Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(2); 2025 > Article
-
Original Article
Characteristics of RET gene mutations in Vietnamese medullary thyroid carcinoma patients: a single-center analysis -
Van Hung Pham1,2
 , Quoc Thang Pham3
, Quoc Thang Pham3 , Minh Nguyen1
, Minh Nguyen1 , Hoa Nhat Ngo3
, Hoa Nhat Ngo3 , Thao Thi Thu Luu3
, Thao Thi Thu Luu3 , Nha Dao Thi Minh1
, Nha Dao Thi Minh1 , Trâm Đặng1
, Trâm Đặng1 , Anh Tu Thai4
, Anh Tu Thai4 , Hoang Anh Vu3
, Hoang Anh Vu3 , Dat Quoc Ngo3
, Dat Quoc Ngo3
-
Journal of Pathology and Translational Medicine 2025;59(2):125-132.
DOI: https://doi.org/10.4132/jptm.2025.01.18
Published online: March 14, 2025
1Faculty of Nursing and Medical Technology, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
2University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam
3Pathology Department, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
4Faculty of Pathology, Ho Chi Minh City Oncology Hospital, Ho Chi Minh City, Vietnam
- Corresponding Author Quoc Thang Pham, MD, PhD Pathology department, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City 748010, Vietnam Tel: +84-783-332-527 Fax: +84-28-3855-2304 E-mail: phamquocthang@ump.edu.vn
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,413 Views
- 176 Download
Abstract
-
Background
- The RET gene point mutation is the main molecular alteration involved in medullary thyroid carcinoma (MTC) tumorigenesis. Previous studies in Vietnam mainly consisted of case reports, with limited data on larger sample sizes. In this study, we investigated RET gene mutations in exons 10, 11, and 16 and analyzed clinicopathological features of a series of Vietnamese MTC patients.
-
Methods
- We collected 33 tissue samples from patients with MTC and analyzed RET mutations using the Sanger sequencing method. The relationship between hotspot RET mutations (exons 10, 11, 16) and clinicopathological features were investigated.
-
Results
- Among the 33 analyzed cases, 17 tumors (52%) harbored RET mutations in exon 10, 11, or 16. A total of 10 distinct genetic alterations were identified, including eight missense mutations and two short indels. Of these, seven were classified as pathogenic mutations based on previous publications, with p.M918T being the most frequent (4 cases), followed by p.C634R (3 cases) and p.C618R (3 cases). Mutations were significantly associated with specific histological patterns, such as the nested/insular pattern (p=.026), giant cells (p=.007), nuclear pleomorphism (p=.018), stippled chromatin (p=.044), and amyloid deposits (p=.024). No mutations were found in germline analyses, suggesting these were somatic alterations.
-
Conclusions
- Our results provided the first comprehensive analysis of RET mutations in Vietnamese MTC patients. The most frequent mutation was p.M918T, followed by p.C634R and p.C618R. Mutations in these three exons were linked to specific histopathological features. Information on mutational profiles of patients with MTC will further aid in the development of targeted therapeutics to ensure effective disease management.
- According to the data from GLOBOCAN 2022, the global incidence of new thyroid cancer cases is 821.214, ranking seventh among all cancer types and claiming the lives of 47.507 individuals annually [1]. In Vietnam, the age-standardized incidence rate of thyroid cancer increased from 2.4 per 100,000 during 1996–2000 to 7.5 per 100,000 during 2011–2015; furthermore, the age of patients at diagnosis decreased gradually [2]. Most thyroid cancer subtypes are derived from follicular cells, except medullary thyroid carcinoma (MTC), which originates from parafollicular C cells. MTC is a well-differentiated thyroid tumor that accounts for about 5% to 10% of all thyroid carcinomas, and shows an intermediate prognostic outcome between papillary and anaplastic thyroid cancer [3,4].
- The primary genetic change implicated in the development of MTC is point mutation in the RET gene [5]. RET-mutant MTC exhibits more aggressive clinical behavior, including a higher incidence of lymph node metastasis and distant metastasis, as well as worse survival [6-8]. Notably, patients have most benefited from the genetic screening for germline mutations of the RET proto-oncogene in the diagnosis, prevention, and treatment of MTC [9]. The allelic frequencies of RET mutations vary in different populations, and thus it is critical to ascertain population-specific mutation frequencies [10].
- Takahashi et al. first described the RET oncogene in 1985 [11]. Situated on chromosome 10q11.2, the RET proto-oncogene encodes a cellular tyrosine kinase transmembrane receptor. Structurally, RET comprises three distinct domains: an extracellular segment at the N-terminus housing four cadherin-like regions, a cysteine-rich region housing a transmembrane domain, and a cytoplasmic domain with tyrosine kinase activity [12]. Upon binding with the ligand-co-receptor complex, RET undergoes dimerization and autophosphorylation on intracellular tyrosine residues, which then recruit adaptor and signaling proteins to activate multiple downstream pathways [13]. The activation of RET stimulates various downstream pathways involved in cell growth, proliferation, survival, and differentiation [14]. Consequently, alterations leading to the dysregulation of RET activity contribute to several human cancers [13].
- For patients with thyroid carcinoma undergoing RET testing, the method typically begins by sequencing the commonly mutated RET cysteine codons within exons 10 and 11, along with hot spots found in exons 13 to 16. Alternatively, all RET exons may be sequenced from the outset [9,15]. Frequently observed somatic mutation M918T occurs in up to 40% of individuals with sporadic MTC and is linked to the aggressive nature of the disease [8,16]. In this study, none of the patients had a family history, thus we selected exons 10, 11, and 16 for analysis. MTC commonly exhibits single amino acid substitutions as well as minor insertions or deletions [15]. Since the Sanger sequencing method is the most suitable technique for analyzing single nucleotide variants and short indels, we employed the Sanger method to achieve the objectives of this study.
- Research examining RET gene mutations in patients with MTC has been conducted in many countries worldwide. However, research in Vietnam remains limited. Therefore, this investigation aims to provide additional data on RET gene mutations in Vietnamese individuals, potentially contributing to diagnostic and molecular-targeted treatment applications.
INTRODUCTION
- Tissue samples
- We retrospectively collected primary tumors of 33 thyroid cancer patients with diagnosed MTC who had undergone thyroid resection at the Oncology Hospital (Ho Chi Minh City, Vietnam) between 2020 and 2022.
- Sections were cut at 3-µm thickness and stained with hematoxylin-eosin. Experienced pathologists Q.T.P. and H.N.N. evaluated indicative regions from both cancerous and non-cancerous tissues on histopathological slides. Pathological features were classified according to the updated World Health Organization (WHO) 2022 criteria [17]. For each sample, 10 slices, each 10 µm thick, were obtained from corresponding paraffin-embedded tissue blocks for subsequent DNA extraction. Normal thyroid tissue was selected from these blocks for germline analysis.
- DNA isolation
- DNA extraction from formalin-fixed paraffin-embedded (FFPE) tissue blocks was carried out using the ReliaPrep FFPE gDNA Miniprep System kit (Promega, Madison, WI, USA) following the instructions of the manufacturer. The purity of the DNA samples was assessed using Nanodrop technology before polymerase chain reaction (PCR) and sequencing. Samples with insufficient DNA yield were excluded from further analysis.
- PCR and Sanger sequencing
- The primers of RET exons 10, 11, and 16 that target hot spot regions are shown in the Supplementary Table S1. All primers used in this study were newly designed. PCR was performed in 15 µL mixtures of 0.1 µM of each forward and reverse primer, 1× PCR Buffer, 1.5 mM MgCl2, 200 µM each dNTP, 0.5 U Taq Hot Start Polymerase (Takara Bio, Shiga, Japan) and 25–50 ng of genomic DNA.
- PCR was denatured at 98°C for 3 minutes followed by 45 cycles of 98°C for 10 seconds, 58°C for 30 seconds and 72°C for 40 seconds, and a final elongation at 72°C for 2 minutes. PCR products were checked for size and purity using 2% agarose gel electrophoresis.
- PCR products were purified enzymatically using the ExoSAP IT PCR Product Cleanup Reagent (Thermo Scientific, Waltham, MA, USA) for removal of excess primers and dNTPs before Sanger sequencing using the BigDye Terminator v3.1 Kit and the ABI 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sequences were compared to the reference sequence of the RET gene (GenBank accession number: NG 07489.1). CLC Main Workbench Software version 5.5 (Qiagen, Frederick, MD, USA) was utilized to analyze mutations. All detected alterations were functionally classified using the available databases (such as NCBI, COSMIC, etc.) and previous reports. Pathogenicity of variants was estimated by Polymorphism Phenotyping-2 (PolyPhen-2; Havard, Boston, MA, USA) or MutationTaster (Neurocure Cluster of Exellence/Berlin Institute of Health, Berlin, Germany).
- Statistical methods
- Statistical analysis was performed using STATA ver. 14.2 (Stata Corp., College Station, TX, USA). Comparisons between the two groups were conducted using the chi-square or Fisher exact test. Differences between the two groups with a significance level of p < .05 were considered statistically significant.
MATERIALS AND METHODS
- Characteristics of MTC patients and their clinicopathological features
- Our research investigated 33 MTC patients. There were more females than males, with a female-to-male ratio of 1.4:1. The average age at diagnosis of MTC was 46.67 years. Of the cases studied, 85% exhibited a solitary tumor, and 88% had the tumor confined to a single lobe of the thyroid gland. The smallest tumor observed grossly measured 6 mm, whereas the largest measured 80 mm (Table 1). Among the morphological features, solid and nest/insular patterns were the most commonly observed in MTC cases (Supplementary Fig. S1). Typical cells were identified in 91% of the cases, exhibiting a round or polyhedral shape with coarsely granular chromatin (Supplementary Fig. S2). Four cases exhibited clearly defined nuclei, while only three cases displayed tumor cells with nuclear inclusions (Supplementary Fig. S3). In the stroma, 52% showed amyloid deposits; in addition, fibrosis was noted in 64% of cases and hemorrhage in 61%, with calcifications occurring in approximately 30% of the cases (Supplementary Fig. S4).
- In total, 45% of MTC patients were diagnosed with lymph node metastasis. High-grade histological features included a high mitotic count (≥5 per 2 mm²) in six cases, necrosis in three cases, and lymphovascular invasion in four cases.
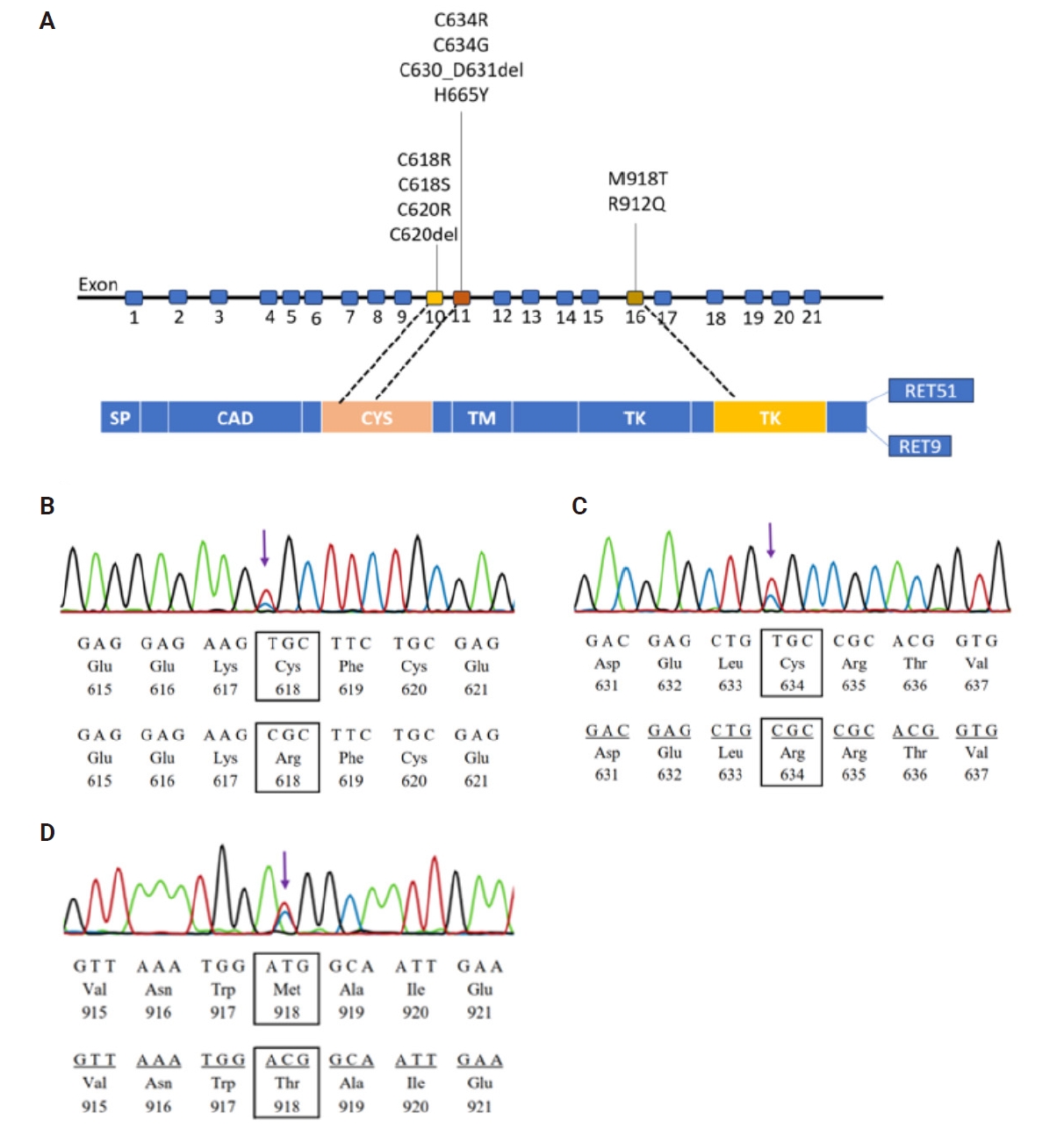
- The landscape of RET gene mutations
- The prevalence of RET gene alterations in the hot spot region was approximately 52% in our population. As shown in Table 2 and Fig. 1, among the 33 analyzed samples, 10 alterations were identified across exons 10, 11, and 16, with frequencies of six (18%), six (18%), and five (15%), respectively. All alterations indicate heterozygous status, and no germline mutations were detected in normal tissue among these cases, suggesting that these variants were somatic mutations. The p.M918T mutation had the highest frequency with four identified cases, followed by p.C634R and p.C618R mutations with three cases each. There were two mutations at the hot spot codon p.C618 (TGC>CGC, TGC>TCC) in exon 10 and two mutations at the hot spot codon p.C634 (TGC>CGC, TGC>GGC) in exon 11.
- Alongside the eight single nucleotide variants (p.C618R, p.C618S, p.C620R, p.C634R, p.C634G, p.H665Y, p.M918T and p.R912Q), two short in-frame insertions/deletions were observed: a 2-amino acid deletion in exon 11 (p.C630_D631del) and a 1-amino acid deletion in exon 16 (p.C620del). Out of the detected alterations, seven were pathogenic mutations according to prior publications. Two alterations were categorized as either ‘disease-causing’ or ‘possibly damaging,’ with PolyPhen-2 and MutationTaster assigning scores of 0.89 and 0.99, respectively. Additionally, one alteration was classified as a polymorphic variant, with a score of 0.58 on MutationTaster (Table 2).
- Our data showed that there are relationships between the detected mutations and histopathological features, including nested or insular pattern, giant cells, nuclear pleomorphism, stippled chromatin, and amyloid deposit (Table 1). The number of cases with detected mutations was also higher in females (p=.024). Other clinicopathological characteristics did not show any statistically significant associations with the presence or absence of mutations.
RESULTS
- In this study, the absence of a family history along with classification of the identified variants as somatic indicated that these MTC cases harbored somatic gene alterations. With an average age of 46.67±13.93 years, this population is consistent with the age range reported by the American Thyroid Association, which notes that sporadic MTC typically presents later, often between the fourth and sixth decades of life, in contrast to hereditary MTC, which tends to manifest at an earlier age [9]. The study results also indicate that most MTC cases presented as a single tumor, with the predominant histological pattern being solid. The typical cells were round or polygonal, exhibiting coarsely granular chromatin, with a low incidence of clear nuclei and nuclear inclusions, consistent with WHO classification [17]. In addition to amyloid deposition in the stroma, our study population was characterized by a high prevalence of fibrosis and hemorrhage, both exceeding 60%.
- Our research described RET gene mutations in a series of cases of Vietnamese patients with MTC. To the best of our knowledge, only one previous study investigated RET gene mutations in Vietnamese patients with MTC; however, that analysis was limited to a single case. Ha et al. [18] reported that a c.2753C>T transition resulted in a missense mutation of methionine to threonine (p.M918T) in the RET protein. According to the American Thyroid Association, around half of sporadic MTC patients exhibit somatic RET mutations [9]. Additionally, Ciampi et al. [8] reported that somatic RET mutations are detected in as many as 55% of sporadic MTC patients. Our data showed mutations in exons 10, 11, and 16 in 17 cases out of a total of 33 cases of MTC with no family history, accounting for 52%.
- In our study, a total of 10 different alterations in the RET gene were identified, including point mutations and short indels. All the point mutations were missense mutations and all the short indels were in-frame changes. In general, missense mutations with a single amino acid change were the most common ones causing loss of function of the RET protein in cases of MTC. Similar results have been reported in other studies [8,16].
- This study detected the p.M918T mutation in exon 16 in four cases, and the p.C634R mutation in exon 11 and the p.C618R mutation in exon 10 in three cases each, suggesting that they are common in Vietnamese patients with MTC. Several publications reported that p.M918T has the highest frequency in similar studies [8,19], and cysteine mutations have been noted in sporadic MTC cases [12]. Moreover, the RET gene p.C634 codon was mutated in four cases with different amino acid alterations, similar to previous report [8]. The p.C634R mutation was observed in three cases. However, the p.C634 codon was mutated in four cases, including three cases of p.C634R and one additional case of p.C634G. Our data differs from a Slovakian study involving patients with MTC, where the p.C618R mutation in exon 10 was more common than p.C634R and p.M918T mutations in patients with a negative family history [20]. In this study, mutations at codon C618 were detected in four cases, more than at codon C620, which were found in two cases. These results differ from those reported by Yeganeh et al. [21], where p.C611Y and p.C620R were the most prevalent mutations in exon 10.
- Asai et al. [22] and Santoro et al. [23] previously elucidated the molecular mechanisms of RET activation by cysteine mutations. When a non-cysteine residue replaces a cysteine residue, it releases a neighboring cysteine normally engaged in forming an intramolecular disulfide bond. This freed cysteine then creates an abnormal intermolecular covalent disulfide bond between two mutant RET molecules, triggering their dimerization and subsequent activation [22,23]. p.M918T mutations enhance kinase activity both as monomers and by presenting substrates for trans-autophosphorylation [23]. This effect arises from structural alterations in the activation loop of the kinase domain [24]. RET mutations of extracellular cysteines, which include mutations in exon 10 and exon 11, facilitate dimerization and kinase activation, whereas mutations in the RET kinase coding domain, including those in exon 16, drive dimerization-independent kinase activation. Thus, RET kinase inhibition is an attractive therapeutic target in patients with RET alterations. Initially, this method was accomplished through multikinase inhibitors, impacting various dysregulated pathways involving RET kinase. In clinical settings, employing multikinase inhibitors for advanced thyroid cancer patients yielded therapeutic benefits, although often accompanied by notable and occasionally severe side effects [15]. Nevertheless, significant advancements have emerged with the discovery of potent and specific RET kinase inhibitors for treating advanced thyroid cancer. While further clinical confirmation through future trials is necessary, the consistent antitumoral effectiveness and enhanced safety profile of these new compounds herald a promising era in precision oncology for RET-driven cancers [15]. In 2020, the U.S. Food and Drug Administration (FDA) sanctioned selpercatinib and pralsetinib for RET-mutated MTC necessitating systemic treatment. Drawing from these data and FDA endorsements, the National Comprehensive Cancer Network Panel advocated for both of these RET inhibitors as primary choices for patients with RET-mutant conditions [25]. Somatic genotyping for RET should be conducted in patients who exhibit germline wild-type status or whose germline status remains undetermined [25].
- Kaserer et al. [26] highlighted that, in comparison to sporadic tumors, hereditary tumors were significantly more likely to exhibit multifocality, bilaterality, association with desmoplastic stroma, and the presence of C cell hyperplasia. Our study identified a significant association between sporadic RET mutations and distinct histological patterns, including a nested/insular pattern and the presence of giant cells, nuclear pleomorphisms, and amyloid deposits. To the best of our knowledge, no previous studies have reported a correlation between RET sporadic mutations and specific histopathological patterns. Interestingly, Verga et al. [27] reported that cutaneous lichen amyloidosis was exclusively identified in MEN2A/FMTC families carrying a RET pathogenic variant at codon 634. Since the number of MTC patients included in our research was relatively small, additional studies are required to better investigate the association between sporadic RET mutations and histological characteristics.
- Our study revealed a landscape of RET gene mutations in exons 10, 11 and 16 in cases of MTC in Vietnamese patients. The most common mutation was p.M918T, followed by p.C634R and p.C618R. Mutations detected in these three exons are associated with histopathological features including histological patterns (nested/insular pattern), cellular features (giant cells, nuclear pleomorphism, and stippled chromatin), and stromal features (amyloid deposition). Moreover, clinical characteristics including patient sex also have a relationship with the detected mutations. The results of this study indicate that when considering RET gene mutations in a Vietnamese population with sporadic MTC, attention should be paid to exons 10, 11, and 16 first.
DISCUSSION
Supplementary Information
Ethics Statement
This study received approval from the Board of Ethics in Biomedical Research of the University of Medicine and Pharmacy in Ho Chi Minh City, Vietnam, under approval number 916/HDDD-DHYD. The consent forms for the patients were obtained.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: VHP, QTP, DQN. Data curation: VHP, HNN, TD, TTTL, NDTM. Formal analysis: VHP, HAV, MN. Funding acquisition: DQN. Investigation: VHP. Methodology: QTP, ATT, HAV, DQN. Project administration: QTP. Resources: ATT, HAV. Software: HAV, VHP. Supervision: QTP, DQN, MN. Validation: ATT, QTP, DQN. Visualization: NDTM. Writing—original draft: VHP, QTP. Writing—review & editing: VHP, QTP, HNN, MN, HAV. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
This study was funded by a grant from the University of Medicine and Pharmacy at Ho Chi Minh City (grant number 114/2023/HĐ-ĐHYD).
Acknowledgments
The authors express their gratitude to the Center for Molecular Biomedicine at the University of Medicine and Pharmacy in Ho Chi Minh City, as well as the Department of Pathology at the Ho Chi Minh City Oncology Hospital, for their support and facilitation in completing this study.

- 1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today [Internet]. Lyon: International Agency for Research on Cancer, 2024 [cited 2024 Dec 10]. Available from: https://gco.iarc.who.int/today.
- 2. Pham DX, Nguyen HD, Phung AH, et al. Trends in incidence and histological pattern of thyroid cancer in Ho Chi Minh City, Vietnam (1996-2015): a population-based study. BMC Cancer 2021; 21: 296.ArticlePubMedPMCPDF
- 3. DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumours: pathology and genetics of tumours of endocrine organs. Lyon: IARC Press, 2004.
- 4. Pusztaszeri MP, Bongiovanni M, Faquin WC. Update on the cytologic and molecular features of medullary thyroid carcinoma. Adv Anat Pathol 2014; 21: 26-35. ArticlePubMed
- 5. Matrone A, Gambale C, Prete A, Elisei R. Sporadic medullary thyroid carcinoma: towards a precision medicine. Front Endocrinol (Lausanne) 2022; 13: 864253.ArticlePubMedPMC
- 6. Romei C, Casella F, Tacito A, et al. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J Med Genet 2016; 53: 729-34. ArticlePubMed
- 7. Moura MM, Cavaco BM, Pinto AE, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer 2009; 100: 1777-83. ArticlePubMedPMCPDF
- 8. Ciampi R, Romei C, Ramone T, et al. Genetic landscape of somatic mutations in a large cohort of sporadic medullary thyroid carcinomas studied by next-generation targeted sequencing. iScience 2019; 20: 324-36. ArticlePubMedPMC
- 9. Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25: 567-610. ArticlePubMedPMC
- 10. Rovcanin B, Damjanovic S, Zivaljevic V, Diklic A, Jovanovic M, Paunovic I. The results of molecular genetic testing for RET proto-oncogene mutations in patients with medullary thyroid carcinoma in a referral center after the two decade period. Hippokratia 2016; 20: 187-91. PubMedPMC
- 11. Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 1985; 42: 581-8. ArticlePubMed
- 12. Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 2016; 12: 192-202. ArticlePubMedPDF
- 13. Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer 2014; 14: 173-86. ArticlePubMedPDF
- 14. Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev 2005; 16: 441-67. ArticlePubMed
- 15. Salvatore D, Santoro M, Schlumberger M. The importance of the RET gene in thyroid cancer and therapeutic implications. Nat Rev Endocrinol 2021; 17: 296-306. ArticlePubMedPDF
- 16. Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008; 93: 682-7. ArticlePubMed
- 17. Ghossein RA, Mete O, Osamura AJ, et al. WHO classification of tumours of endocrine and neuroendocrine tumours. 5th ed. Vol. 8. WHO Classification of Tumours online. Lyon: International Agency for Research on Cancer, 2022.
- 18. Ha NH, Yen TT, Nhung VP, Thuong MT, Ton ND, Hai NV. Identification of mutation in the RET gene in a patient with medullary thyroid cancer. Viet J Biotechnol 2016; 14: 405-10. ArticlePDF
- 19. Marsh DJ, Learoyd DL, Andrew SD, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 1996; 44: 249-57. ArticlePubMedPDF
- 20. Milicevic S, Bergant D, Zagar T, Peric B. Crude annual incidence rate of medullary thyroid cancer and RET mutation frequency. Croat Med J 2021; 62: 110-9. ArticlePubMedPMC
- 21. Yeganeh MZ, Sheikholeslami S, Dehbashi Behbahani G, Farashi S, Hedayati M. Skewed mutational spectrum of RET proto-oncogene Exon10 in Iranian patients with medullary thyroid carcinoma. Tumour Biol 2015; 36: 5225-31. ArticlePubMedPDF
- 22. Asai N, Iwashita T, Matsuyama M, Takahashi M. Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol 1995; 15: 1613-9. ArticlePubMedPMCPDF
- 23. Santoro M, Carlomagno F, Romano A, et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science 1995; 267: 381-3. ArticlePubMed
- 24. Takahashi M, Kawai K, Asai N. Roles of the RET proto-oncogene in cancer and development. JMA J 2020; 3: 175-81. ArticlePubMedPMC
- 25. Haddad RI, Bischoff L, Ball D, et al. Thyroid carcinoma, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2022; 20: 925-51. ArticlePubMed
- 26. Kaserer K, Scheuba C, Neuhold N, et al. Sporadic versus familial medullary thyroid microcarcinoma: a histopathologic study of 50 consecutive patients. Am J Surg Pathol 2001; 25: 1245-51. ArticlePubMed
- 27. Verga U, Fugazzola L, Cambiaghi S, et al. Frequent association between MEN 2A and cutaneous lichen amyloidosis. Clin Endocrinol (Oxf) 2003; 59: 156-61. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure


Fig. 1.
Graphical abstract
| Variable | Wild-type tumors (n=16) | Tumor with RET mutations (n=17) | p-value |
|---|---|---|---|
| Sex (male/female) | 10/6 | 4/13 | .024 |
| Age (yr), median (range) | 45 (30–65) | 50 (35–70) | .112 |
| Tumor size (mm) | 25 (6–80) | 30 (10–75) | .145 |
| Morphological features | |||
| Solid pattern | 13 | 17 | .103 |
| Nested/insular pattern | 8 | 15 | .026 |
| Trabecular pattern | 2 | 4 | .656 |
| Papillary pattern | 4 | 2 | .398 |
| Follicular pattern | 4 | 3 | .688 |
| Others | 4 | 4 | >.99 |
| Tumor cell characteristics | |||
| Admixtures | 9 | 14 | .141 |
| Round or polyhedral cells | 14 | 16 | .601 |
| Spindle cells | 4 | 6 | .708 |
| Plasmacytoid cells | 4 | 5 | >.99 |
| Giant cells | 0 | 7 | .007 |
| Oncocytic cells | 2 | 3 | >.99 |
| Small cells | 1 | 0 | .485 |
| Nuclear pleomorphism | 0 | 6 | .018 |
| Nuclear inclusions | 2 | 1 | .601 |
| Stippled chromatin | 12 | 17 | .044 |
| Nucleoli | 2 | 2 | >.99 |
| Stromal tissue characteristics | |||
| Necrosis | 3 | 0 | .103 |
| Sclerosis | 11 | 10 | .554 |
| Amyloid deposits | 5 | 12 | .024 |
| Hemorrhage | 9 | 11 | .619 |
| Calcification | 3 | 7 | .259 |
| Prognostic features | |||
| Vascular invasion | 3 | 1 | .335 |
| Nodal metastases | 9 | 6 | .227 |
| High mitotic count (≥5 per 2 mm2) | 4 | 2 | .398 |
| TNM staging | |||
| pT1 | 7 | 5 | .412 |
| pT2 | 8 | 9 | .209 |
| pT3 | 1 | 3 | .112 |
| pN0 | 10 | 7 | .354 |
| pN1 | 6 | 10 | .227 |
| pM0 | 16 | 17 | N/A |
| AJCC stage | |||
| Stage I | 9 | 5 | .121 |
| Stage II | 7 | 10 | .278 |
| Stage III | 0 | 2 | .189 |
| Location | Variants | Protein changes | No. of cases | Type of variants | Classification |
|---|---|---|---|---|---|
| Exon 10 | c.1852T>C | p.C618R (p.Cys618Arg) | 3 | Missense | Pathogenic |
| c.1853G>C | p.C618S (p.Cys618Ser) | 1 | Missense | Pathogenic | |
| c.1858T>C | p.C620R (p.Cys620Arg) | 1 | Missense | Pathogenic | |
| c.1858_1860del | p.C620del (p.Cys620del) | 1 | Deletion | Disease causing (predicted score by MutationTaster: 0.99) | |
| Exon 11 | c.1900T>C | p.C634R (p.Cys634Arg) | 3 | Missense | Pathogenic |
| c.1900T>G | p.C634G (p.Cys634Gly) | 1 | Missense | Pathogenic | |
| c.1887_1893delinsA | p.C630_D631del (p.Cys630_Asp631del) | 1 | Deletion | Polymorphysm (predicted score by MutationTaster: 0.58) | |
| c.1993C>T | p.H665Y (p.His665Tyr) | 1 | Missense | Posibly Damaging (predicted score by PolyPhen-2: 0.89) | |
| Exon 16 | c.2753T>C | p.M918T (p.Met918Thr) | 4 | Missense | Pathogenic |
| c.2735G>A | p.R912Q (p.Arg912Gln) | 1 | Missense | Pathogenic |
AJCC, American Joint Committee on Cancer.

 E-submission
E-submission