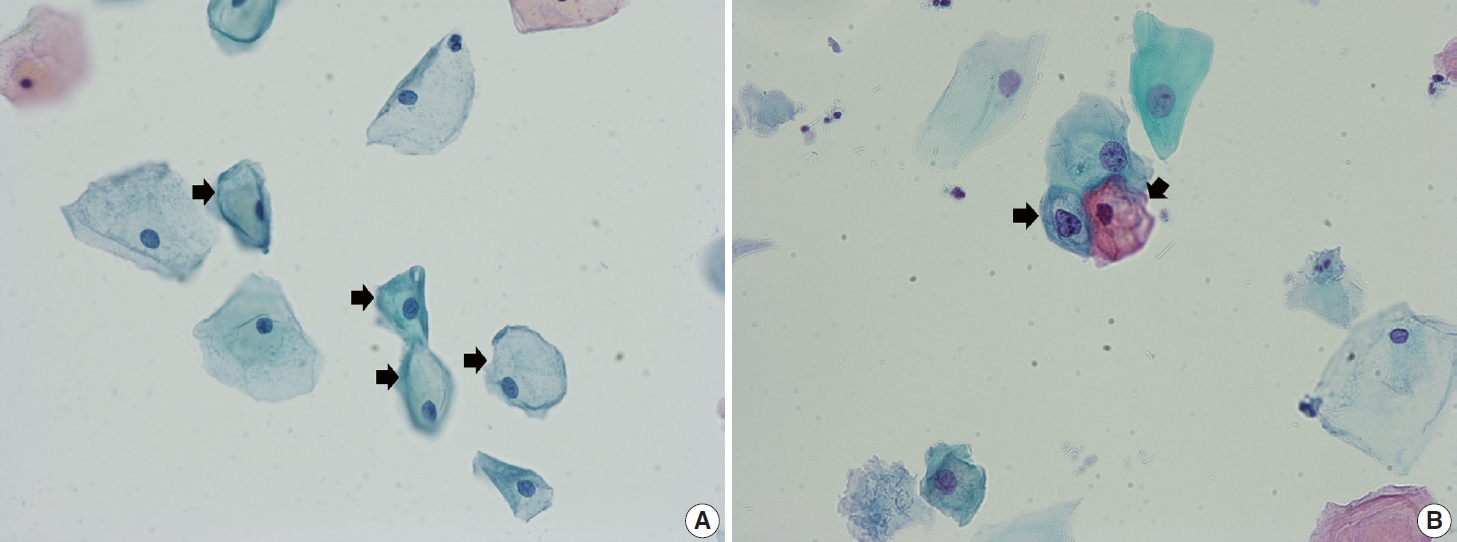
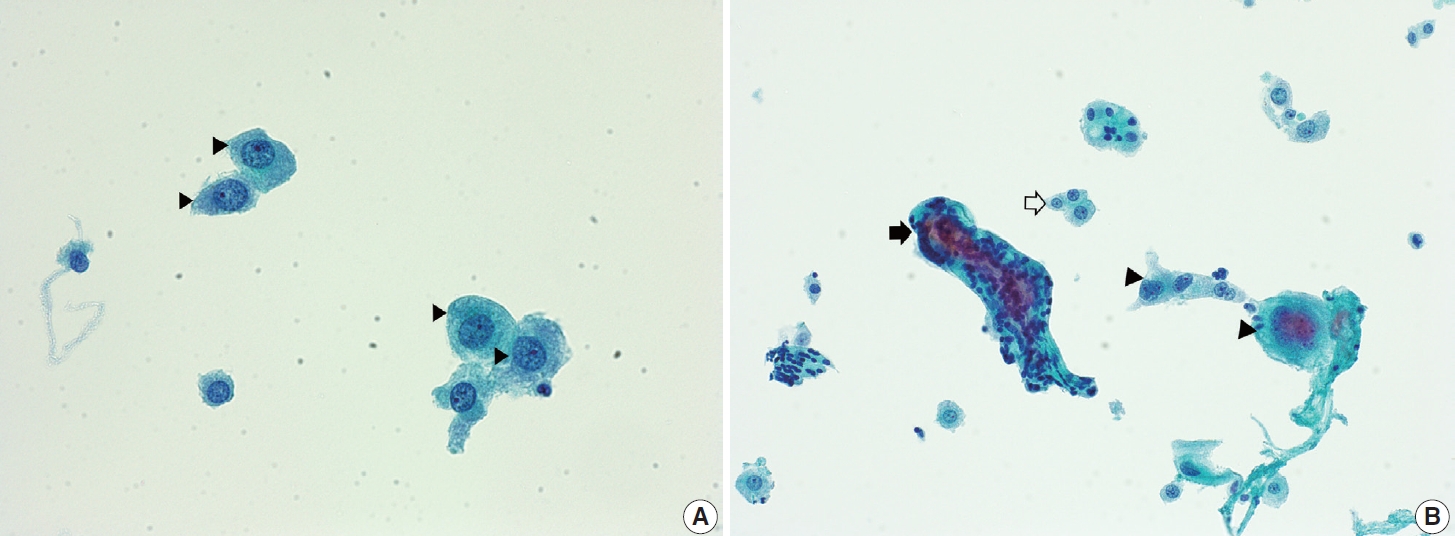
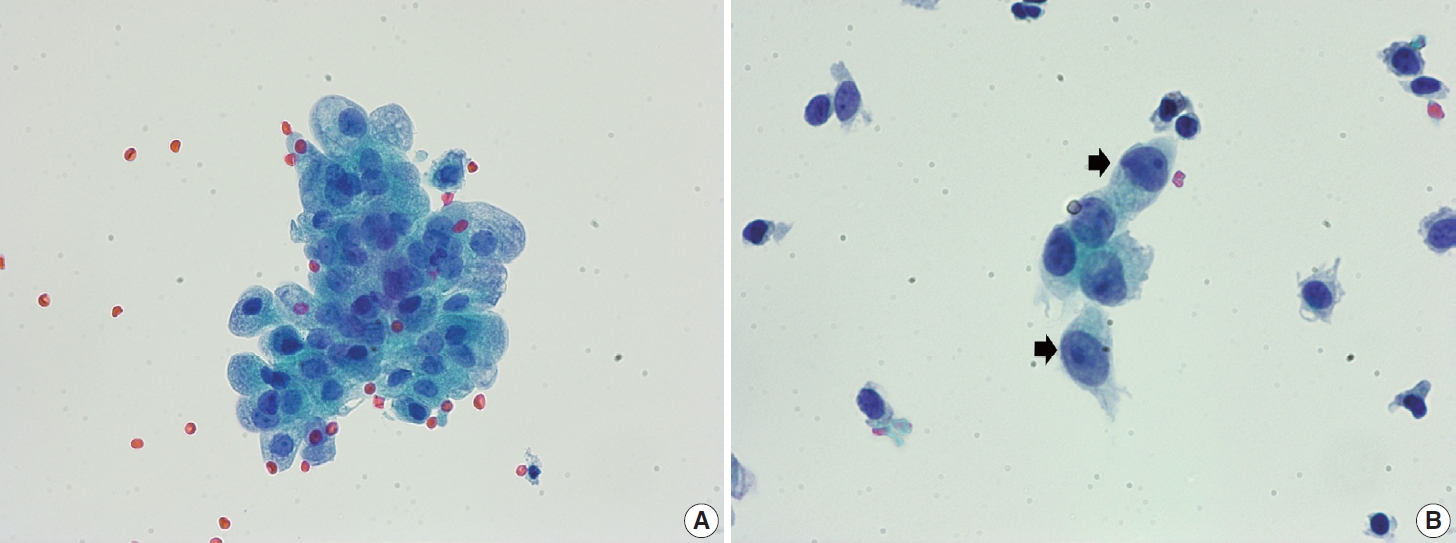
- Navicular cells are intermediate squamous epithelial cells with glycogen-rich cytoplasm and a boat-like (navicular) shape (Fig. 1A) [15]. These cells may be present in the normal secretory phase, but the number increases as gestation proceeds. These cells sometimes can be misinterpreted as koilocytes. The differential aspect is the presence of pale yellowish cytoplasm with a vague outline instead of the clear halo around the nuclei with defined border corresponding to the prefix “koilo” (meaning empty) in koilocytes. The nuclei of navicular cells are small and typical, while those of koilocytes are enlarged, raisinoid, hyperchromatic, and sometimes multinucleated (Fig. 1B).
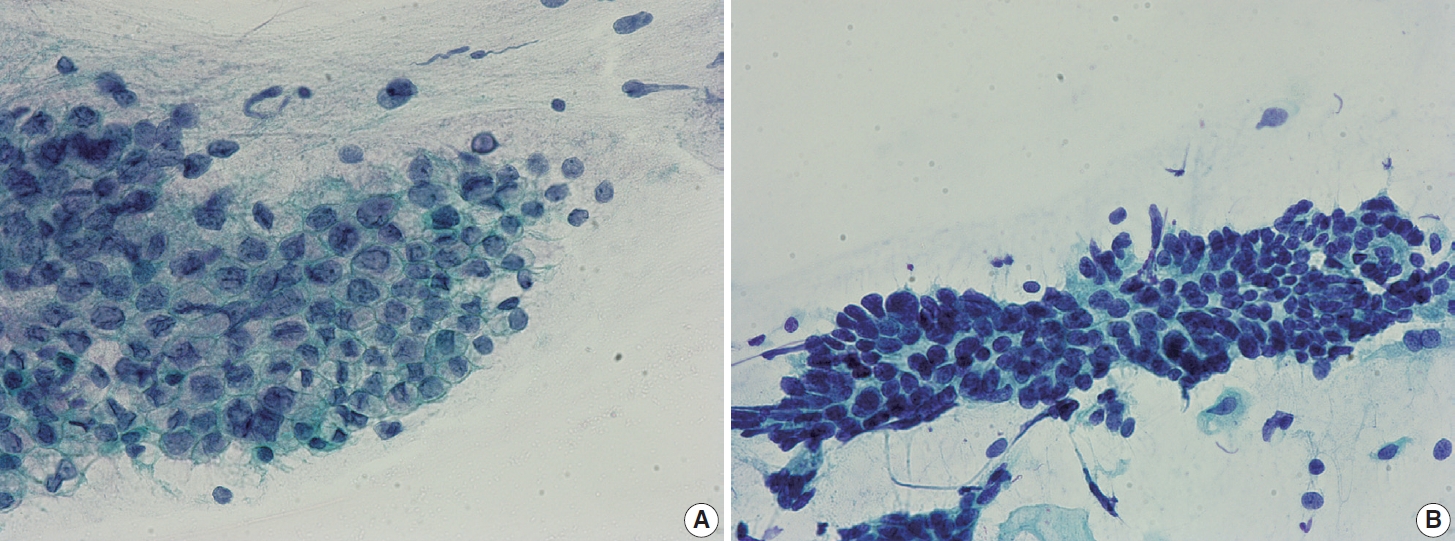
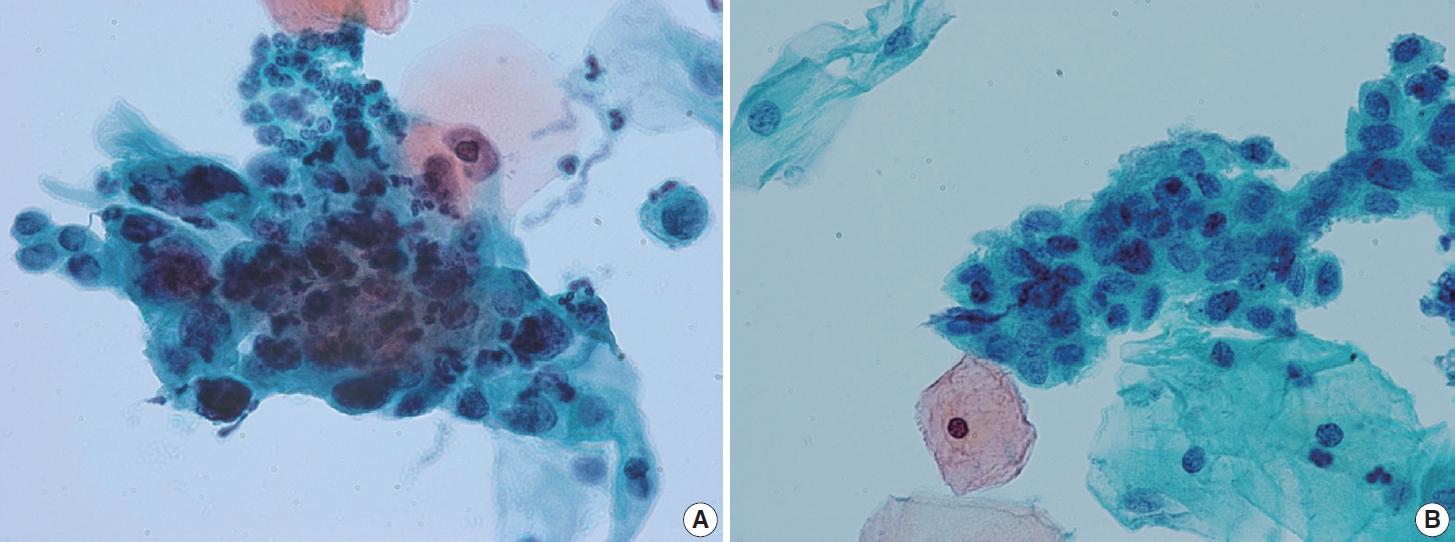
- Endocervical glandular hyperplasia often results in an abundance of glandular epithelium on sampling, with increased mucin. These glandular cells are sometimes confused with adenocarcinoma in situ (AIS) and may be diagnosed as atypical glandular cells (AGCs). The differential diagnosis is based on the regular honeycomb appearance of the glandular cluster with low nuclear-cytoplasmic (N/C) ratio. The cytoplasm of these glandular clusters is always full of clear mucin (Fig. 2A). Conversely, AIS cells usually have less mucinous cytoplasm and stratified nuclei and are pencil-like and hyperchromatic. The cells often show typical feathering at the outer edges of the clusters (Fig. 2B).
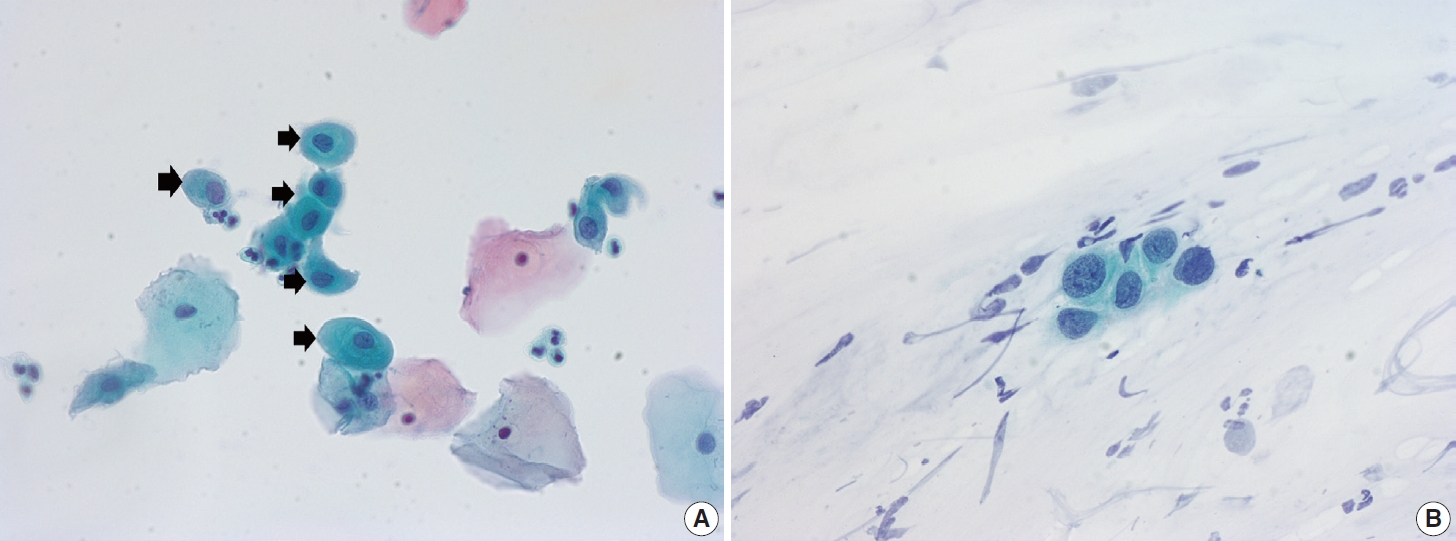
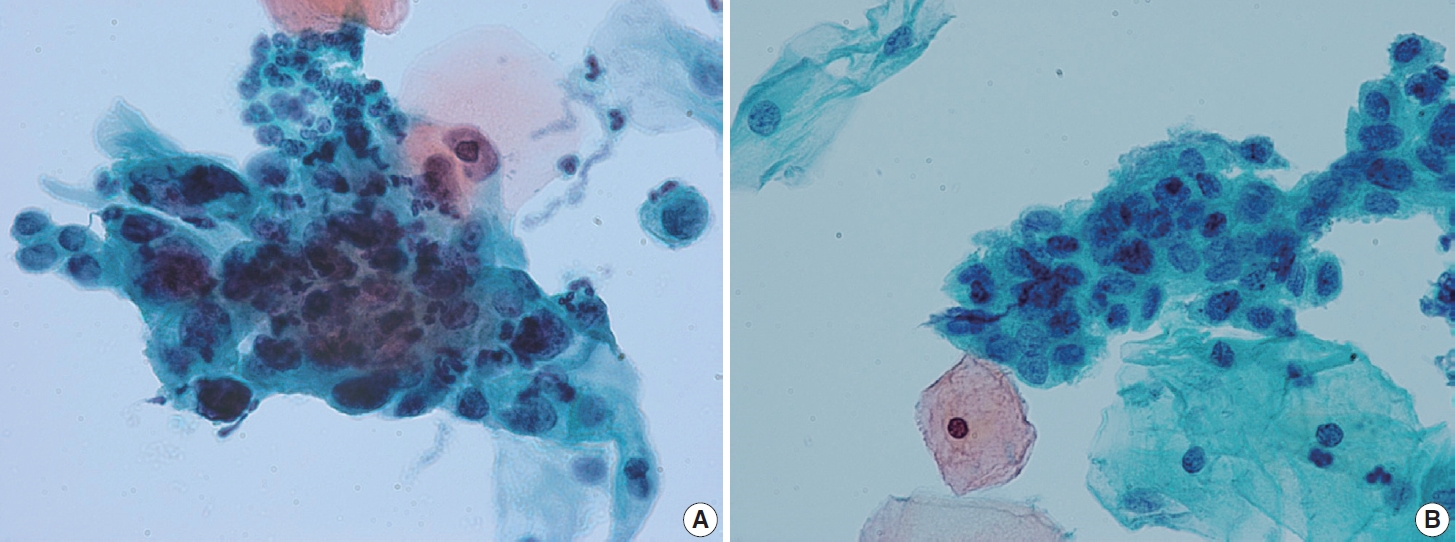
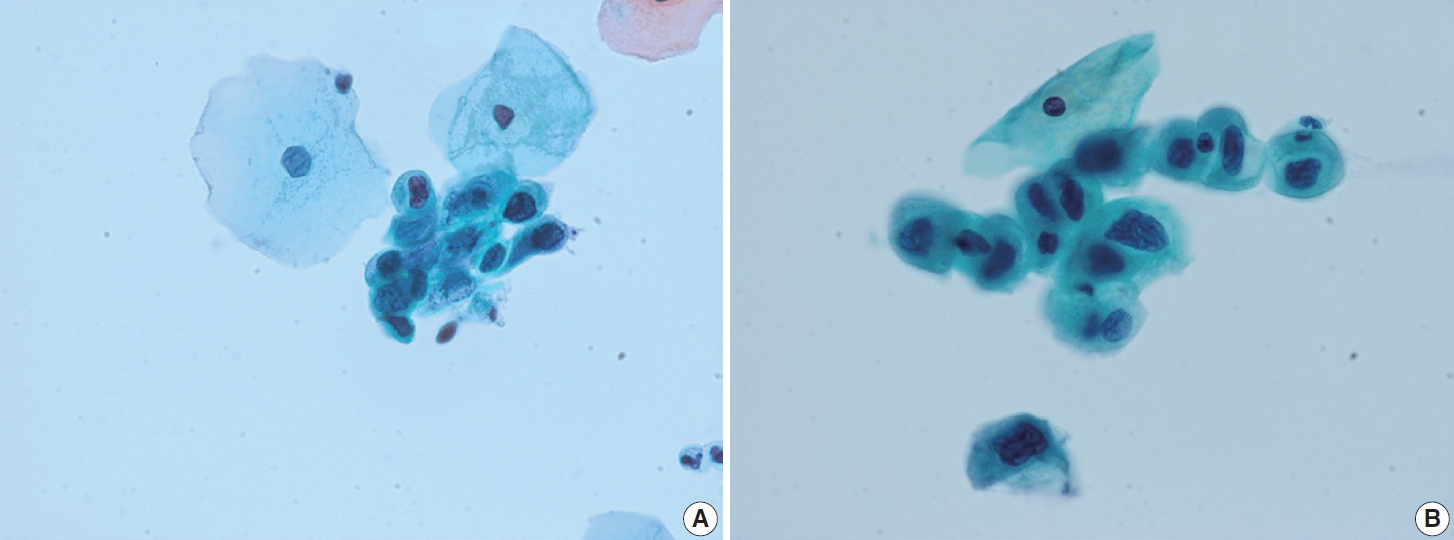
- Ectropion, eversion of the uterine cervix, can result in exposure of the transformation zone, which in combination with the decreased maturation, results in numerous immature metaplastic cells in the cytologic specimen. These cells can be confused with ASC-H (atypical squamous cells - cannot exclude high grade squamous intraepithelial lesion) or high-grade squamous intraepithelial lesion (HSIL), causing difficulty in diagnosis (Fig. 3A). Differentiation between ASC-H and HSIL is mainly based on scrutinization of the nuclear features. ASC-H and HSIL have enlarged hyperchromatic nuclei with coarse chromatin. The nuclear membrane is often irregular and thickened in ASC-H and HSIL (Fig. 3B).
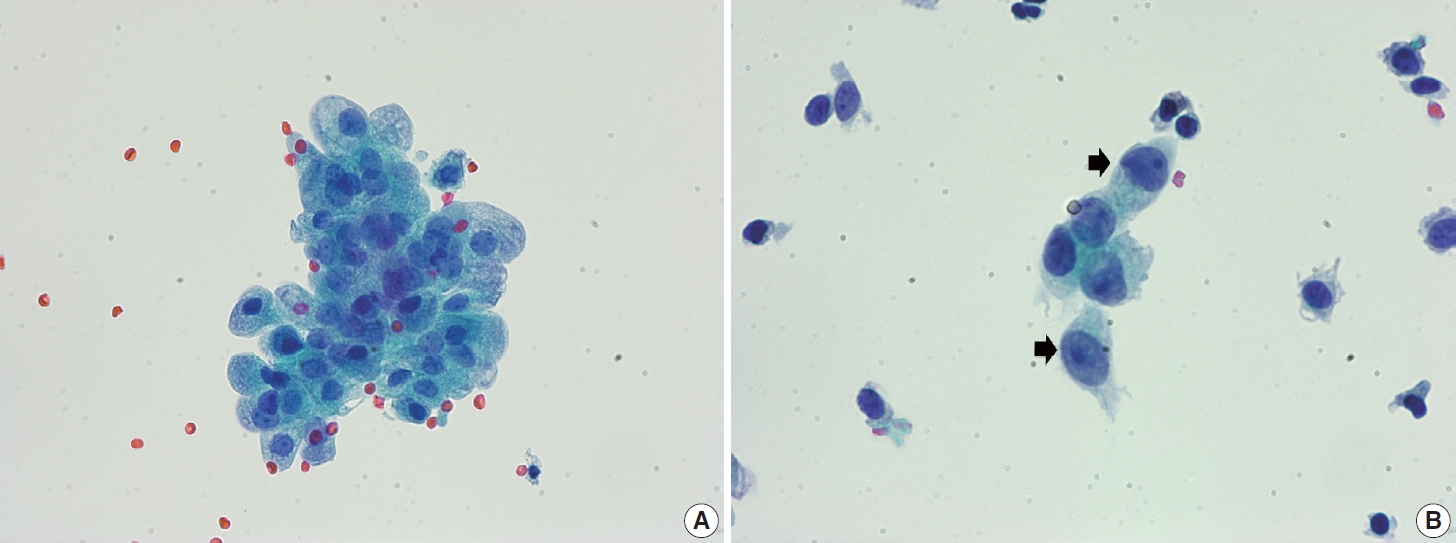
- The Arias-Stella reaction is a specific cellular change in gestation, usually observed in endometrial and sometimes endocervical glandular cells. Affected cells show markedly pleomorphic, atypical, hyperchromatic nuclei with occasional prominent nucleoli, sometimes having a hobnail appearance. The cells have abundant clear or vacuolated cytoplasm. On cytologic samples, detached glandular cells with Arias-Stella reaction can easily be misdiagnosed as AGC or adenocarcinoma, especially if the pathologist is not informed of the pregnancy status. Careful attention to the cytological details including low N/C ratio, ample lacy cytoplasm, smooth or indistinct nuclear outline, and dull opaque rather than coarse chromatin pattern aids in the differentiation of this specific benign condition from malignancy (Fig. 4).
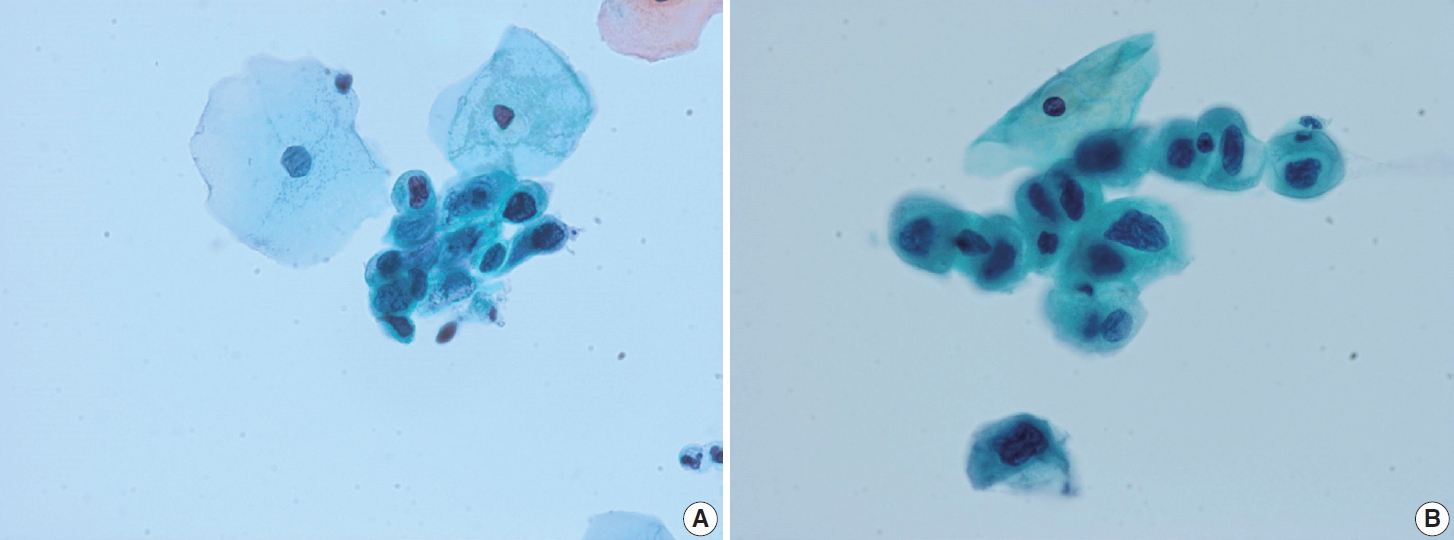
- Degenerated decidual cells or trophoblasts may be released and sampled in cytologic specimens, although infrequently. These cells may be considered as abnormal squamous cells due to their glossy ample cytoplasm and slightly large hyperchromatic nuclei and misdiagnosed as ASC or ASC-H [16-18]. Decidual cells usually do not demonstrate the high N/C ratio and coarse chromatin pattern of HSIL, although they sometimes show prominent nucleoli (Fig. 5).
CYTOLOGIC CHANGES DURING PREGNANCY AND ISSUES IN CYTOLOGICAL INTERPRETATION
- Although the natural course of CIN in pregnant women does not differ significantly from non-pregnant women, CIN during pregnancy has a few notable features. Progression to invasion is extremely rare in CIN during pregnancy. Most cases remain stable and many even regress. The overall regression rate for CIN during pregnancy is estimated to be as high as 76% for low-grade squamous intraepithelial lesions (LSILs) and up to 59% for HSILs [19-23]. Although some heterogeneous results have been reported, the regression rate of CIN during pregnancy is generally accepted to be much higher than for non-pregnant women [21].
- In general, irrespective of pregnancy status, more than 80% of HPV infections resolve spontaneously, with approximately 10%–20% of HPV-infected women developing CIN [24]. In LSIL/CIN 1, approximately 57% of cases regress, 32% persist, and approximately 12% progress to high-grade lesions [25]. For high-grade lesions, such as CIN 3, the regression rates decrease significantly, with almost half persisting and more than 12% of the cases progressing to invasive carcinoma [25].
- For LSIL/CIN 1 occurring in pregnancy, regression rates are higher, in the range of 63%–76%, and progression rates are low (6%–8%). The overall regression rate for HSIL (CIN 2/CIN 3) during pregnancy ranges from 29%–59% [19-23]. In a previous meta-analysis, the pooled regression rate for HSIL during pregnancy was 40% (95% confidence interval [CI], 35% to 45%) [21]. CIN 2 had a significantly higher regression rate compared with CIN 3 (59%–88% vs. 21%–29%) [19-23]. Most regression tends to occur within the first 2 years postpartum. In a previous study, approximately 68%–70% of regressions (for both CIN 2 and CIN 3) were observed within the first 2 years after diagnosis [22]. The progression rate to invasive cancer is very low, typically around 1% (95% CI, 0% to 2%) [21], which becomes the basis for a more conservative approach in management of CIN during pregnancy.
NATURAL HISTORY OF CERVICAL INTRAEPITHELIAL NEOPLASIA AND RATE OF REGRESSION DURING PREGNANCY
- The mechanism of frequent CIN regression during pregnancy is not yet completely understood. Some proposed hypotheses include pregnancy-induced immunologic alteration, inflammatory process, and cervical repair related to delivery. Cervical trauma during vaginal delivery and subsequent repair were suggested to contribute to regression and was supported in a few studies in which regression was more frequent in vaginal delivery [19,26,27]. However, other studies have shown that regression rates do not differ significantly between vaginal deliveries and cesarean sections, challenging this hypothesis [28-30]. In a recent meta-analysis, delivery mode did not affect regression [31]. The hypothesis of cervical repair and the impact of delivery mode on CIN regression should be further investigated.
- The observation that CIN 2 regresses more frequently than CIN 3 is consistent across multiple studies [21] and reasonable considering that CIN 2 more commonly regresses than CIN 3 in non-pregnant cases.
- High-risk HPV infection, particularly HPV 16/18, and HPV E6/E7 mRNA expression are reported to correlate with disease persistence and/or progression [30,32,33].
- There are no other prominent cytological or histological features known to predict regression. Stromal inflammation was reportedly associated with regression and can be associated with an immunologic reaction that may have a role in regression [34]. However, measuring stromal inflammation accurately is difficult and can be interpreted as non-specific. Morphological differences do not exist between regressed and persisted/progressed cases (Figs. 6, 7) [34].
POSSIBLE MECHANISMS OF REGRESSION AND PREDICTING FACTORS
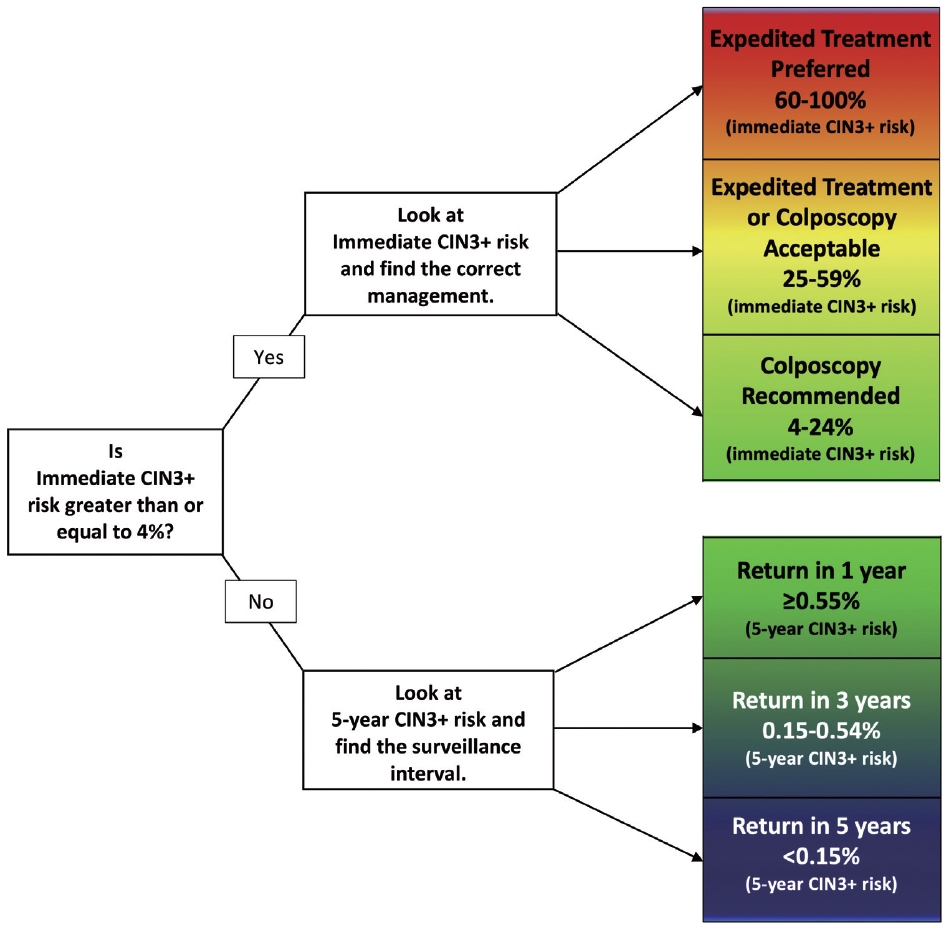
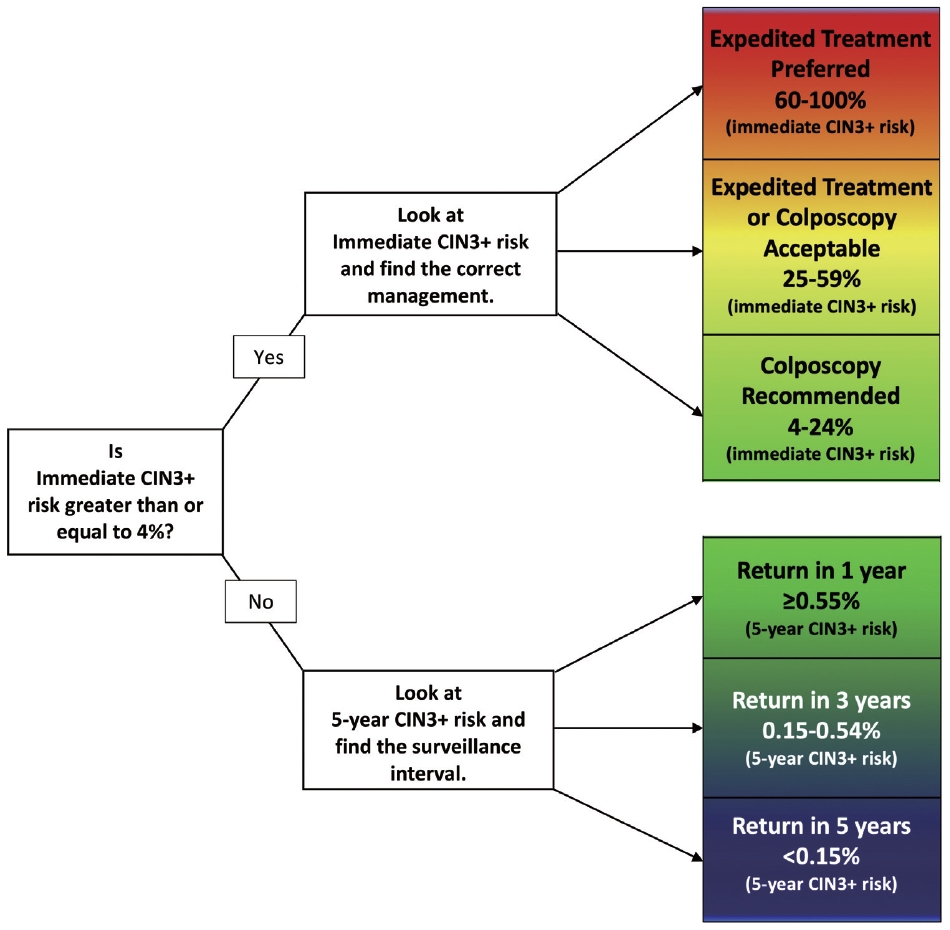
- Pregnancy does not alter the management approach of abnormal screening test results. According to the 2019 American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines, the management algorithm is divided based on immediate risk of CIN 3+ (CIN 3 or worse) > 4% (Fig. 8) [35,36]. This risk is calculated using a complex tabulation including patient age, interval since last screening, and current and prior test results. HSIL, ASC-H, and AGC on cytology as well as HPV-16 and HPV-18 on HPV test always require colposcopy and/or biopsy [37]. Although the risk threshold of 4% for colposcopy referral are not altered during pregnancy, endocervical curettage, endometrial biopsy, and expedited treatment are not acceptable [35]. In pregnant women, all diagnostic approaches should be directed to exclude invasive cervical carcinoma [38]. During pregnancy, patients with histologically confirmed CIN 2 or CIN 3 are recommended to be under active surveillance with repeat colposcopy every 12 to 24 weeks. However, it is acceptable to defer colposcopy until postpartum [35]. Treatment of histologic HSIL is not recommended. Diagnostic excisional procedure or repeat biopsy should be postponed until after delivery unless invasive carcinoma is suspected [35].
MANAGEMENT OF ABNORMAL CYTOLOGY DURING PREGNANCY
- Cervical cytology has become an integral part of routine antenatal care and remains a crucial step in screening for cervical cancer in women. Pathologists should consider patient age and pregnancy status when examining cytology samples. Understanding the cytologic features of pregnancy-related changes and potential issues is important to avoid misdiagnosis. Generally, caution is advises to not overdiagnose cytology samples from pregnant women because HSIL frequently regresses and may not be detected during postpartum follow-up. However, unequivocal HSIL should be classified as HSIL regardless of the patient’s pregnancy status.
CONCLUSION
Ethics Statement
Not applicable.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: JYK, JYS. Data curation: JYK. Investigation: JYK, JYS. Supervision: JYS. Writing—original draft: JYK. Writing—review & editing: JYS. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.





- 1. Nguyen C, Montz FJ, Bristow RE. Management of stage I cervical cancer in pregnancy. Obstet Gynecol Surv 2000; 55: 633-43. ArticlePubMed
- 2. Norstrom A, Jansson I, Andersson H. Carcinoma of the uterine cervix in pregnancy: a study of the incidence and treatment in the western region of Sweden 1973 to 1992. Acta Obstet Gynecol Scand 1997; 76: 583-9. ArticlePubMed
- 3. Pentheroudakis G, Pavlidis N. Cancer and pregnancy: poena magna, not anymore. Eur J Cancer 2006; 42: 126-40. ArticlePubMed
- 4. Al-Halal H, Kezouh A, Abenhaim HA. Incidence and obstetrical outcomes of cervical intraepithelial neoplasia and cervical cancer in pregnancy: a population-based study on 8.8 million births. Arch Gynecol Obstet 2013; 287: 245-50. ArticlePubMedPDF
- 5. Kumari S. Screening for cervical cancer in pregnancy. Oncol Rev 2023; 17: 11429.ArticlePubMedPMC
- 6. Eibye S, Kjaer SK, Mellemkjaer L. Incidence of pregnancy-associated cancer in Denmark, 1977-2006. Obstet Gynecol 2013; 122: 608-17. ArticlePubMed
- 7. Eibye S, Kruger Kjaer S, Nielsen TS, Mellemkjaer L. Mortality among women with cervical cancer during or shortly after a pregnancy in Denmark 1968 to 2006. Int J Gynecol Cancer 2016; 26: 951-8. ArticlePubMed
- 8. Yang KY. Abnormal pap smear and cervical cancer in pregnancy. Clin Obstet Gynecol 2012; 55: 838-48. ArticlePubMed
- 9. Suzuki S, Hayata E, Hoshi SI, et al. Current status of cervical cytology during pregnancy in Japan. PLoS One 2021; 16: e0245282. ArticlePubMedPMC
- 10. Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol 2015; 125: 330-7. PubMed
- 11. Watson M, Benard V, King J, Crawford A, Saraiya M. National assessment of HPV and Pap tests: changes in cervical cancer screening, National Health Interview Survey. Prev Med 2017; 100: 243-7. ArticlePubMedPMC
- 12. Xhaja A, Ahr A, Zeiser I, Ikenberg H. Two years of cytology and HPV co-testing in Germany: initial experience. Geburtshilfe Frauenheilkd 2022; 82: 1378-86. ArticlePubMedPMC
- 13. Gu L, Hu Y, Wei Y, et al. Optimising cervical cancer screening during pregnancy: a study of liquid-based cytology and HPV DNA cotest. Epidemiol Infect 2024; 152: e25. ArticlePubMedPMC
- 14. Zagorianakou N, Mitrogiannis I, Konis K, Makrydimas S, Mitrogiannis L, Makrydimas G. The HPV-DNA test in pregnancy: a review of the literature. Cureus 2023; 15: e38619. ArticlePubMedPMC
- 15. Kamal M, Topiwala F. Nonneoplastic cervical cytology. Cytojournal 2022; 19: 25.ArticlePubMedPMC
- 16. Cibas ES, Ducatman BS. Cytology: diagnostic principles and clinical correlates. Philadelphia: Elsevier, 2021; 5th.
- 17. Origoni M, Salvatore S, Perino A, Cucinella G, Candiani M. Cervical Intraepithelial Neoplasia (CIN) in pregnancy: the state of the art. Eur Rev Med Pharmacol Sci 2014; 18: 851-60. PubMed
- 18. Grimm D, Lang I, Prieske K, et al. Course of cervical intraepithelial neoplasia diagnosed during pregnancy. Arch Gynecol Obstet 2020; 301: 1503-12. ArticlePubMedPDF
- 19. Stuebs FA, Mergel F, Koch MC, et al. Cervical intraepithelial neoplasia grade 3: development during pregnancy and postpartum. Arch Gynecol Obstet 2023; 307: 1567-72. ArticlePubMedPDF
- 20. Dasgupta S. The fate of cervical dysplastic lesions during pregnancy and the impact of the delivery mode: a review. Cureus 2023; 15: e42100. ArticlePubMedPMC
- 21. Chen C, Xu Y, Huang W, Du Y, Hu C. Natural history of histologically confirmed high-grade cervical intraepithelial neoplasia during pregnancy: meta-analysis. BMJ Open 2021; 11: e048055. ArticlePubMedPMC
- 22. Ehret A, Bark VN, Mondal A, Fehm TN, Hampl M. Regression rate of high-grade cervical intraepithelial lesions in women younger than 25 years. Arch Gynecol Obstet 2023; 307: 981-90. ArticlePubMedPDF
- 23. Han B, Yuan M, Gong Y, et al. The clinical course of untreated CIN2 (HPV16/18+) under active monitoring: a protocol of systematic reviews and meta-analysis. Medicine (Baltimore) 2023; 102: e32855. ArticlePubMedPMC
- 24. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338: 423-8. ArticlePubMed
- 25. Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol 1993; 12: 186-92. PubMed
- 26. Ahdoot D, Van Nostrand KM, Nguyen NJ, et al. The effect of route of delivery on regression of abnormal cervical cytologic findings in the postpartum period. Am J Obstet Gynecol 1998; 178: 1116-20. ArticlePubMed
- 27. Chung SM, Son GH, Nam EJ, et al. Mode of delivery influences the regression of abnormal cervical cytology. Gynecol Obstet Invest 2011; 72: 234-8. ArticlePubMedPDF
- 28. Coppola A, Sorosky J, Casper R, Anderson B, Buller RE. The clinical course of cervical carcinoma in situ diagnosed during pregnancy. Gynecol Oncol 1997; 67: 162-5. ArticlePubMed
- 29. Bracic T, Reich O, Taumberger N, Tamussino K, Trutnovsky G. Does mode of delivery impact the course of cervical dysplasia in pregnancy? A review of 219 cases. Eur J Obstet Gynecol Reprod Biol 2022; 274: 13-8. ArticlePubMed
- 30. Cubo-Abert M, Centeno-Mediavilla C, Franco-Zabala P, et al. Risk factors for progression or persistence of squamous intraepithelial lesions diagnosed during pregnancy. J Low Genit Tract Dis 2012; 16: 34-8. ArticlePubMed
- 31. Douligeris A, Pergialiotis V, Pappa K, et al. The effect of the delivery mode on the evolution of cervical intraepithelial lesions during pregnancy: a meta-analysis. J Gynecol Obstet Hum Reprod 2022; 51: 102462.ArticlePubMed
- 32. Frega A, Verrone A, Manzara F, et al. Expression of E6/E7 HPV-DNA, HPV-mRNA and colposcopic features in management of CIN2/3 during pregnancy. Eur Rev Med Pharmacol Sci 2016; 20: 4236-42. PubMed
- 33. Hong DK, Kim SA, Lim KT, Lee KH, Kim TJ, So KA. Clinical outcome of high-grade cervical intraepithelial neoplasia during pregnancy: a 10-year experience. Eur J Obstet Gynecol Reprod Biol 2019; 236: 173-6. ArticlePubMed
- 34. Pongsuvareeyakul T, Eaton S, Quddus MR, Sung CJ, Singh K. Comparison of cervical HSIL outcome between pregnant and non-pregnant women. Ann Clin Lab Sci 2022; 52: 544-55. PubMed
- 35. Perkins RB, Guido RS, Castle PE, et al. 2019 ASCCP Risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2020; 24: 102-31. ArticlePubMedPMC
- 36. Nayar R, Chhieng DC, Crothers B, et al. Moving forward-the 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors and beyond: implications and suggestions for laboratories. J Am Soc Cytopathol 2020; 9: 291-303. ArticlePubMed
- 37. Larish A, Long ME. Diagnosis and management of cervical squamous intraepithelial lesions in pregnancy and postpartum. Obstet Gynecol 2024; 144: 328-38. ArticlePubMed
- 38. Perrone AM, Bovicelli A, D’Andrilli G, Borghese G, Giordano A, De Iaco P. Cervical cancer in pregnancy: analysis of the literature and innovative approaches. J Cell Physiol 2019; 234: 14975-90. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- The significance of biological samples from pregnant women in cervical intraepithelial neoplasia
Xue Mi, Maharjan Rashmi, Zangyu Pan, Di Wu, Jinwei Miao
Frontiers in Medicine.2025;[Epub] CrossRef - Oncologic and pregnancy outcomes of cervical high-grade intraepithelial lesions and delivery mode
Olga P. Matylevich, Ilya A. Tarasau, Sviatlana Y. Shelkovich, Aliaksandr F. Martsinkevich
Academia Oncology.2025;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure








 E-submission
E-submission