Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(2); 2025 > Article
-
Original Article
Low Ki-67 labeling index is a clinically useful predictive factor for recurrence-free survival in patients with papillary thyroid carcinoma -
Takashi Masui1
 , Katsunari Yane1
, Katsunari Yane1 , Ichiro Ota1
, Ichiro Ota1 , Kennichi Kakudo2,3
, Kennichi Kakudo2,3 , Tomoko Wakasa2
, Tomoko Wakasa2 , Satoru Koike1
, Satoru Koike1 , Hirotaka Kinugawa1
, Hirotaka Kinugawa1 , Ryuji Yasumatsu4
, Ryuji Yasumatsu4 , Tadashi Kitahara5
, Tadashi Kitahara5
-
Journal of Pathology and Translational Medicine 2025;59(2):115-124.
DOI: https://doi.org/10.4132/jptm.2024.11.08
Published online: February 18, 2025
1Department of Otolaryngology-Head and Neck Surgery, Kindai University Nara Hospital, Ikoma, Japan
2Department of Diagnostic Pathology, Kindai University Nara Hospital, Ikoma, Japan
3Department of Pathology and Thyroid Disease Center, Izumi City General Hospital, Izumi, Japan
4Department of Otolaryngology-Head and Neck Surgery, Kindai University, Osakasayama, Japan
5Department of Otolaryngology-Head and Neck Surgery, Nara Medical University, Kashihara, Japan
- Corresponding Author: Takashi Masui, MD, PhD Department of Otolaryngology-Head and Neck Surgery, Kindai University Nara Hospital, 1248-1 Otoda-cho, Ikoma, Nara 630-0293, Japan Tel: +81-743-77-0880, FAX: +81-743-77-0902, E-mail: 1813a5@med.kindai.ac.jp
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- We report a new risk stratification of invasive stage papillary thyroid carcinomas (PTCs) by combining invasive status, using extrathyroid invasion (Ex) status, and tumor growth speed using the Ki-67 labeling index (LI).
-
Methods
- We examined tumor recurrence in 167 patients with PTC who were surgically treated at the Kindai University Nara Hospital between 2010 and 2022. The patients were classified according to the degree of invasion [negative (Ex0) or positive (Ex1, Ex2, and Ex3)] and tumor growth speed expressed with Ki-67 LI, as low (<5%) or high (≥5%). This study confirmed previous findings that the disease-free survival (DFS) rate in PTCs significantly differed between patients with a high and low Ki-67 index.
-
Results
- When combining Ex status (negative or positive) and Ki-67 proliferation status (low or high), the DFS rate of invasion in the negative, low Ki-67 LI group was only 1.1%, while that of invasion in the positive, high Ki-67 LI was 44.1%. This study reports for the first time that recurrence risks can be stratified accurately when combining carcinoma’s essential two features of extrathyroid invasion status and tumor growth speed.
-
Conclusions
- We believe the evidence for low tumor recurrence risk may contribute to use of more conservative treatment options for invasive-stage PTCs and help alleviate patient anxiety about tumor recurrence and death.
- Papillary thyroid carcinoma (PTC) usually has an indolent nature with a very slow rate of tumor growth, even though it often (7%–23%) involves cervical lymph node metastases [1-4]. Due to this evidence, a choice of surgery or clinical follow-up of patients with early invasive PTC can be challenging for endocrinologists and endocrine surgeons. Clinical factors such as age, sex, and tumor size and histopathologic parameters such as extrathyroidal invasion, lympho-vascular invasion, and lateral cervical lymph node metastasis are predictive factors for PTC recurrence [5-7]. However, those clinical parameters do not distinguish PTC, and a number of cases with these characteristics does not develop recurrence. Simultaneously, a number of patients without these characteristics can present with locoregional recurrence. Thus, more accurate risk stratification of PTC is needed for proper postoperative follow-up strategy to reduce unnecessary surgery. The 2015 American Thyroid Association (ATA) published management guidelines regarding estimated risk of structural disease recurrence in patients without structurally identifiable disease after initial therapy into low-risk, intermediate-risk, and high-risk [8]. However, more than 10 parameters were used in this risk stratification, included more than 10 independent features seen in PTCs, including (1) gross extrathyroidal extension (Ex2/3), (2) incomplete tumor resection, (3) distant metastasis or (4) lymph node metastasis more than 3 cm in size for ATA high-risk for disease recurrence, (5) aggressive histological subtypes of PTC, (6) minor extrathyroidal extension (Ex1), (7) vascular invasion, or (8) more than five involved lymph nodes (2–30 mm in size) for ATA intermediate-risk for recurrence, and (9) intrathyroidal differentiated thyroid carcinomas (Ex0) or (10) less than five involved lymph node micrometastasis (<2 mm) for ATA low-risk for recurrence, in addition to encapsulation, multifocality, TERT promoter mutation, and BRAFV600E mutation. Thus, we believe that outcome prediction of well-differentiated thyroid carcinomas (PTC, follicular subtype PTC, oncocytic thyroid carcinoma and follicular thyroid carcinoma) using only one of these clinicopathological parameters is not reliable. Although all 14 variables are useful prognostic and predictive factors, this ATA risk stratification for structural disease recurrence is complicated and not perfect in real-world practice. Although a small fraction of ATA low-risk and intermediate-risk patients develop tumor recurrence, most patients and clinicians choose treatment with total thyroidectomy (TTX) just in case. This results in significant overtreatment of low-risk small PTCs. More than 80% of small (less than 20 mm) PTCs in the United States in 2014 were treated with TTX [9], and more than 40% of PTCs eligible for lobectomy in two North American institutes in 2019 were treated with TTX [10]. These findings underscore the need for carefully consideration in medical treatment policies for this subgroup.
- The Ki-67 labeling index (LI) is a well-established method in pathology practice in a variety of malignancies including medullary thyroid carcinoma [11-13] but rarely is used for clinical prognostic risk stratification of PTC. We report a new method to predict risk of tumor recurrence in invasive PTC by combining invasion status, positive or negative extrathyroid extension, and tumor growth rate (high or low using the Ki-67 LI).
- Staining for Ki-67 is routinely conducted for neuroendocrine tumors, breast carcinomas, malignant lymphomas, brain tumors, and sarcoma [14-16]. However, the Ki-67 LI does not play a diagnostic role in other organs, and its prognostic value remains a controversial issue [17].
- The grade of extrathyroid invasion (Ex0, Ex1, Ex2, and Ex3) is a well-known prognostic and predictive factor in PTCs and constitutes a significant component in TNM stage category [18] and other prognostic categories [19] such as AGES [20] and MACIS [21]. As some studies demonstrated that the Ki-67 LI alone has promising results in predicting recurrence-free survival rates [6,22,23] and cause-specific survival rates of PTCs regardless of TNM stage, the present study examined Ki-67 LI combined with invasion capacity (extrathyroid extension as Ex0, Ex1, Ex2, or Ex3) in 167 cases of curatively treated PTCs in a single tertiary hospital in Japan. This is the first study to successfully demonstrate prognostic and predictive values of Ki-67 LI after risk stratification based on a combination of invasive capacity and Ex category.
INTRODUCTION
- Patients and our protocol
- The demographic characteristics, TN category, and stage of the 167 patients are detailed in Tables 1 and 2 [18]. Between 2010 and 2022, 167 patients with PTC underwent either lobectomy (113 cases, 67.7%) or TTX (54 cases, 32.3%) (Table 2), with or without paratracheal and lateral cervical lymph node dissection, at Kindai University Nara Hospital. Patients that were excluded included those with distant metastasis diagnosed by computed tomography (CT) at initial presentation, severe calcification or bone formation unsuitable for sensitive Ki-67 immunohistochemistry, benign lesions, malignant lymphoma, follicular variant papillary carcinoma, oncocytic carcinoma, follicular carcinoma, medullary carcinoma, poorly differentiated carcinoma, high-grade differentiated carcinoma, anaplastic carcinoma including anaplastic transformation, and incidental carcinoma. Additionally, cases in which patient consent was not obtained for publication were excluded.
- Surgical treatment strategies for patients with PTC at the Kindai University Nara Hospital
- We recommend TTX for patients younger than 70 years with high-risk clinical features (tumors > 40 mm, metastatic lymph nodes > 30 mm, gross Ex2 and 3, or metastatic lymph nodes in the lateral cervical region). In contrast, lobectomy is generally recommended in patients younger than 70 years without high-risk clinical features and in patients older than 70 years of age. Lymph node dissection in the central (paratracheal) compartment is usually performed including the lateral compartment if nodal metastatic disease is clinically suspected. Radioisotope therapy (30 mCi) is mainly administered to high-risk patients < 70 years of age who have undergone TTX and to patients > 70 years of age with 131I uptake in distant metastases.
- Thyroid nodule practice at the Kindai University Nara Hospital
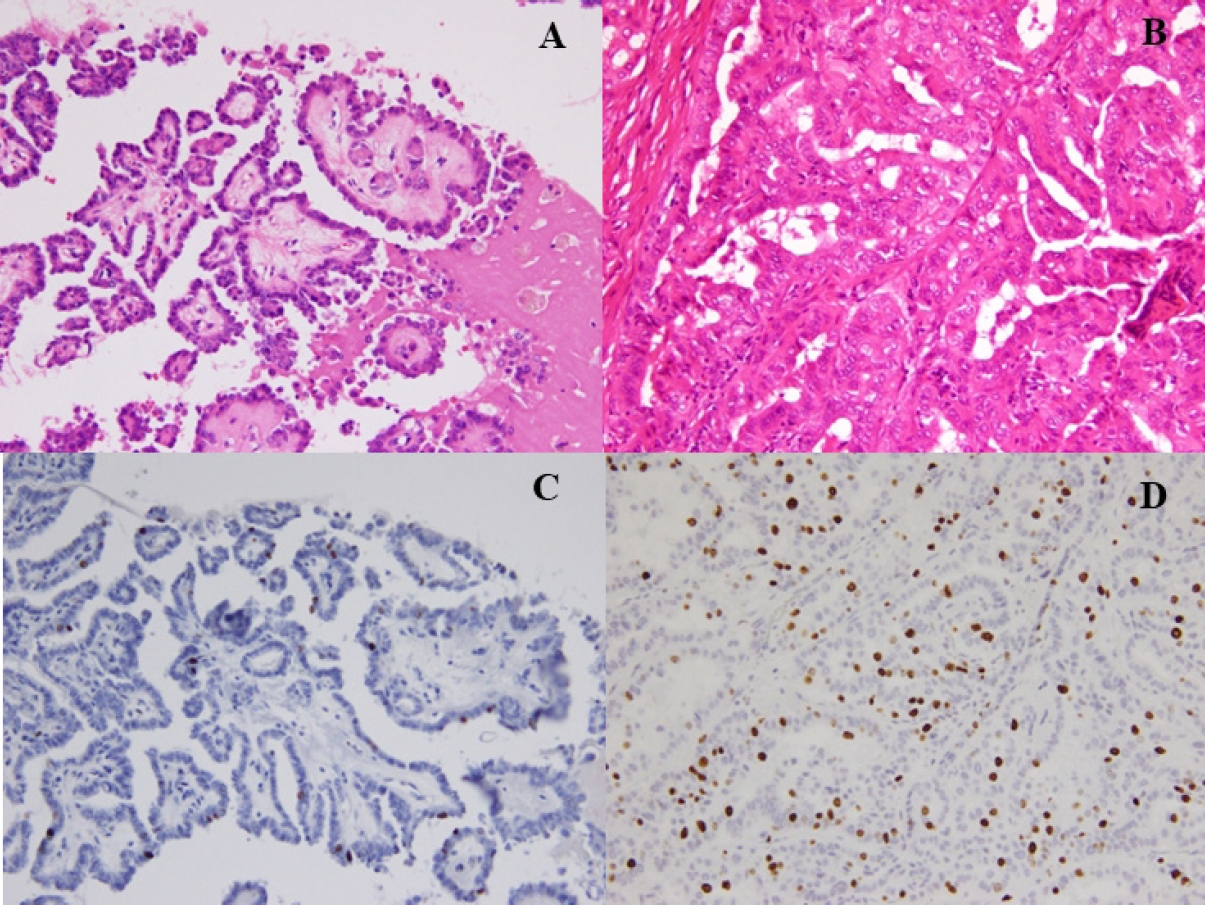
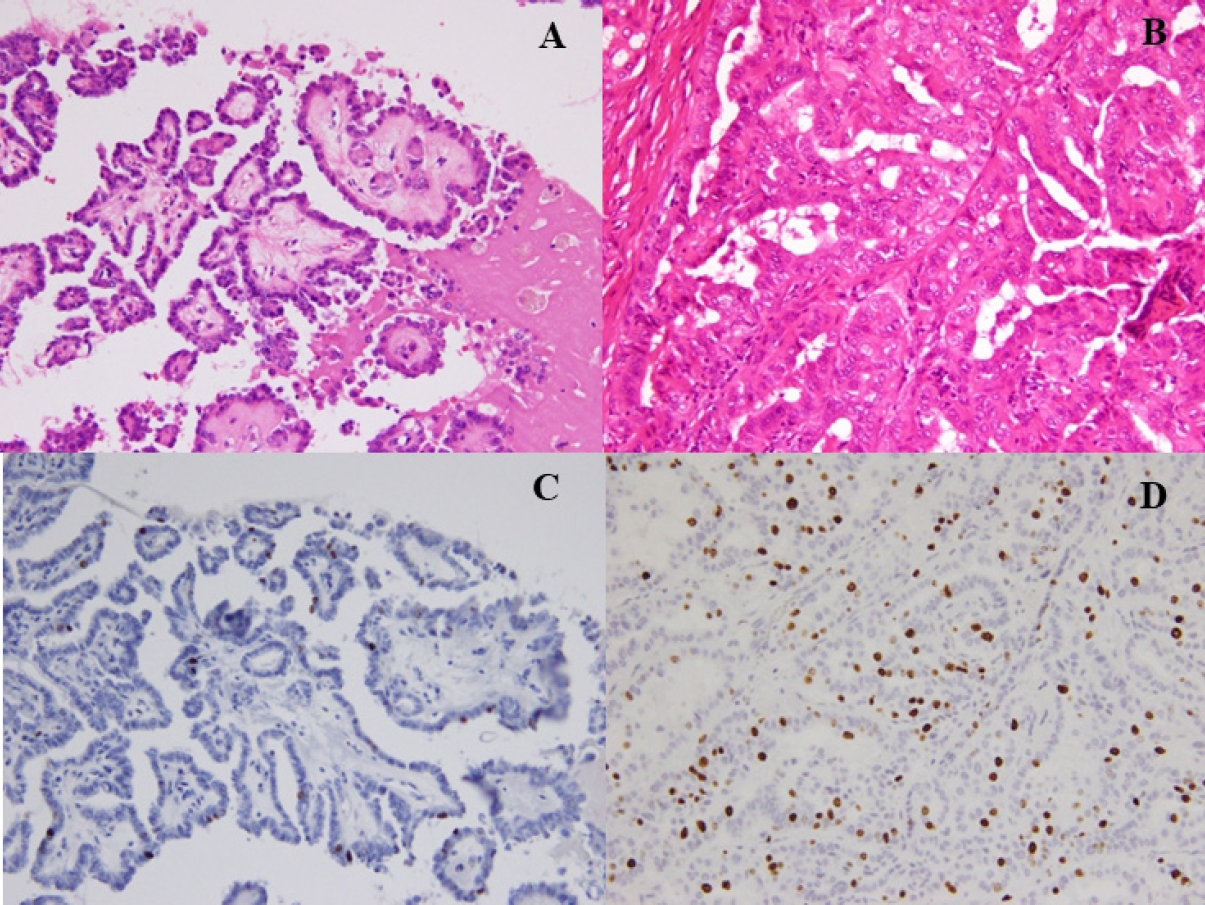
- Fine-needle aspiration cytology was used to diagnose PTC and facilitate the preoperative evaluation. We conducted thyroid function tests, including thyroglobulin (TG), and performed neck ultrasonography to detect and localize tumor recurrence during the postoperative follow-up. CT was performed annually. Recurrence was defined as the appearance of a new postoperative lesion that was not present prior to the initial radical surgery. After histological confirmation of lymph node metastasis at the second surgery, we usually conducted an image review to identify suspicious nodal lesions before the initial surgery. Recurrence was noted after confirmation of no suspicious nodal disease before the initial surgery. When suspicious nodal enlargements were present, we excluded those cases from recurrence and classified them as residual diseases curatively treated with a second surgery. Immunohistochemistry was performed using a Histostainer 36 A (Nichirei Biosciences, Tokyo, Japan) according to the manufacturers’ instructions (SP6 Anti Ki67 rabbit monoclonal antibody). The Ki-67 LI was calculated by two pathologists (K.K. and T.W.) by manually counting the Ki-67-positive tumor cell nuclei and dividing by the total tumor cell nuclei in the hotspot regions, counting at least 1,000 cells twice each. Ki-67 LI was expressed as the range between the lower and higher values in the percentile. Representative PTCs, low-risk (Fig. 1A, C) and high-risk (Fig. 1B, D) cases are shown (Fig. 1).
- First, the 167 patients were divided into Ki-67 LI low-risk (<5%) and moderate (5%–10%)-to-high-proliferation index (10%–30%) groups to examine the differences in recurrence rates. Next, to identify independent prognostic factors for disease-free survival (DFS), univariate analyses were performed for each patient characteristic, and multivariate analyses were performed for factors that differed significantly (Tables 2, 3). The patients were stratified into the following three groups according to the degree of extrathyroid invasiveness (there were no Ex3 cases in this patient series): Ex–group 1, early non-invasive stage (Ex0, anyN, and M0); Ex–group 2, early invasive stage (Ex1, any N, and M0); and Ex–group 3, advanced invasive stage (Ex2 and 3, anyN, and M0). Tested combinations of these two features were examined for tumor recurrence: (1) tumor growth rate as the Ki-67 LI and (2) invasive capacity (Ex–group 1, Ex–group 2, and Ex–group 3) (Table 4).
- Statistical analysis
- All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). The chi-square test and the log-rank test were used for univariate analysis, and the Cox proportional hazards model was used for multivariate analysis. p-values < .05 were considered statistically significant.
MATERIALS AND METHODS
- Multivariate analysis for DFS
- To identify independent prognostic factors for disease-free survival, univariate analyses were performed for each patient characteristic, and multivariate analyses were performed for factors that differed significantly (Tables 2, 3). Univariate analysis showed that male, tumor diameter >20 mm, Ex1/2, and Ki-67 LI >5% were the four possible prognostic factors. Therefore, multivariate analysis of these four factors showed that Ki-67 LI >5% was an independent prognostic factor (Tables 2, 3).
- Ki-67 LI and other clinicopathologic factors of recurrence-free survival rate
- In this study, we classified the cases into three groups based on Ex status (Ex–group 1 for Ex0, any N, and M0; Ex–group 2 for Ex1, any N, and M0; and Ex–group 3 for Ex2 and 3, any N and M0) and into three groups based on Ki-67 LI (Ki-67–group 1 for Ki-LI of 0%–5%, Ki-67–group 2 of 5%–10%, and Ki-67–group 3 of 10%–30%) (Table 4). The recurrence rate in Ex–group 1 was 1/89 (1.1%), and the recurrent patients exhibited a Ki-67 <5%; however, only one patient with Ex–group 1 PTC exhibited Ki-67 ≥5%. In contrast, recurrence occurred in 11 of 62 patients (17.7%) in Ex–group 2 and in five of 16 patients (31.3%) in Ex–group 3 PTCs. A statistically significant difference between Ex–group 1 and the other two groups (p < .001) (Table 4) was observed. When recurrence was examined only in cases with high (≥5%) Ki-67 LI, it was found in zero of 1 (0%) in group 1 (Ki-67 LI ≥5% and Ex0), 10 of 23 (43.5%) in group 2 (Ki-67 LI ≥5% and Ex1), and five of 11 (45.5%) in Group 3 (Ki-67 LI ≥5% and Ex2) (Table 4). As the disease progressed, both the number of cases with Ki-67 LI ≥5% and the recurrence rate increased. However, there were no significant differences (p = .425).
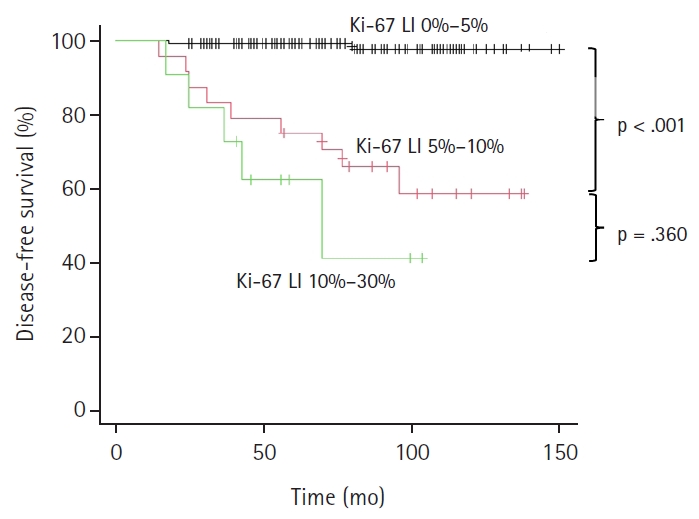
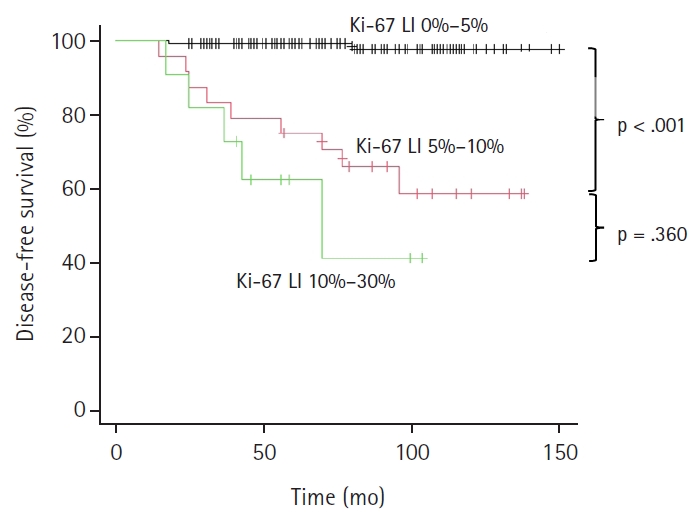
- As illustrated in the Kaplan-Meier method of Fig. 2, patients were classified based on Ki-67 LI status, Ki-67–group 1 (Ki-LI of 0%–5%), Ki-67–group 2 (Ki-67 LI of 5%–10%), and Ki-67–group 3 (Ki-67 LI of 10%–30%), and their relationship with tumor recurrence was investigated. A higher recurrence rate in the Ki-67–group 2 (37.5%, 9/24 cases, p < .001) and Ki-67–group 3 (54.5%, 6/11 cases, p < .001) than that of the Ki-67–group 1 (1.5%, 2/132 cases) is shown in the Fig. 2. A statistically significant difference was observed between the Ki-67–group 1 and group 2, and between Ki-67–group 1 and group 3 (p < .001), but not between Ki-67–group 2 and group 3 (p = .360) (Fig. 2). As there were a small number of cases in this study, we combined Ki-67 LI 5%–10% and 10%–30% groups in one group (Ki-67 LI of ≥5%) and Ex1 and Ex2 groups in a second group (Ex1 and 2) (Table 5), and the recurrence rates were analyzed. We confirmed the recurrence is rare (1.5%) in cases with <5% Ki-67 LI regardless of Ex status. There was only one case with Ex0 and Ki-67 LI of 10%–30%, and that case did not recur.
- There were two cases of tumor death. They were a case of Ex1 and Ki-67 LI 5%–10% and a case of Ex2 and Ki-67 LI 20%–30%. Because of the small number of carcinoma deaths, analysis for cause-specific death was not carried out.
- Further studies in larger patient cohorts are necessary to confirm our observation (negligible risk of recurrence in Ki-67 LI of <5% patients regardless of Ex status and in Ex0 patients regardless of Ki-67 LI).
RESULTS
- Overdiagnosis and overtreatment of carcinomas, including thyroid carcinoma, are significant issues in healthcare communities worldwide [9,22]. Identifying potential lethal malignancies for radical surgical intervention (TTX) and indolent thyroid carcinomas for more conservative surgery (lobectomy) or non-surgical observation is essential to reducing overdiagnosis and overtreatment of thyroid carcinomas. Several strategies have been proposed for thyroid carcinoma treatment to resolve these uncertainties: (1) do not include asymptomatic adults in thyroid carcinoma screening [23], (2) do not apply fine-needle aspiration cytology to low-risk small thyroid nodules [24], (3) downgrade low-risk neoplasms from carcinoma category to non-malignant (borderline) tumor category [25], (4) active surveillance (non-surgical option) to low-risk small PTCs [8,26,27], and (5) do not apply TTX and radioactive iodine to low-risk thyroid carcinomas [24]. However, these five strategies did not improve the overtreatment of thyroid carcinomas sufficiently, and a paradoxical phenomenon that early-stage thyroid carcinoma patients can live longer than the general population has been reported [28]. We speculated that there are a significant number of invasive thyroid carcinomas (Ex1 or Ex2) that do not cause disease-specific death as they are not targets of the above five strategies and are still treated with unnecessary aggressive carcinoma surgery (TTX). This study was conducted to identify low-risk carcinomas for recurrence in invasive PTCs, which are almost synonymous with the so-called “very-slow growing carcinoma” defined by Welch and Black [9] to never progress to clinically significant carcinoma. Among 167 surgically treated PTCs in a single institute under a conservative treatment strategy in Japan were few studies focusing on recurrence, comprising 17 (10.2%) cases who developed tumor recurrence with a median of 64 months of follow-up and two cases of cause-specific death (1.2%).
- Recurrence rates between Ex–group 1 (Ex0, any N and M0) and 2 (Ex1, any N and M0) were statistically different (p < .001), as were those between Ex–group 1 and 3 (Ex2, any N and M0) (p < .001). Recurrence rates in Ki-67–groups 1 (<5%), 2 (5%–10%), and 3 (10%–30%) were 1.5% (2/132), 37.5% (9/24), and 54.5% (6/11), respectively (Table 3). When recurrence rates based on Ex status (Ex–groups 1, 2, and 3) were examined in only cases with Ki-67 LI ≥5% (combined Ki-67 LI ≥5% and Ex status), recurrence rates of group 1 (Ex0 and Ki-67 LI ≥5%), group 2 (Ex1 and Ki-67 LI ≥5%), and group 3 (Ex2/3 and Ki-67 LI ≥5%) were 0% (0/1), 43.5% (10/23), and 45.5% (5/11), respectively (Tables 3, 4). There were no significant differences. When combining Ex status into two groups (Ex0 and Ex1/2/3) and Ki-67 proliferation status into two groups (<5% and ≥5%), the DFS rate of the Ex0 and Ki-67 LI <5% group was only 1.1%, while that of Ex1/2/3 and Ki-67 LI 5%–30% was 44.1% (Table 4). No recurrence was found among patients with Ex0, N0, and Ki-67 LI <5% (data not shown).
- Dwivedi et al. [29] analyzed the expression of Ki-67 and observed a greater expression of this marker in PTCs than in non-neoplastic lesions. Miyauchi et al. [23] found that Ki-67 in PTCs was an independent prognostic factor for disease-free survival. However, most previous studies neglected tumor stage and invasion status (T, N, M, and Ex status). We further incorporated invasive status (Ex0, Ex1, and Ex2/3) into analysis of the Ki-67 proliferation index for tumor recurrence for the first time because both are essential criteria of carcinoma (uncontrolled proliferation and invasion into nearby tissue). This means that a tumor cannot be a biologically malignant progressive lethal carcinoma when either one of the two essential carcinoma criteria is missing or insufficient. This study is the first to successfully show a higher recurrence rate in cases with both high (≥5%) Ki-67 LI and Ex 1 or Ex 2/3 invasive status, while there is a negligible risk of recurrence in cases with either one or without these two features (Ex0 [no invasion into nearby tissue] and Ki-67 LI <5% [low tumor growth rate]).
- Matsuse et al. [30] reported that Ki67 LI and BRAF/TERT gene mutations are risk factors for the recurrence of PTCs. Although BRAF and TERT mutations are prognostically important oncogene mutations in patients with PTC, TERT mutations are rarely present in patients younger than 45 years. Therefore, this method cannot be applied to many young patients with PTC. Our method, using invasive status and Ki-67 LI, can be applied in more patients and does not involve the cost burden related to genetic testing. However, TERT, BRAF, and Ki-67 LI may be used to extract and confirm high-grade PTCs in older patients with advanced-stage tumors.
- A cutoff of 5% for Ki-67 LI was introduced in the 3rd edition World Health Organization (WHO) classification [31] and by several authors, including Kakudo et al. in 2015 [30,32]. Ki-67 LI is a continuous variable that should not be divided into two groups, <5% and ≥5%. Even in cases higher than 5%, a difference between 5%–10% and 10%–30% is expected, as confirmed in this study (Table 4). These PTCs with a high Ki-67 LI of 10%–30% might have covered lesions equivalent to poorly differentiated carcinoma introduced in the 3rd edition WHO classification [31] and high-grade differentiated thyroid carcinoma introduced in the 5th edition WHO classification [33]. In their diagnostic criteria, increased mitoses and/or tumor necrosis have very similar implications to Ki-67 LI greater than 10%. Therefore, we recommend pathologists include the absolute value of Ki-67 LI in their pathology reports so that clinical doctors can understand risk of recurrence and carcinoma death more precisely. This can inform clinicians about the risk of recurrence and grading of tumor death risk, as Ki-67 LI is a continuous variable with a higher value associated with worse prognosis.
- Using a larger number of cases than ours (312 cases with 5% or less), Miyauchi et al. [23] reported a recurrence prognosis of 86.4% at 10 years for PTC with a Ki-67 LI <5%, a lower figure (higher recurrence rate) than our 98.5%. Possible explanations for this include the following. (1) There are differences in the methods of measuring Ki-67 LI. Standardization of measurement methods is desired as a solution. (2) Tumor specimens in advanced stages of disease may show diversity in proliferative potential within the specimen. In such cases, the value of the examined site with the highest proliferative potential (hot spot) was used in this study, although it is possible that the hot spot did not have the maximum potential in the specimen. In addition, (3) if the values used for comparison are not hot spots but average values, differences in the conclusions are expected. However, the studies agree about the importance of a higher Ki-67 LI and a higher recurrence rate. (4) Two different cutoff values, 3% and 5%, have been proposed to identify high-grade MTCs by two independent groups [10,34]. While the international consensus has adopted the cutoff of 5% [12], Australia (Sydney Group) is still using 3% [8]. Thus, the value of the cutoff for the Ki-67 labeling rate needs to be determined by the pathology laboratory.
- We also considered a situation with a Ki-67 labeling rate cutoff of 4%, but this resulted in a recurrence rate of 1.6% for the 0%–4% Ki-67 labeling group (n = 123) and 20.7% for the 4%–10% Ki-67 labeling group (n = 29). Due to this large difference, we ultimately adopted the cutoff value of 5% as in the study by Kakudo et al. [32].
- In the present study, we stratified 167 PTCs according to invasive status combined with tumor growth rate using Ki-67 LI for recurrence. At the same time, we excluded exceptional PTCs that were likely to show anaplastic transformation (high-grade PTCs with a high Ki-67 labeling rate >30% and with an anaplastic carcinoma component). We believe that this strategy allowed us to elucidate a clear difference between cases with Ki-67 LI ≥5% and <5% in PTCs, and recurrence was seen in PTCs with Ki-67 LI <5% was only 2/132 patients (1.5%). Therefore, this prognostic characteristic (a negligible risk of recurrence at 1.5% for a median 64-month follow-up in PTCs with Ki-67 LI <5%) is essential information for a patient immediately after surgery, when the typical patient most frequently experiences carcinoma anxiety. As there are rare exceptional occurrences in low Ki-67 LI cases, we cannot guarantee absence of recurrence. However, a 1.5% risk is negligible in invasive PTCs, and no recurrence was found among patients with Ex0, N0, and Ki-67 LI <5%. Doctors could comfort patients with Ki-67 LI <5%, N0, and Ex0 suffering from severe carcinoma anxiety immediately after surgery when planning postoperative follow-up strategies. Even in patients with Ex0-3 and/or N1/2 PTCs, curative surgery was possible in 98.5% if the Ki-67 LI was <5%. This is important data for alleviating the fear of carcinoma recurrence, metastasis, and tumor mortality. However, for advanced-stage PTCs in which curative resection is not possible and the disease is persistent, an alternative strategy is required to predict patient outcomes, and the serum TG doubling time and doubling rate, proposed by Miyauchi et al. [23], play a significant role in predicting patient outcomes in such cases. Based on the results obtained in this study, we believe that patients with a Ki-67 LI <5% should be followed up as usual. Conversely, patients with a Ki-67 LI ≥5% are at high risk of recurrence and should be followed with imaging studies at relatively short intervals.
- Ki-67 LI is a clinically useful predictive factor for recurrence-free survival in patients with papillary thyroid carcinoma. We believe the evidence for low tumor recurrence risk may contribute to use of more conservative treatment options for invasive-stage PTCs and help alleviate patient anxiety about tumor recurrence and death.
- A limitation of this study was the small number of patients analyzed. However, the results showed significant differences even in this small patient cohort, which indicates that the difference between high (≥5%) Ki-67 LI PTCs and low (<5%) Ki-67 LI PTCs is clear and biologically meaningful. However, further confirmatory studies in a large patient series and different ethnic populations are required before our proposal is widely accepted worldwide. A second limitation is our indication for thyroid surgery. The choice of lobectomy or TTX deviated slightly from Western thyroid nodule practice, and most patients were treated with lobectomy even in a significant number of patients with lateral nodal metastasis (N1b). However, all surgeries were performed by a single surgeon (K.Y.) and his team, and we believe that the indications for surgery and choice of surgery type were constant during this rather extended (2010–2022) study period. A third limitation of this study is the lack of a genetic profile of the PTCs studied, as the health insurance system in Japan does not cover genetic tests for diagnosis of PTCs. Last, another key limitation is the lack of interlaboratory reproducibility of Ki-67 LI measurements due to multiple sources of variation, including antibody clones, antibody formats, staining methods, testing personnel, and staining platforms. Technical standardization of the Ki-67 immunohistochemical assay among laboratories is essential for establishing a reliable risk classification for thyroid carcinomas. As expected, our determination of the Ki-67 LI threshold varies from those reported in other publications by Miyauchi et al. [22,23] due to inherent challenges in the Ki-67 immunostaining and LI measurement methods, particularly antigen preservation, antibody clones, antibody incubation time, DAB reaction time, reaction temperature, and immuno-staining method used. Consequently, a cutoff value established for one assay system might not universally apply to others. In clinical practice, it is imperative to account for the specific measurement system employed and its validated cutoff values rather than relying exclusively on generalized thresholds.
DISCUSSION
Ethics Statement
This study was performed was approved by the Kindai University, Nara Hospital (protocol no. 23-54). Informed consent was obtained from the participant included in the study.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: TM, KY, IO, KK, TW, SK, HK, RY, TK. Data curation: TM, KK, SK, HK. Formal analysis: TM, KK, KY, IO. Funding acquisition: TM. Methodology: TM, KK, TW, RY, TK. Resources: TM, KK. Writing—original draft: TM, KK. Writing—review & editing: TM, KY, IO, KK, TW, SK, HK, RY, TK. Approval of final manuscript: all authors.
Conflicts of Interest
K.K., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
No funding was received for this study.
|
< 55 years old |
≥ 55 years old |
|||
|---|---|---|---|---|
| N category 0 | N category 1 | N category 0 | N category 1 | |
| T category | ||||
| 1a | 10 | 6 | 23 | 4 |
| 1b | 3 | 8 | 10 | 6 |
| 2 | 4 | 10 | 3 | 1 |
| 3 | 7 | 20 | 8 | 26 |
| 4 | 1 | 4 | 0 | 13 |
| Stage | ||||
| I | 73 | 36 | ||
| II | 0 | 45 | ||
| III | - | 13 | ||
| Characteristic | Total patients | Patients with recurrence (n = 19) | Patients without recurrence (n = 154) | Univariate analysis by log-rank test, p-value |
|---|---|---|---|---|
| Age (yr) | .254 | |||
| ≤55 | 78 | 6 | 72 | |
| >55 | 89 | 11 | 78 | |
| Sex | .023 | |||
| Male | 31 | 6 | 25 | |
| Female | 136 | 11 | 125 | |
| Tumor diameter (mm) | <.001 | |||
| >20 | 57 | 14 | 43 | |
| ≤20 | 110 | 3 | 107 | |
| Lympho-vascular invasion | .059 | |||
| Yes | 17 | 4 | 13 | |
| No | 150 | 13 | 137 | |
| Extrathyroidal invasion | <.001 | |||
| Yes | 78 | 16 | 62 | |
| No | 89 | 1 | 88 | |
| Lateral cervical lymph node metastasis | .233 | |||
| Yes | 39 | 6 | 33 | |
| No | 128 | 11 | 117 | |
| Ki-67 LI | <.001 | |||
| >5% | 35 | 15 | 20 | |
| ≤5% | 132 | 2 | 130 | |
| Surgical methodsa | ||||
| Lobectomy | 113 | |||
| Total thyroidectomy | 54 | |||
| Recurrence case | 17 | |||
| Local | 3 | |||
| Cervical lymph node | 11 | |||
| Lung | 9 | |||
| Liver | 1 | |||
| Skin | 1 | |||
| Outcome | ||||
| Alive and well | 160 | |||
| Cancer-bearing survival | 5 | |||
| Cause-specific death | 2 | |||
| Observation period (mo) | 13–144 (median, 77) |
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Male sex | 2.74 | 0.94–7.99 | .065 |
| Tumor diameter >20 mm | 3.15 | 0.86–11.45 | .081 |
| Ex1/2 | 2.88 | 0.33–25.24 | .339 |
| Ki-67 LI >5% | 13.26 | 2.66–66.12 | .002 |
|
Ki-67 labeling index |
Subtotal | ||
|---|---|---|---|
| <5% | 5%–30% | ||
| Ex0 | 1/88 (1.1) | 0/1 (0) | 1/89 (1.1) |
| Ex1 and 2 | 1/44 (2.3) | 15/34 (44.1) | 16/78 (20.5) |
| Subtotal | 2/132 (1.5) | 15/35 (42.9) | |
Values are presented as number (%).
Low-risk: Risk of recurrence is 1.5% (0%–2.3%) 2/132.
High-risk: Risk of recurrence is 44.1% (15/34).
Ex0: no Ex, Ex1: microscopic Ex; Ex2: gross and clinical Ex.
The recurrence rate of 34 patients with both Ki-67 LI 5%–30% and Ex1/2/3 is significantly higher (44.1%) than the other groups (either Ki-67 LI <5% or Ex0) (1.1%) with a median follow-up period of 64 months. Statistically significant differences were observed (p < .001).
PTC, papillary thyroid carcinoma; Ex, extrathyroid invasion; LI, labeling index.
- 1. Popadich A, Levin O, Lee JC, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery 2011; 150: 1048-57. ArticlePubMed
- 2. Hartl DM, Mamelle E, Borget I, Leboulleux S, Mirghani H, Schlumberger M. Influence of prophylactic neck dissection on rate of retreatment for papillary thyroid carcinoma. World J Surg 2013; 37: 1951-8. ArticlePubMedPDF
- 3. Liu J, Zhang Z, Huang H, et al. Total thyroidectomy versus lobectomy for intermediate-risk papillary thyroid carcinoma: a single-institution matched-pair analysis. Oral Oncol 2019; 90: 17-22. ArticlePubMed
- 4. Song E, Han M, Oh HS, et al. Lobectomy is feasible for 1-4 cm papillary thyroid carcinomas: a 10-year propensity score matched-pair analysis on recurrence. Thyroid 2019; 29: 64-70. ArticlePubMed
- 5. Guo K, Wang Z. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Clin Exp Pathol 2014; 7: 5393-403. PubMedPMC
- 6. Ito Y, Higashiyama T, Takamura Y, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg 2007; 31: 2085-91. ArticlePubMedPDF
- 7. Baek SK, Jung KY, Kang SM, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid 2010; 20: 147-52. ArticlePubMed
- 8. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016; 26: 1-133. ArticlePubMedPMC
- 9. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst 2010; 102: 605-13. ArticlePubMed
- 10. Caulley L, Eskander A, Yang W, et al. Trends in diagnosis of noninvasive follicular thyroid neoplasm with papillarylike nuclear features and total thyroidectomies for patients with papillary thyroid neoplasms. JAMA Otolaryngol Head Neck Surg 2022; 148: 99-106. ArticlePubMedPMC
- 11. Fuchs TL, Nassour AJ, Glover A, et al. A proposed grading scheme for medullary thyroid carcinoma based on proliferative activity (Ki-67 and mitotic count) and coagulative necrosis. Am J Surg Pathol 2020; 44: 1419-28. ArticlePubMedPMC
- 12. Xu B, Fuchs TL, Ahmadi S, et al. International medullary thyroid carcinoma grading system: a validated grading system for medullary thyroid carcinoma. J Clin Oncol 2022; 40: 96-104. ArticlePubMedPMC
- 13. Bai Y, Guo T, Niu D, et al. Clinical significance and interrelations of PD-L1 expression, Ki-67 index, and molecular alterations in sporadic medullary thyroid carcinoma from a Chinese population. Virchows Arch 2022; 481: 903-11. ArticlePubMedPDF
- 14. Weigel MT, Dowsett M. Current and emerging biomarkers in breast cancer: prognosis and prediction. Endocr Relat Cancer 2010; 17: R245-62. ArticlePubMed
- 15. Berney DM, Gopalan A, Kudahetti S, et al. Ki-67 and outcome in clinically localised prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study. Br J Cancer 2009; 100: 888-93. ArticlePubMedPMCPDF
- 16. Kloppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2011; 18 Suppl 1: S1-16. ArticlePubMed
- 17. La Rosa S. Diagnostic, prognostic, and predictive role of Ki67 proliferative index in neuroendocrine and endocrine neoplasms: past, present, and future. Endocr Pathol 2023; 34: 79-97. ArticlePubMedPMCPDF
- 18. Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual. 8th ed. Cham: Springer International Publishing, 2016.
- 19. D'Avanzo A, Ituarte P, Treseler P, et al. Prognostic scoring systems in patients with follicular thyroid cancer: a comparison of different staging systems in predicting the patient outcome. Thyroid 2004; 14: 453-8. ArticlePubMed
- 20. Hay ID, Grant CS, Taylor WF, McConahey WM. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery 1987; 102: 1088-95. PubMed
- 21. Voutilainen PE, Siironen P, Franssila KO, Sivula A, Haapiainen RK, Haglund CH. AMES, MACIS and TNM prognostic classifications in papillary thyroid carcinoma. Anticancer Res 2003; 23: 4283-8. PubMed
- 22. Miyauchi A. Clinical trials of active surveillance of papillary microcarcinoma of the thyroid. World J Surg 2016; 40: 516-22. ArticlePubMedPMCPDF
- 23. Miyauchi A, Kudo T, Hirokawa M, et al. Ki-67 labeling index is a predictor of postoperative persistent disease and cancer growth and a prognostic indicator in papillary thyroid carcinoma. Eur Thyroid J 2013; 2: 57-64. ArticlePubMedPMC
- 24. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic": screening and overdiagnosis. N Engl J Med 2014; 371: 1765-7. ArticlePubMed
- 25. U. S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2017; 317: 1882-7. ArticlePubMed
- 26. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. 4th ed. Lyon: International Agency for Research on Cancer, 2017.
- 27. Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 2021; 31: 183-92. ArticlePubMedPMC
- 28. Sugitani I, Ito Y, Miyauchi A, Imai T, Suzuki S. Active surveillance versus immediate surgery: questionnaire survey on the current treatment strategy for adult patients with low-risk papillary thyroid microcarcinoma in Japan. Thyroid 2019; 29: 1563-71. ArticlePubMedPMC
- 29. Dwivedi SS, Khandeparkar SG, Joshi AR, et al. Study of immunohistochemical markers (CK-19, CD-56, Ki-67, p53) in differentiating benign and malignant solitary thyroid nodules with special reference to papillary thyroid carcinomas. J Clin Diagn Res 2016; 10: EC14-9. ArticlePubMedPMC
- 30. Matsuse M, Yabuta T, Saenko V, et al. TERT promoter mutations and Ki-67 labeling index as a prognostic marker of papillary thyroid carcinomas: combination of two independent factors. Sci Rep 2017; 7: 41752.ArticlePubMedPMCPDF
- 31. DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumours: pathology and genetics of tumours of endocrine organs. Lyon: IARC Press, 2004.
- 32. Kakudo K, Wakasa T, Ohta Y, Yane K, Ito Y, Yamashita H. Prognostic classification of thyroid follicular cell tumors using Ki-67 labeling index: risk stratification of thyroid follicular cell carcinomas. Endocr J 2015; 62: 1-12. ArticlePubMed
- 33. Baloch ZW, Asa SL, Barletta JA, et al. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol 2022; 33: 27-63. ArticlePubMedPDF
- 34. Alzumaili B, Xu B, Spanheimer PM, et al. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod Pathol 2020; 33: 1690-701. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
References
Citations

- Research Progress on the Correlation between Three Biomarkers, Ki-67, CAIX and VEGF and Clear Cell Renal Cell Carcinoma
锦容 马
Advances in Clinical Medicine.2025; 15(09): 326. CrossRef - Immunophenotypic Panel for Comprehensive Characterization of Aggressive Thyroid Carcinomas
Mihail Ceausu, Mihai Alin Publik, Dana Terzea, Carmen Adina Cristea, Dumitru Ioachim, Dana Manda, Sorina Schipor
Cells.2025; 14(19): 1554. CrossRef - High Ki-67 labeling index correlates with aggressive clinicopathological features in papillary thyroid carcinoma: a retrospective study
Defi Nurlia Erdian, Maria Francisca Ham, Dina Khoirunnisa, Agnes Stephanie Harahap
Thyroid Research.2025;[Epub] CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
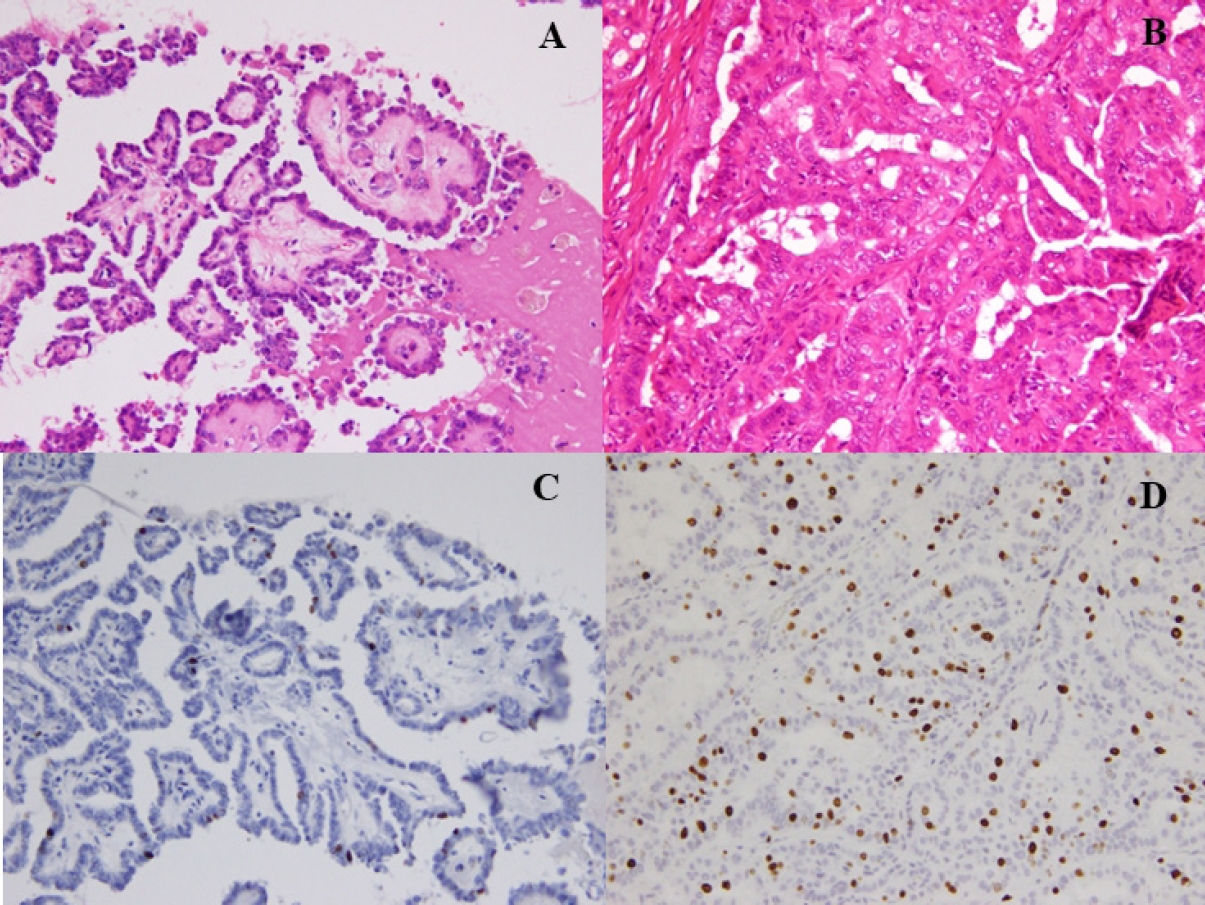
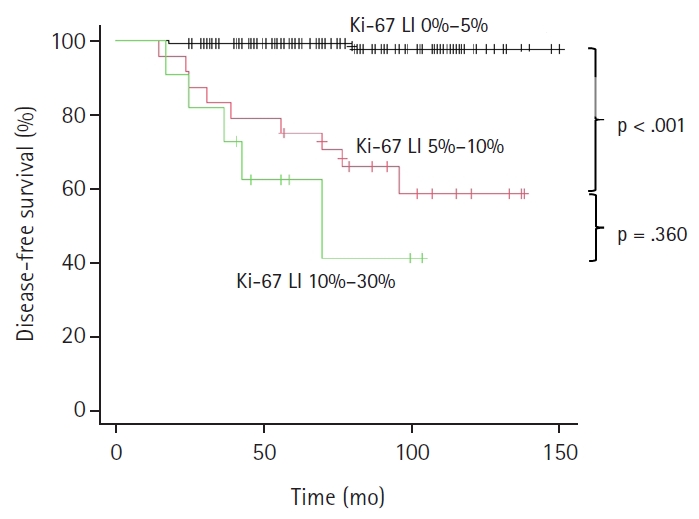
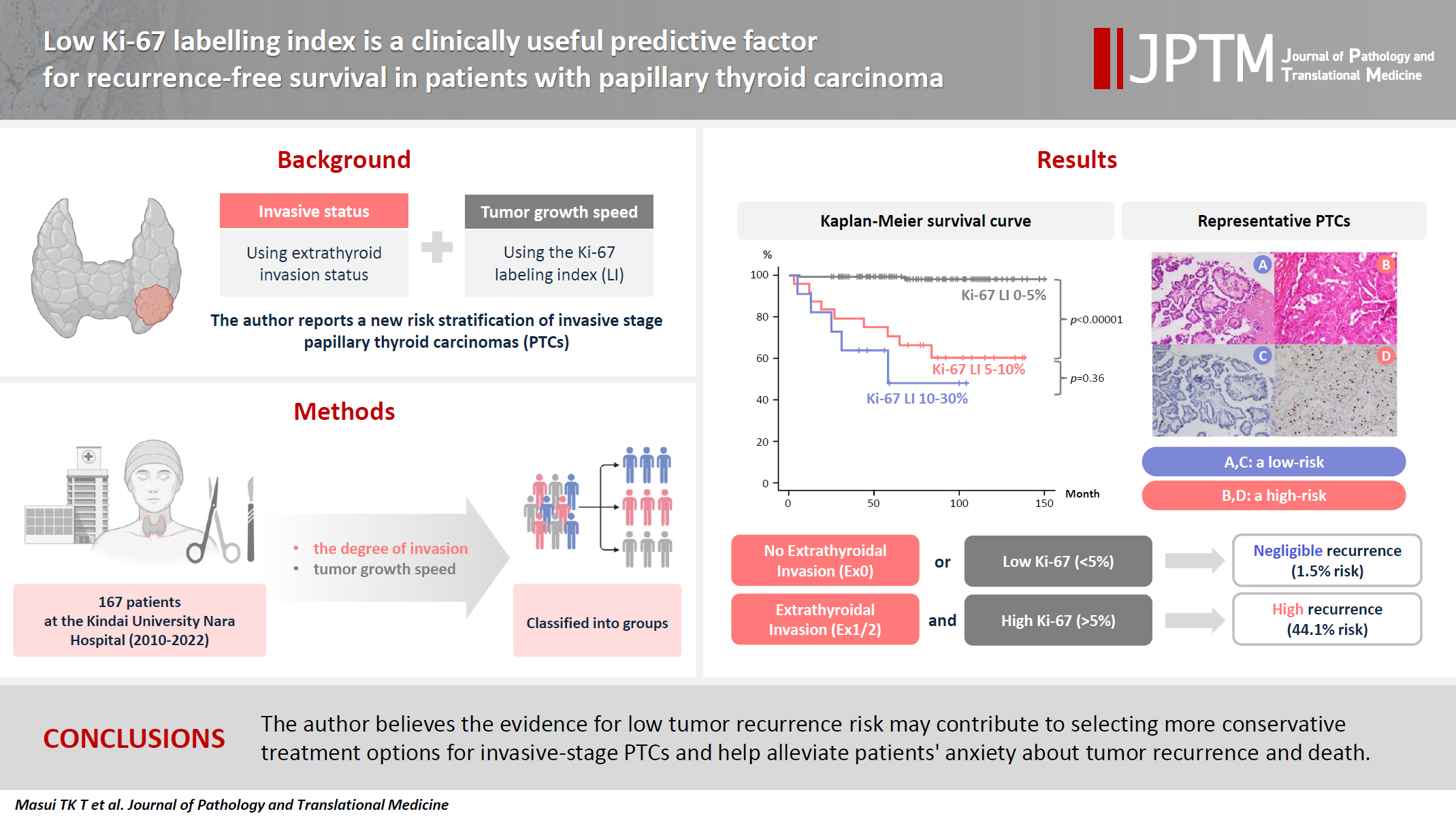
Fig. 1.
Fig. 2.
Graphical abstract
| < 55 years old |
≥ 55 years old |
|||
|---|---|---|---|---|
| N category 0 | N category 1 | N category 0 | N category 1 | |
| T category | ||||
| 1a | 10 | 6 | 23 | 4 |
| 1b | 3 | 8 | 10 | 6 |
| 2 | 4 | 10 | 3 | 1 |
| 3 | 7 | 20 | 8 | 26 |
| 4 | 1 | 4 | 0 | 13 |
| Stage | ||||
| I | 73 | 36 | ||
| II | 0 | 45 | ||
| III | - | 13 | ||
| Characteristic | Total patients | Patients with recurrence (n = 19) | Patients without recurrence (n = 154) | Univariate analysis by log-rank test, p-value |
|---|---|---|---|---|
| Age (yr) | .254 | |||
| ≤55 | 78 | 6 | 72 | |
| >55 | 89 | 11 | 78 | |
| Sex | .023 | |||
| Male | 31 | 6 | 25 | |
| Female | 136 | 11 | 125 | |
| Tumor diameter (mm) | <.001 | |||
| >20 | 57 | 14 | 43 | |
| ≤20 | 110 | 3 | 107 | |
| Lympho-vascular invasion | .059 | |||
| Yes | 17 | 4 | 13 | |
| No | 150 | 13 | 137 | |
| Extrathyroidal invasion | <.001 | |||
| Yes | 78 | 16 | 62 | |
| No | 89 | 1 | 88 | |
| Lateral cervical lymph node metastasis | .233 | |||
| Yes | 39 | 6 | 33 | |
| No | 128 | 11 | 117 | |
| Ki-67 LI | <.001 | |||
| >5% | 35 | 15 | 20 | |
| ≤5% | 132 | 2 | 130 | |
| Surgical methods |
||||
| Lobectomy | 113 | |||
| Total thyroidectomy | 54 | |||
| Recurrence case | 17 | |||
| Local | 3 | |||
| Cervical lymph node | 11 | |||
| Lung | 9 | |||
| Liver | 1 | |||
| Skin | 1 | |||
| Outcome | ||||
| Alive and well | 160 | |||
| Cancer-bearing survival | 5 | |||
| Cause-specific death | 2 | |||
| Observation period (mo) | 13–144 (median, 77) |
| Variable | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Male sex | 2.74 | 0.94–7.99 | .065 |
| Tumor diameter >20 mm | 3.15 | 0.86–11.45 | .081 |
| Ex1/2 | 2.88 | 0.33–25.24 | .339 |
| Ki-67 LI >5% | 13.26 | 2.66–66.12 | .002 |
| Total cases | rec+ | rec– | p-value | |
|---|---|---|---|---|
| Ex–group 1 (Ex0, any N and M0) | 89 | 1 | 88 | <.001 |
| Ex–group 2 (Ex1, any N and M0) | 62 | 11 | 51 | |
| Ex–group 1 (Ex0, any N and M0) | 89 | 1 | 88 | <.001 |
| Ex–group 3 (Ex2/3, any N and M0) | 16 | 5 | 11 | |
| Ex–group 2 (Ex1, any N and M0) | 62 | 11 | 51 | .298 |
| Ex–group 3 (Ex2/3, any N and M0) | 16 | 5 | 11 | |
| Group 1 (Ki-67 LI >5% and Ex0) | 1 | 0 | 1 | .425 |
| Group 2 (Ki-67 LI >5% and Ex1) | 23 | 10 | 13 | |
| Group 3 (Ki-67 LI >5% and Ex2/3) | 11 | 5 | 6 | |
| Ki-67–group 1 (Ki-67 LI <5%) | 132 | 2 | 130 | <.001 |
| Ki-67–group 2 (Ki-67 LI 5%–10%) | 24 | 9 | 15 | |
| Ki-67–group 3 (Ki-67 LI 10%–30%) | 11 | 6 | 5 |
| Ki-67 labeling index |
Subtotal | ||
|---|---|---|---|
| <5% | 5%–30% | ||
| Ex0 | 1/88 (1.1) | 0/1 (0) | 1/89 (1.1) |
| Ex1 and 2 | 1/44 (2.3) | 15/34 (44.1) | 16/78 (20.5) |
| Subtotal | 2/132 (1.5) | 15/35 (42.9) | |
LI, labeling index. Duplicate counted if there are more than 2.
Ki-67 LI >5% was an independent prognostic factor. CI, confidence interval; Ex, extrathyroid invasion; LI, labeling index.
After combining these two (Ex status and Ki-67 LI of ≥5%) parameters, recurrence (rec) rates were compared. Ex, extrathyroid invasion; LI, labeling index.
Values are presented as number (%). Low-risk: Risk of recurrence is 1.5% (0%–2.3%) 2/132. High-risk: Risk of recurrence is 44.1% (15/34). Ex0: no Ex, Ex1: microscopic Ex; Ex2: gross and clinical Ex. The recurrence rate of 34 patients with both Ki-67 LI 5%–30% and Ex1/2/3 is significantly higher (44.1%) than the other groups (either Ki-67 LI <5% or Ex0) (1.1%) with a median follow-up period of 64 months. Statistically significant differences were observed (p < .001). PTC, papillary thyroid carcinoma; Ex, extrathyroid invasion; LI, labeling index.

 E-submission
E-submission