Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(1); 2025 > Article
-
Review
Post-transplant liver biopsies: a concise and practical approach for beginners -
Mohamad Besher Ourfali,1,*
 , David Hirsch1,*
, David Hirsch1,* , Marianna Scranton2,3
, Marianna Scranton2,3 , Tony El Jabbour1
, Tony El Jabbour1
-
Journal of Pathology and Translational Medicine 2025;59(1):1-10.
DOI: https://doi.org/10.4132/jptm.2024.11.15
Published online: January 15, 2025
1Department of Pathology and Laboratory Medicine, Hartford Hospital, Hartford, CT, USA
2Connecticut GI, Hartford, CT, USA
3The Comprehensive Liver Center, Hartford Hospital, Hartford, CT, USA
- Corresponding Author: Mohamad Besher Ourfali Department of Pathology and Laboratory Medicine, Hartford Hospital 80 Seymour Street, Hartford, CT 06106, USA Tel: +1-860-972-2488, Fax: +1-860-545-2204 E-mail: Mohamad.Ourfali@hhchealth.org
- *Mohamad Besher Ourfali and David Hirsch contributed equally to this work.
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Exposure to post-transplant liver biopsies varies among pathology residencies and largely depends on the institution's training program, particularly if the hospital has a liver transplant program. The interpretation of biopsies from transplanted livers presents its own set of challenges, even for those with a solid understanding of non-transplant medical liver biopsies. In this review, we aim to provide a succinct, step-by-step approach to help you interpret liver transplant biopsies. This article may be beneficial for residents interested in liver pathology, gastrointestinal and liver pathology fellows in the early stages of training, clinical gastroenterology and hepatology fellows, hepatologists and general pathologists who are curious about this niche.
- Liver transplantation has emerged as a vital therapeutic option for patients with liver failure and/or end-stage liver disease. While it offers significant benefits, it also presents risks and necessitates thorough monitoring to prevent rejection or failure of the transplanted liver. This monitoring is done radiologically and through serial blood liver function tests. The liver function test panel includes alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, albumin, gamma-glutamyl transferase, bilirubin, prothrombin time, and international normalized ratio. If abnormalities in the liver function tests cannot be explained clinically or radiologically, a biopsy is needed to determine any treatable etiology.
- Assessing post-transplant liver biopsies can be challenging because of the wide range of potential pathologies. Breaking down the assessment of these biopsies into six steps may provide a simplified approach, particularly for beginners. In each step, assess the biopsy for one broad category of etiologies and begin building a differential diagnosis list. After the final step, the comprehensive list of possible diagnoses can be further refined based on the clinical scenario.
INTRODUCTION
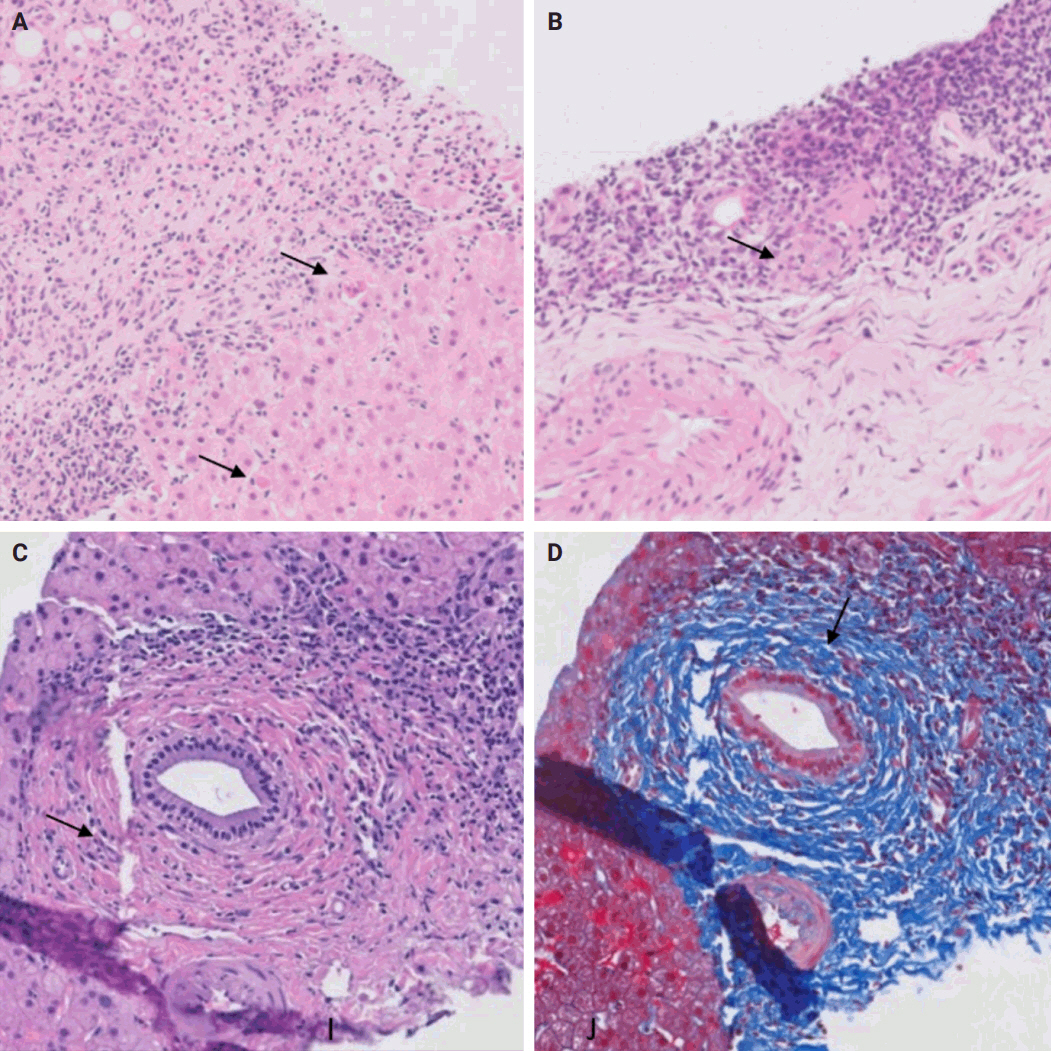
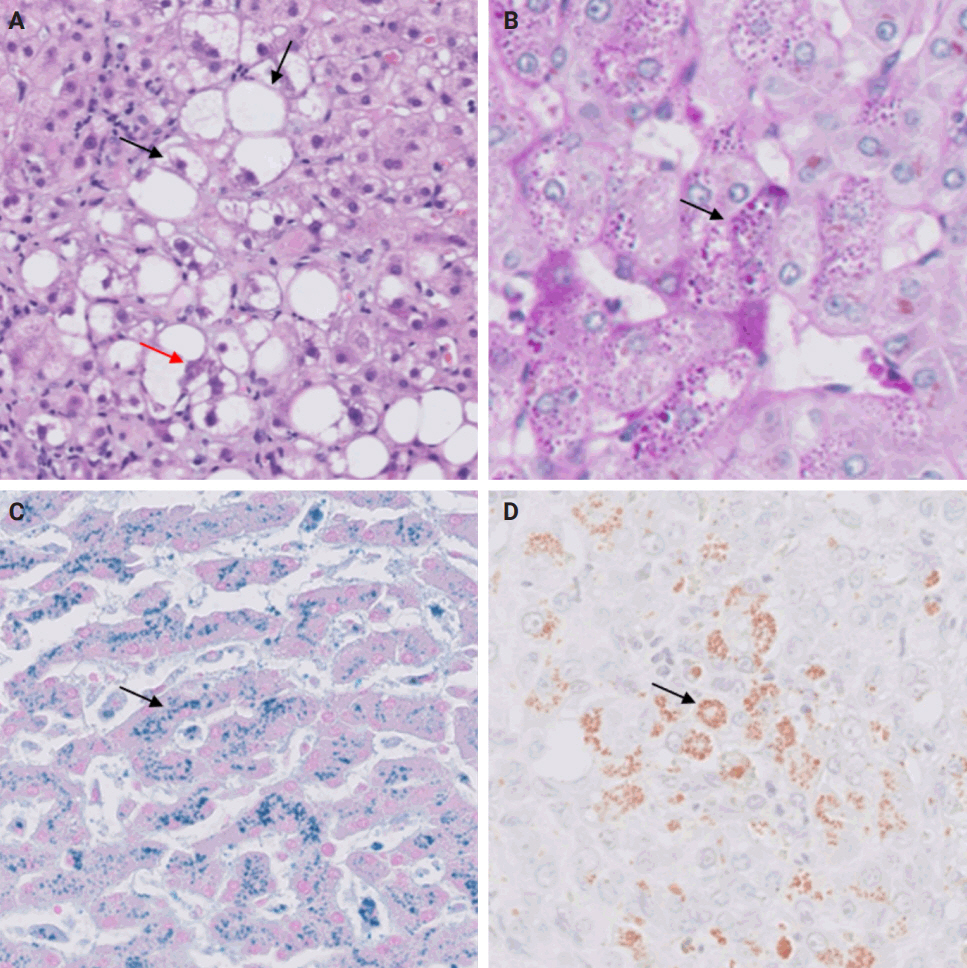
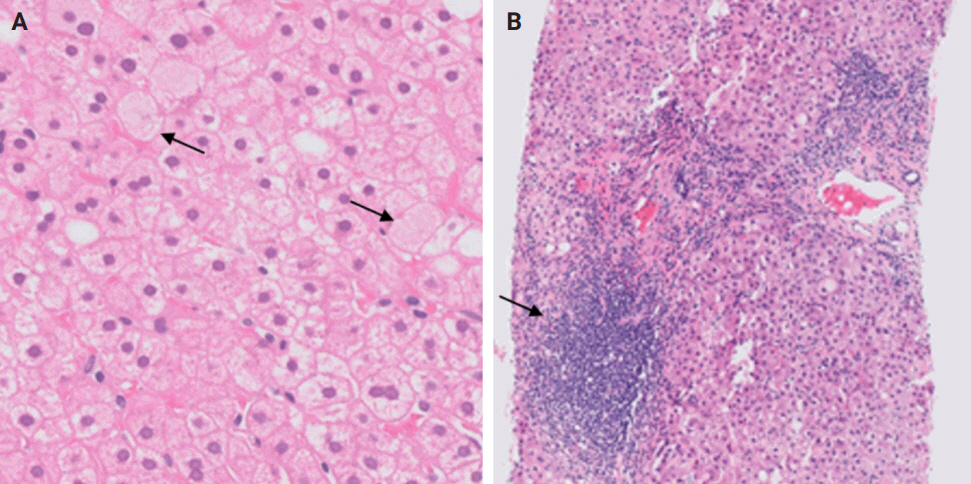
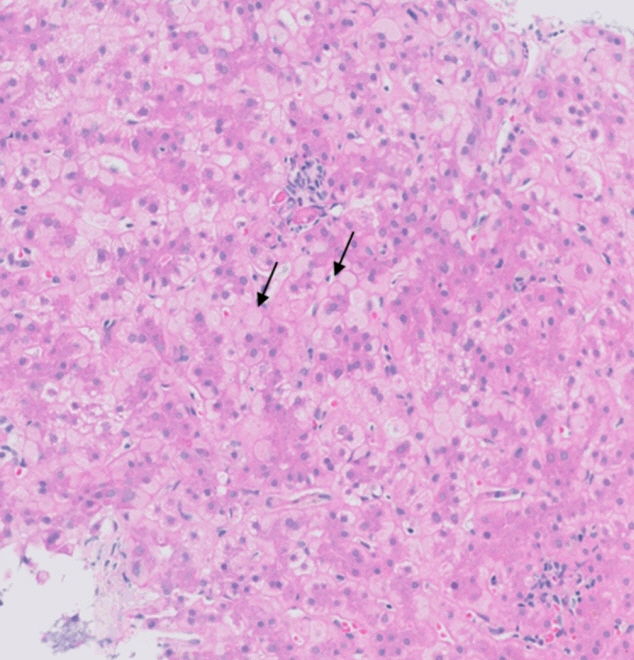
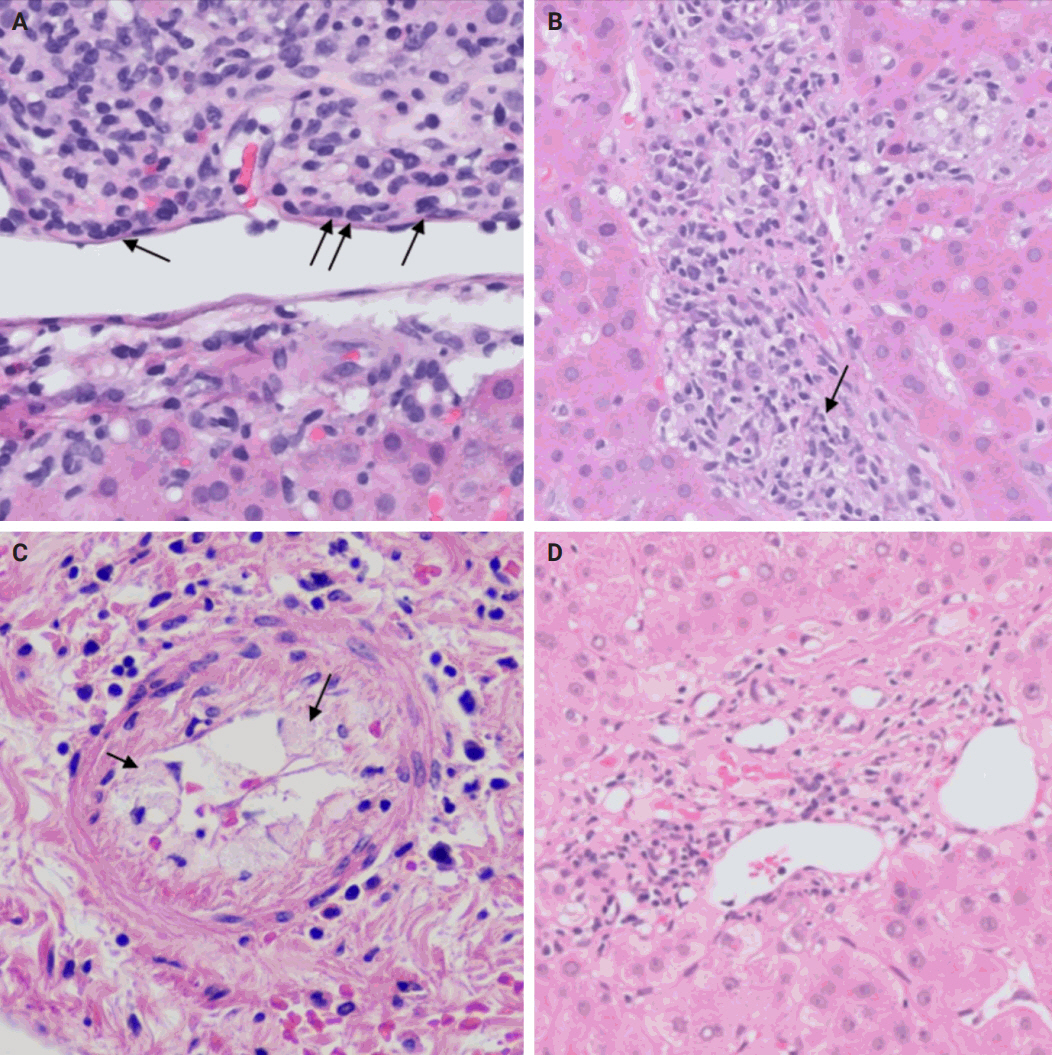
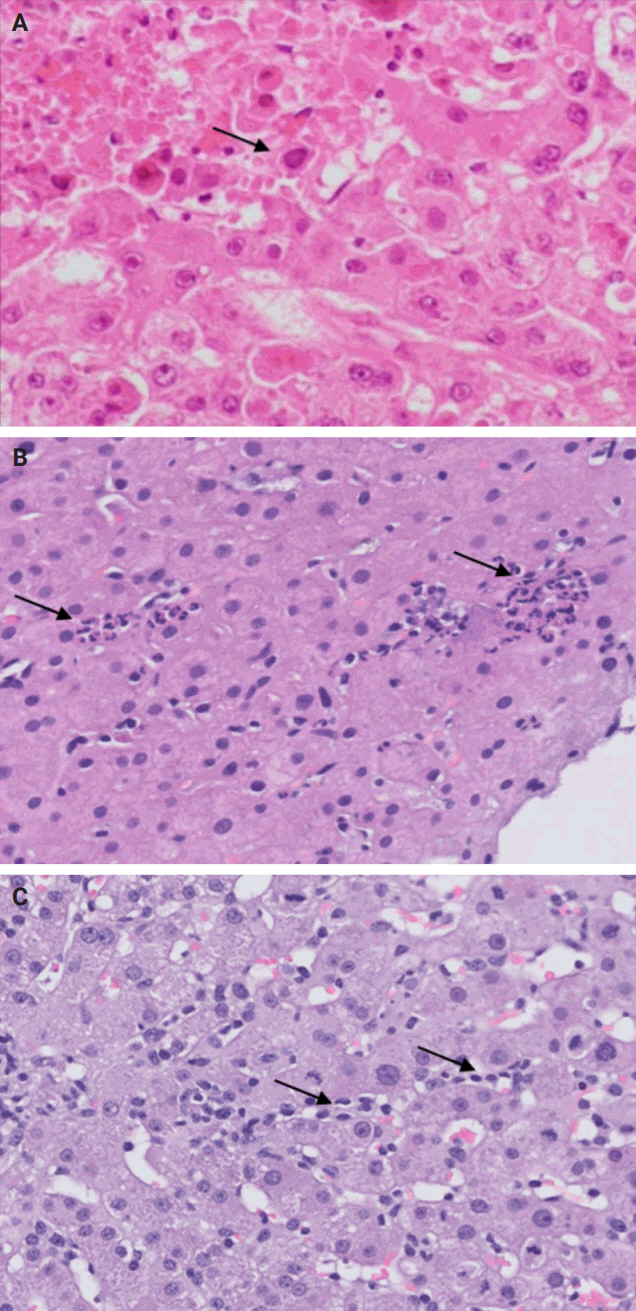
- De novo liver disease refers to the development of new liver pathology that was not present in the liver before transplantation. To assess this category of entities, begin by disregarding the fact that this is a post-transplant biopsy and assess whether the microscopic findings are pathognomonic of any known liver pathology. Employ the same approach you would use for non-transplant medical liver biopsies. Table 1 summarizes the pathognomonic histologic findings of the most commonly encountered liver pathologies. These findings are also illustrated in Figs. 1–3.
STEP 1: DE NOVO DISEASES
- In this step, evaluate the primary liver disease that necessitated the transplant. If the disease is prone to recur, examine the biopsy for its distinct histologic features. Incorporate the possibility of disease recurrence into your growing list of differential diagnoses.
- Wilson’s disease and alpha-1 antitrypsin deficiency are two primary liver diseases that typically do not recur following liver transplantation. Wilson’s disease arises from a mutation in the ATP7B gene, responsible for encoding a copper-transporting ATPase pivotal in excreting excess copper from the liver into bile [1]. Liver transplantation is considered curative as the transplanted liver possesses a functional ATP7B gene, facilitating effective regulation of copper metabolism [2]. Alpha-1 antitrypsin deficiency stems from mutations in the SERPINA1 gene, leading to the production of abnormal alpha-1 antitrypsin protein, which accumulates in hepatocytes, causing inflammation and fibrosis [3]. Liver transplantation is curative for liver dysfunction caused by alpha-1 antitrypsin deficiency as the transplanted liver can produce normal alpha-1 antitrypsin protein [4].
- Hereditary hemochromatosis, characterized by increased intestinal iron absorption due to a mutation in the HFE gene, poses a different challenge [5]. It is controversial whether hemochromatosis can recur post-liver transplantation, although it is usually curative [5,6]. Additionally, due to their pathophysiology, primary biliary cholangitis, primary sclerosing cholangitis, autoimmune hepatitis, steatohepatitis, and hepatotropic viral hepatitis are also known to recur after liver transplantation [7-12].
STEP 2: RECURRENCE OF PRIMARY LIVER DISEASE
- In this step, you should assess the possibility of rejection and include it in the list of potential diagnoses. There are two types of rejection that can occur after liver transplantation: T cell–mediated rejection (TCMR) and antibody-mediated rejection (AMR).
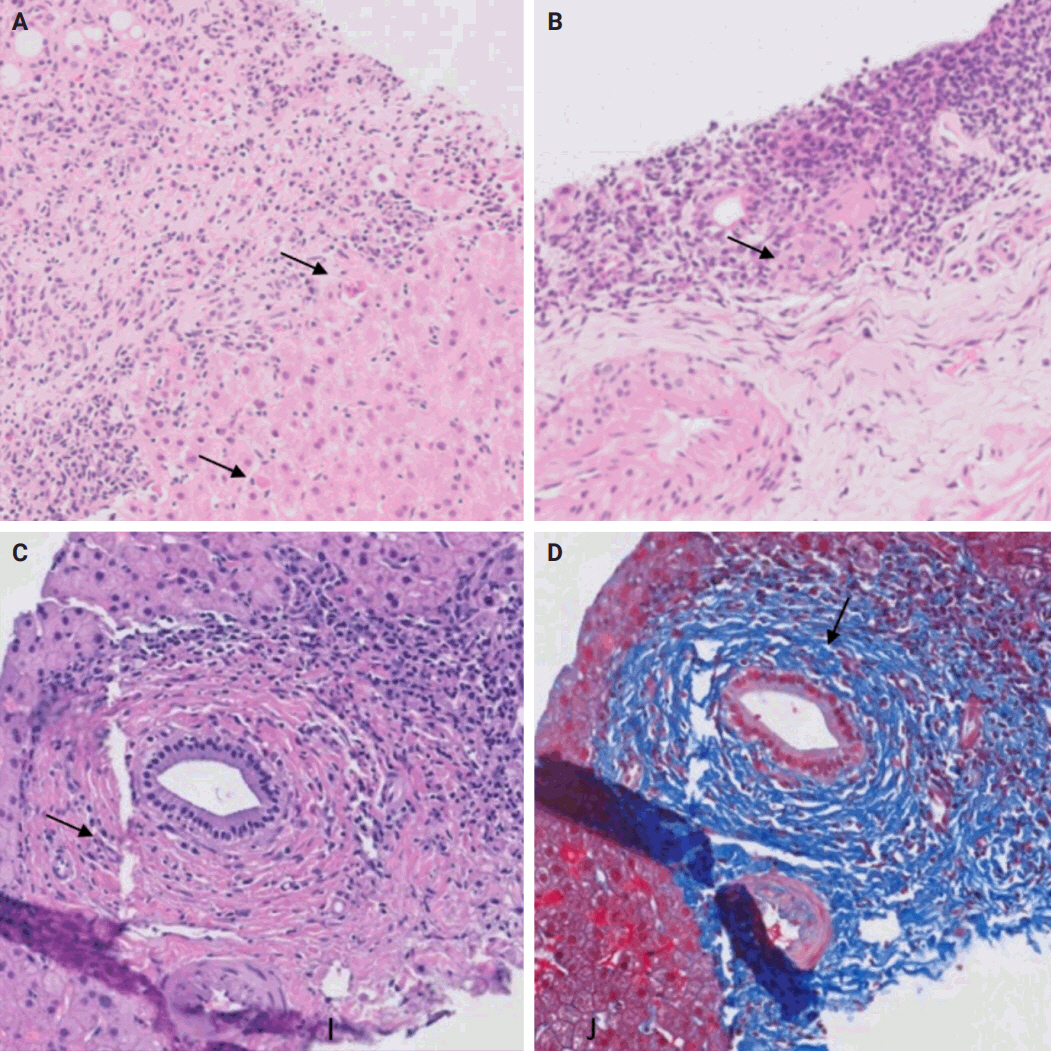
- TCMR is more commonly encountered and encompasses acute cellular rejection, chronic ductopenic rejection, and chronic rejection with foam cell arteriopathy. Given its prevalence in clinical practice, it is important to highlight the Banff scoring system. This system is commonly employed for both diagnosing and grading acute cellular rejection. It categorizes the typical histopathological findings of acute cellular rejection into three main areas: portal inflammation, bile duct inflammation, and venous endothelial inflammation. These categories are then graded on a scale ranging from none (0) to severe (3). A cumulative score of 0–1 indicates a negative result for acute cellular rejection, while a score of 2–3 is considered indeterminate or borderline. Mild rejection is denoted by a score of 3–4, moderate rejection by a score of 5–7, and severe rejection by a score of 8–9 [15,16].
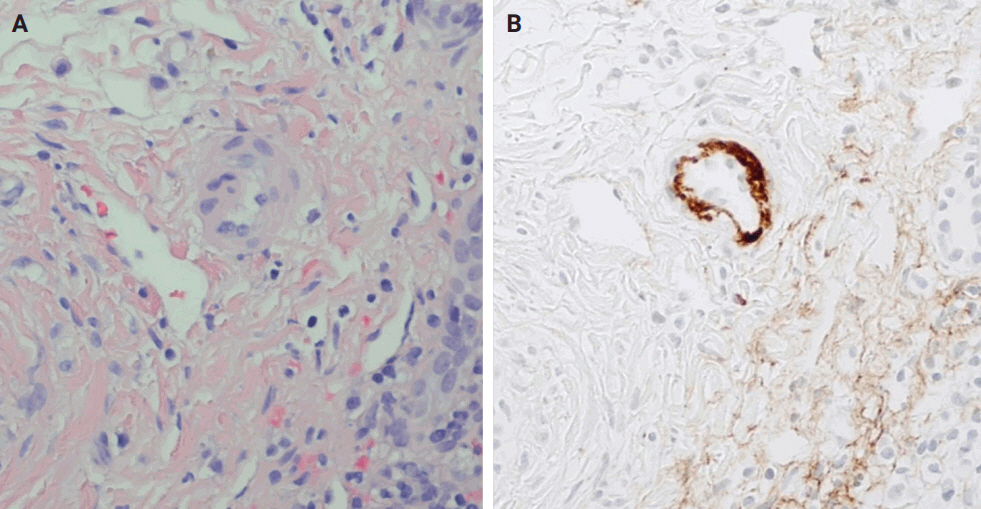
- AMR includes hyperacute AMR (almost eliminated due to its rarity), acute AMR (aAMR), and chronic active AMR. Both of the latter two entities are associated with an elevated serologic titer of donor-specific antibodies, which are antibodies specific to the donor's human leukocyte antigens and C4d deposition in portal microvascular endothelium (portal vein and capillaries) (best seen on C4d immunostain) [16,17].
- Plasma cell-rich rejection is a mixed TCMR and aAMR which occurs in patients with original disease other than autoimmune hepatitis [16].
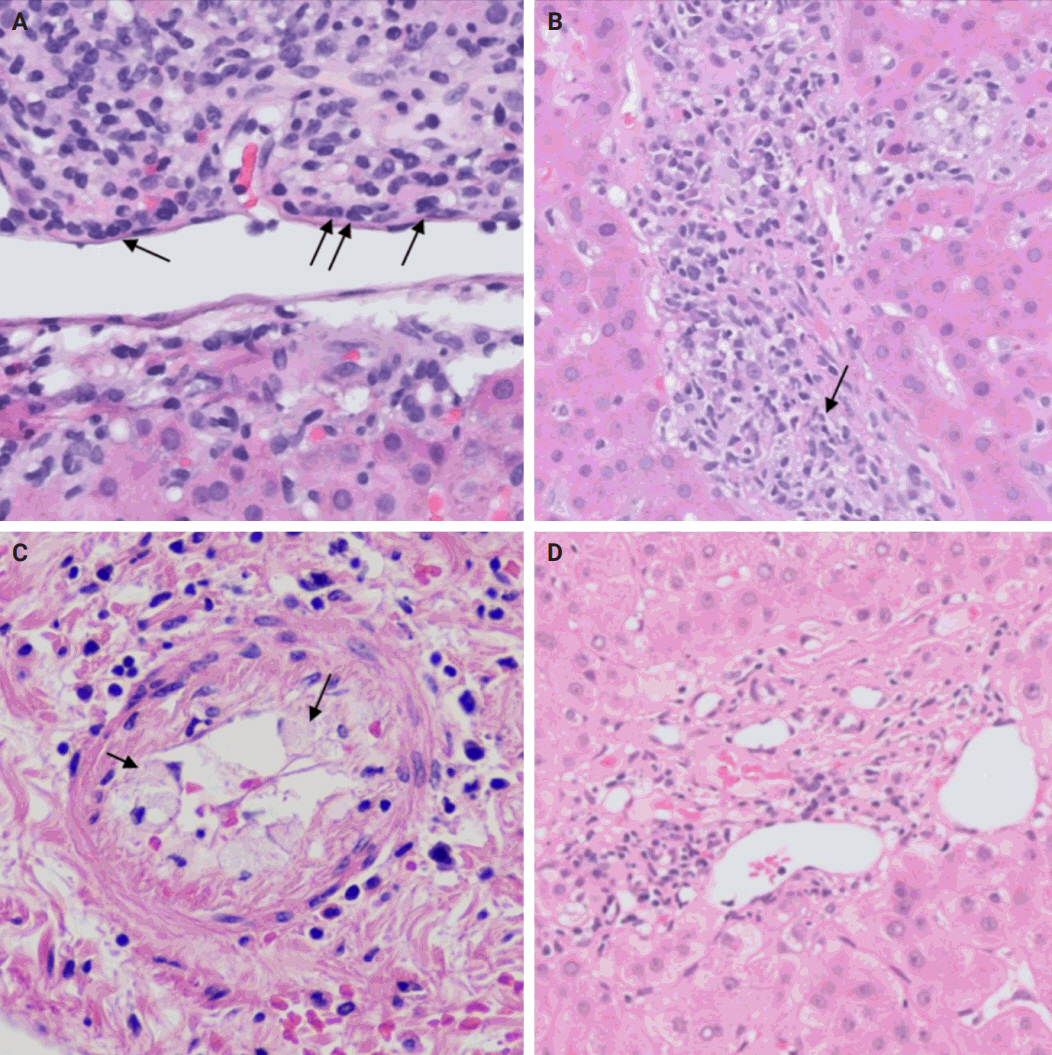
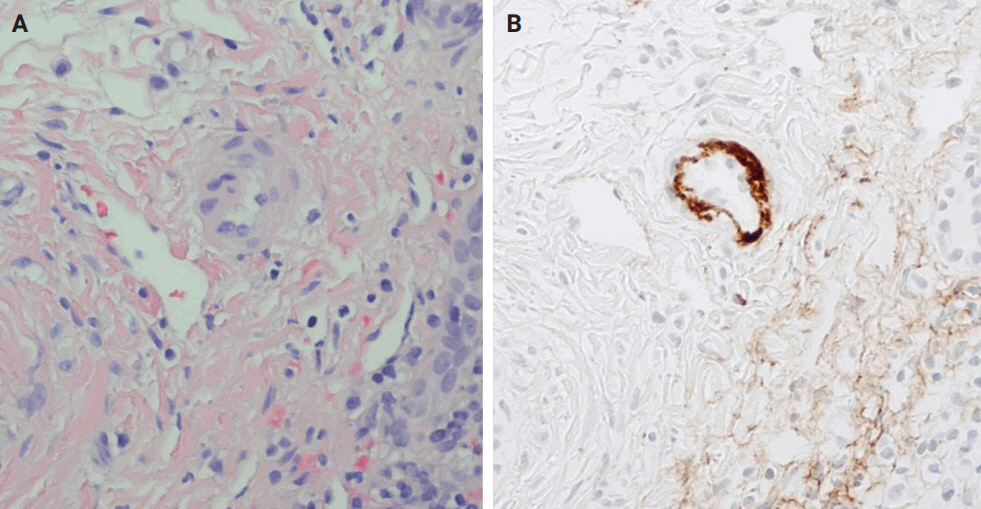
- The distinctive histologic features of these rejections are outlined in Table 2. Figs. 4 and 5 highlight the pathognomonic features of each subtype of rejection.
STEP 3: REJECTION
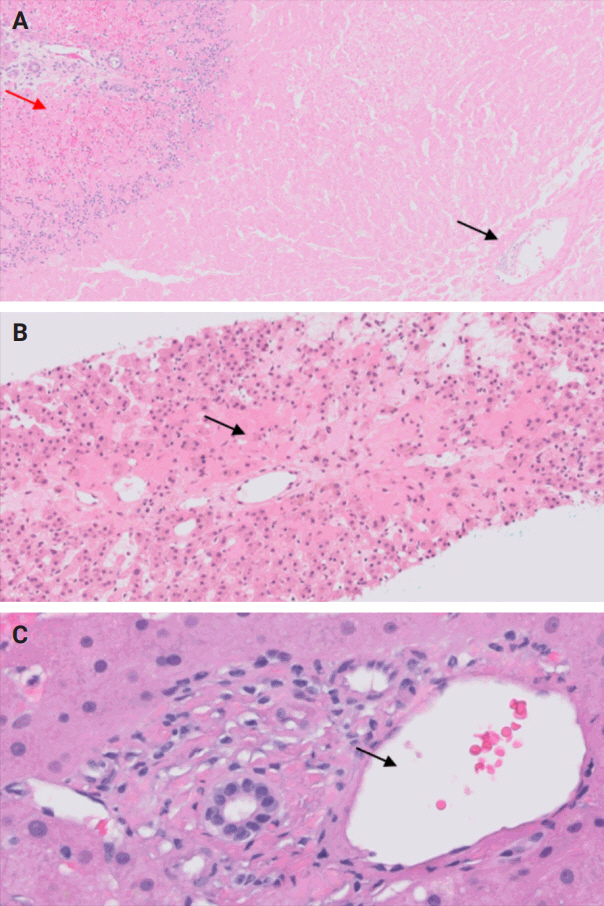
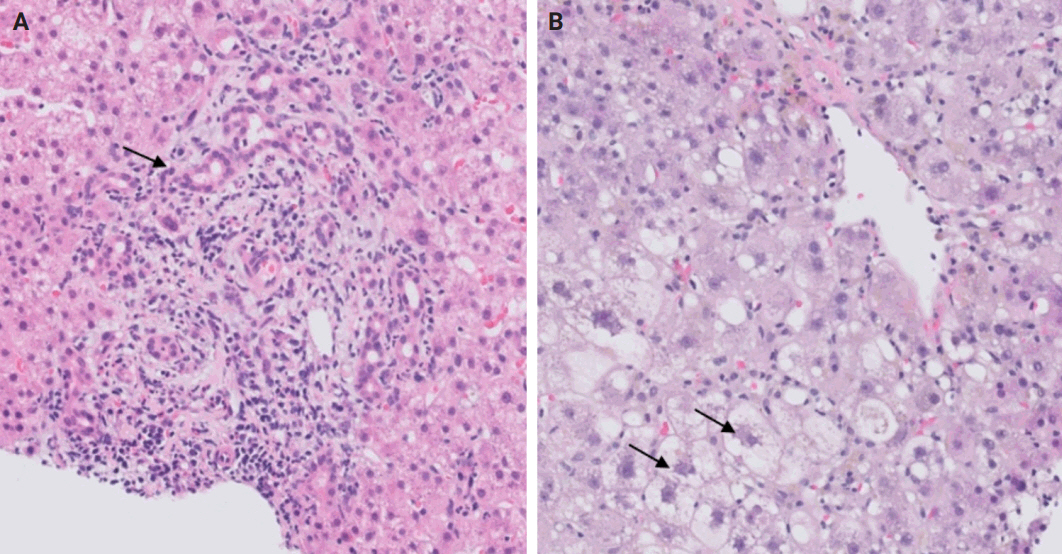
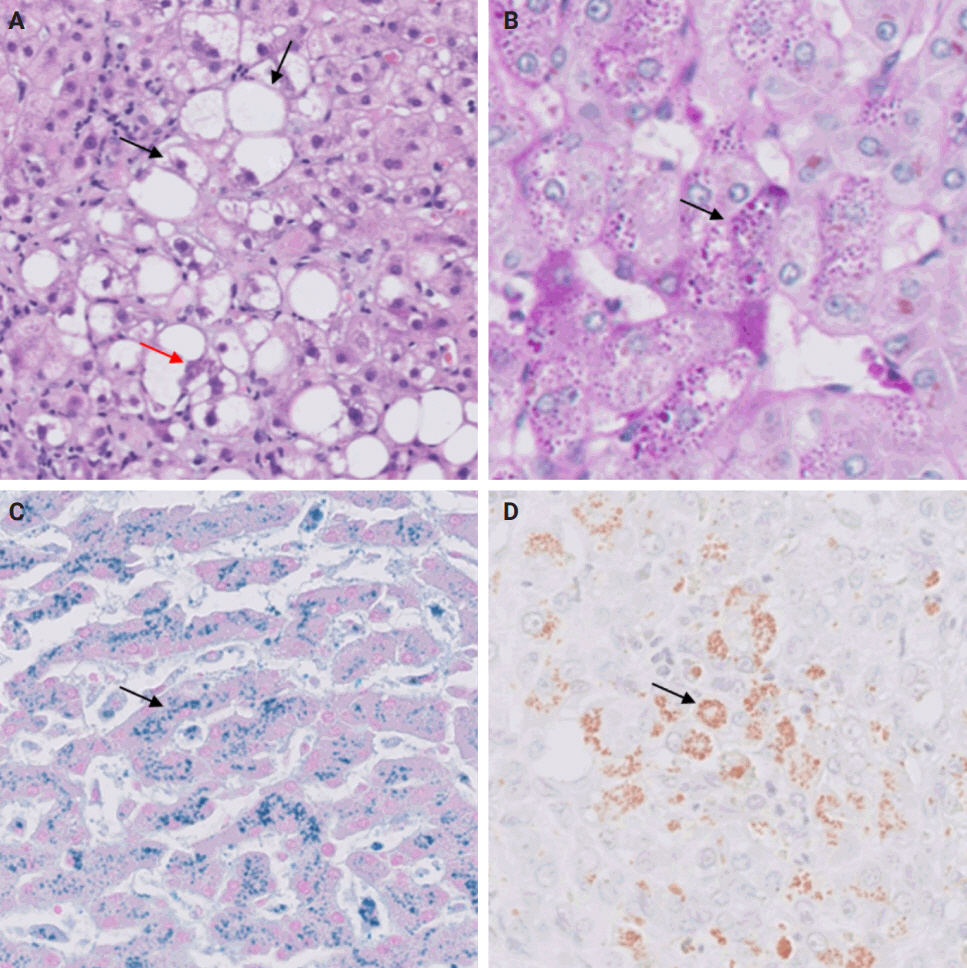
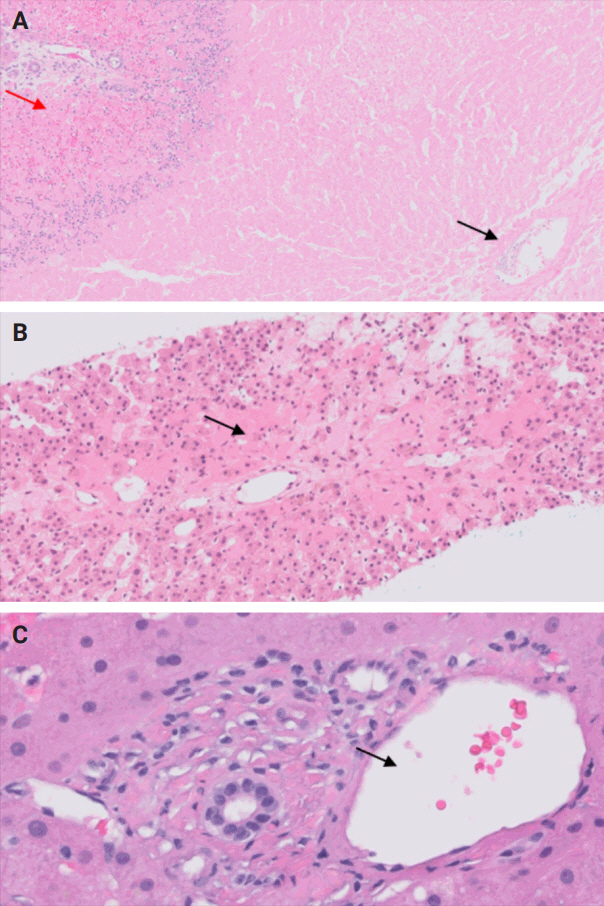
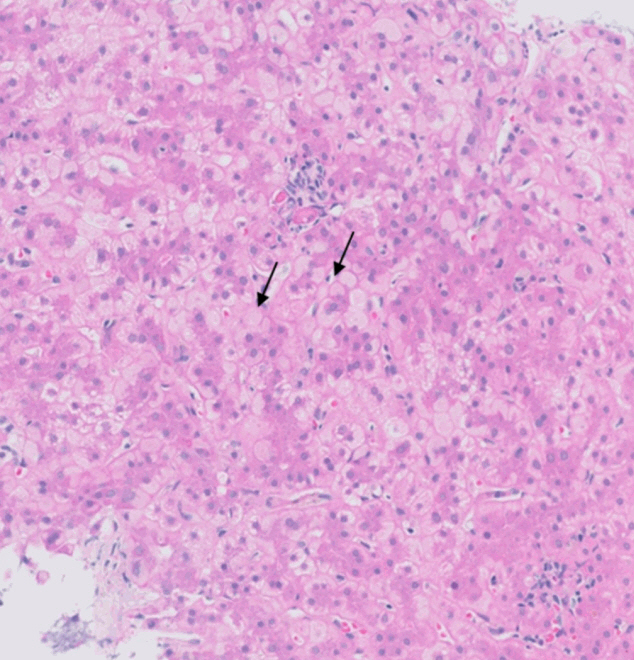
- During liver transplantation, precise vascular and biliary anastomoses are crucial for ensuring the proper function and integration of the transplanted liver into the recipient’s body. However, these anastomoses carry the risk of obstruction and failure. Therefore, it is necessary to assess for obstruction or thrombosis of the hepatic artery, hepatic vein, portal vein, and bile ducts when evaluating a post-transplant liver. Table 3 summarizes the histologic features associated with these clinical scenarios. Additionally, the restoration of blood flow to the transplanted liver after ischemia can lead to liver damage known as reperfusion injury [18]. Table 3 also outlines the histologic characteristics of reperfusion injury [19-23]. All the histologic characteristics are also illustrated in Figs. 6 and 7.
STEP 4: BLOOD AND BILE FLOW OBSTRUCTION
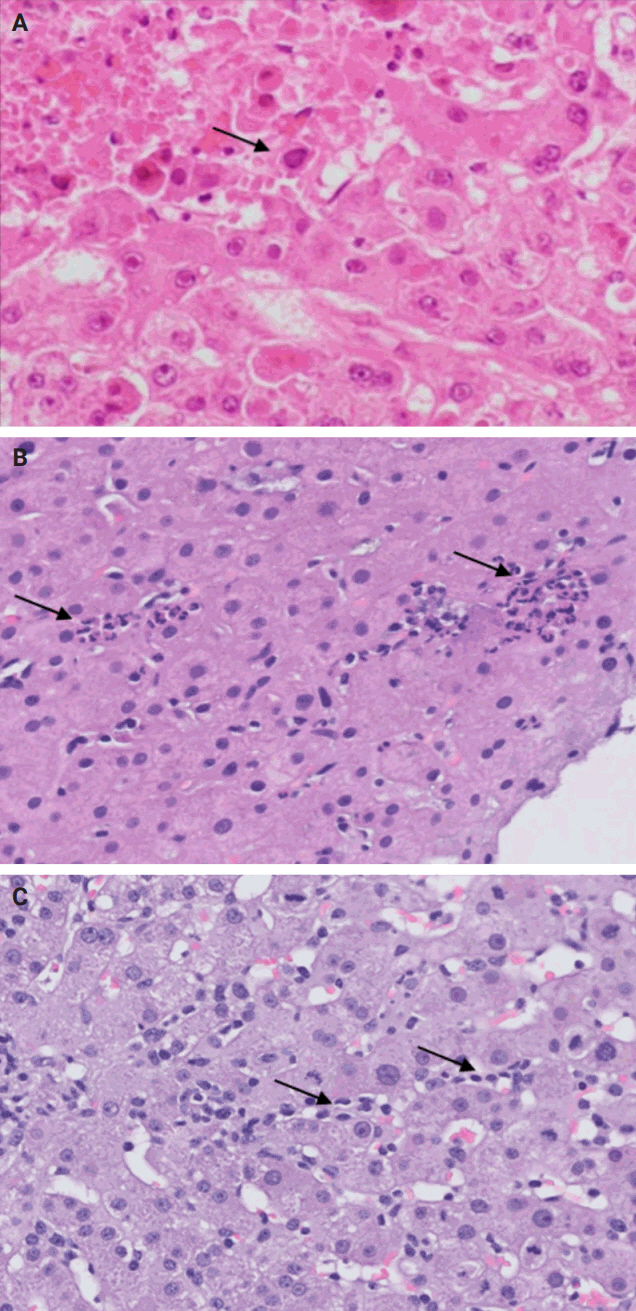
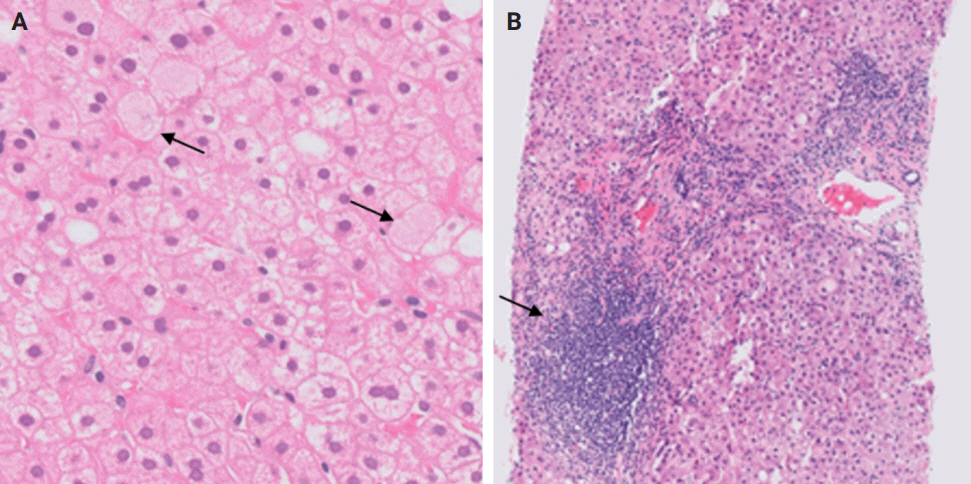
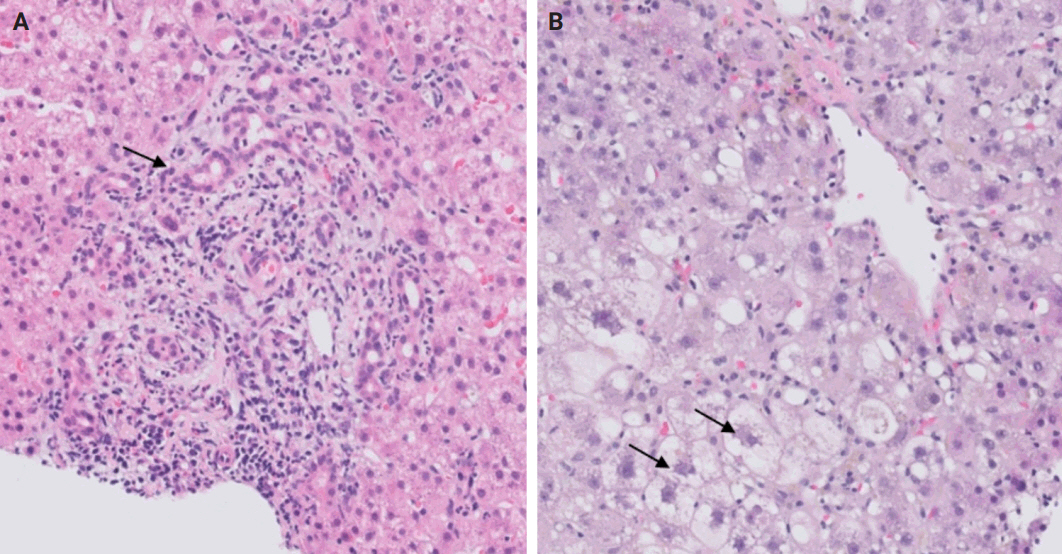
- Following liver transplantation, patients receive immunosuppressive therapy, increasing their susceptibility to various opportunistic infections and/or reactivation of dormant infections, such as cytomegalovirus (CMV), Epstein-Barr virus, and other opportunistic pathogens. Bacterial and fungal infections commonly affect organs other than the liver and often do not require biopsy. However, viral infections can directly involve the liver and may necessitate biopsy to distinguish them from rejection. During this step, the focus is on identifying histologic features specific to non-hepatotropic viral infections, which could also be confirmed by immunostaining. Table 4 provides a summary of the histologic characteristics observed in viral infections following liver transplant [24,25]. Some of these features are shown in Fig. 8.
STEP 5: OPPORTUNISTIC NON-HEPATOTROPIC VIRAL INFECTIONS
- Following liver transplantation, patients receive immunosuppressive therapy, medical prophylaxis as well as ongoing treatment for existing comorbidities. Each of these medications have varying degrees of likelihood to cause drug-induced liver injury (DILI) and should be considered as potential offending agents when developing your differential diagnosis. However, diagnosing DILI is challenging due to the diverse histopathological patterns observed, which may overlap with patterns seen in other post-transplant diseases. During this step, it's important to review the patient's medication list to identify any drugs that match any pathognomonic histologic findings observed. The inclusion of DILI in the differential diagnosis may also be reasonable if the histologic findings cannot be explained by other steps in this evaluation process.
- Keep in mind that patients typically receive prophylactic antimicrobial medications and immunosuppressants in the postoperative period, many of which can induce DILI. In terms of antiviral medications, valganciclovir (Valcyte) is indicated for CMV prophylaxis for the first 3–6 months post-transplantation in all patients [26]. For antibiotic coverage, prophylaxis against Pneumocystis jirovecii pneumonia is typically administered for 12 months after transplantation [26]. Trimethoprim/sulfamethoxazole is the most common medication used; however, atovaquone or dapsone can be used as an alternative in patients with a sulfa allergy [26]. In patients with latent tuberculosis, prophylactic isoniazid is warranted for 9 months duration [26]. Finally, fungal prophylaxis is indicated in low-risk patients for 1 month after transplant but may be used for longer duration in higher risk patients [26]. This is typically accomplished with clotrimazole, fluconazole, or itraconazole [26]. Commonly used medications for immunosuppression and antimicrobial prophylaxis, along with their likelihood of causing DILI, are listed in Table 5.
- A rare manifestation of DILI is the development of pseudo-ground-glass inclusions, which can occur with immunosuppressive therapy. These inclusions can mimic the appearance of hepatitis B inclusions but will be negative for hepatitis B virus surface antigen [28]. These inclusions are highlighted in Fig. 9.
STEP 6: DRUG-INDUCED LIVER INJURY
- Evaluation of post-transplant liver biopsies presents a unique challenge in pathology training, influenced significantly by institutional exposure and the presence of specialized liver transplant programs. By emphasizing key diagnostic considerations such as de novo diseases, disease recurrence, rejection, vascular complications, viral infections, and DILI, our structured, step-by-step approach outlined in this review aims to facilitate a systematic interpretation of these biopsies, bridging the knowledge gap for pathologists at various stages of their careers.
CONCLUSION
Ethics Statement
Institutional Review Board approval was waived due to the use of retrospective, de-identified data. This study adhered to the guidelines enacted by the Office of Human Research Protection that is supported by the U.S. Department of Health & Human Services. All information included in this report is de-identified and no personal details that may be used to identify the patient are included in this report.
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Code Availability
Not applicable.
Author Contributions
Conceptualization: TEJ. Investigation: DH, MBO, TEJ, MS. Project administration: TEJ, MBO, DH, MS. Resources: TEJ. Supervision: TEJ. Visualization: TEJ, MBO, DH. Writing—original draft: MBO, DH, MS. Writing—review & editing: TEJ, DH, MBO, MS. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to disclose.
Acknowledgments
Hwa Jeong Lee, MD for contributing a case of herpes simplex virus hepatitis. Maria Isabel Fiel, MD for contributing a case of chronic rejection with foam cell arteriopathy.
| Disease | Characteristic histologic findings |
|---|---|
| Autoimmune hepatitis | Plasma cell-rich portal inflammation, interface activity, apoptotic hepatocytes, and lobular hepatitis [10] |
| Primary biliary cholangitis | Lymphocytes-predominant portal inflammation, damaged bile ducts with intraepithelial lymphocytes and epithelioid granulomas [7,10] |
| Primary sclerosing cholangitis | Edematous portal tracts with bile ductular reaction and neutrophils (early stages) fibrous cholangitis (late stages) [10] |
| Alcoholic and non-alcoholic steatohepatitis | Steatosis with lobular inflammation and ballooning degeneration with Mallory-Denk bodies [11] |
| Alpha-1 antitrypsin deficiency | Presence of intrahepatocytic alpha-1 antitrypsin globules visible on PAS-D stain [4] |
| Hereditary hemochromatosis | Panacinar intrahepatocytic deposition of iron visible on Prussian blue stain [13] |
| Wilson’s disease | Panacinar intrahepatocytic deposition of copper visible on Rhodanin stain [14] |
| Hepatitis B virus hepatitis | Lymphocytes-predominant portal inflammation, interface activity, and ground glass hepatocytes inclusion [12] |
| Hepatitis C virus hepatitis | Lymphocytes-predominant portal inflammation, interface activity, and lymphoid follicles formation [12] |
| Type of rejection | Characteristic histologic findings |
|---|---|
| Acute cellular rejection | Lymphocytes-rich portal inflammation, bile duct injury, and venous endothelial inflammation (endotheliitis) [15,16] |
| Chronic ductopenic rejection | Ductopenia (more than half of the portal tracts without native bile ducts), bile ductular reaction, and metaplastic hepatocytes (best seen on CK7 immunostain) [15,16] |
| Chronic rejection with foam cell arteriopathy | Arteriolar damage with accumulation of foam cells within the vessel walls [15,16] |
| Acute antibody-mediated rejection | Portal microvascular injury including microvascular endothelial cell enlargement, microvasculitis/capillaritis, capillary dilatation, and microvascular disruption [15,16] |
| Chronic active antibody-mediated rejection | Mononuclear portal or perivenular inflammation, with interface or perivenular necroinflammatory activity and at least moderate portal/periportal, sinusoidal or perivenular fibrosis [15,16] |
| Plasma cell-rich rejection | Plasma cell-rich (>30%) portal infiltrate or central infiltrate, with interface or perivenular necroinflammatory activity in patients without history of autoimmune hepatitis [15,16] |
| Complication | Characteristic histologic findings |
|---|---|
| Hepatic artery thrombosis | Centrilobular (zone 3) necrosis and/or localized coagulative necrosis (could involve zone 1 and zone 2) and possible subsequent ischemic cholangiopathy [19,20] |
| Hepatic vein thrombosis | Centrilobular (zone 3) congestion and/or necrosis [21] |
| Portal vein thrombosis | Sinusoidal dilatation, portal veins dystrophy (dilatation, occlusion, and herniation), and possible centrilobular (zone 3) necrosis [22] |
| Bile duct obstruction | Portal tracts edema with bile ductular reaction and neutrophils [20] |
| Reperfusion injury | Centrilobular (zone 3) hepatocytes ballooning, cholestasis, and neutrophilic infiltrate with possible subsequent centrilobular (zone 3) hepatocyte necrosis [23] |
| Virus | Characteristic histologic findings |
|---|---|
| Cytomegalovirus | Scattered foci of apoptotic hepatocytes with neutrophilic microabscesses and rare nuclear inclusions [24] |
| Human simplex virus | Non-zonal necrosis of the hepatocytes with viral cytopathic effect in the remaining hepatocytes [24] |
| Epstein-Barr virus | Prominent sinusoidal mildly atypical lymphocytic infiltrate with rare hepatocytic/parenchymal necrosis [24] |
| Adenovirus | Non-zonal necrosis with smudge cells (cells with basophilic nuclei and indistinct chromatin) [25] |
| Medication | Likelihood scorea of DILI [27] |
|---|---|
| Immunosuppressants | |
| Glucocorticoids | A |
| Calcineurin inhibitors | C |
| Everolimus | E |
| Mycophenolate | D |
| Sirolimus | C |
| Azathioprine | A |
| Anti-thymocyte globulin | D |
| Basiliximab | E |
| Antimicrobial prophylaxis | |
| Valganciclovir | C |
| Valacyclovir | D |
| Trimethoprim-sulfamethoxazole | A |
| Atovaquone | D |
| Dapsone | A |
| Clotrimazole | E |
| Fluconazole | B |
| Itraconazole | B |
| Isoniazid | A |
DILI, drug-induced liver injury; NIH, National Institutes of Health.
aLikelihood scoring: A, well established cause of liver injury; B, highly likely case of liver injury; C, probable rare cause of clinically apparent liver injury; D, possible rare cause of clinically apparent liver injury; E, unproven and also unlikely cause of clinically apparent liver injury.
- 1. Wu F, Wang J, Pu C, Qiao L, Jiang C. Wilson's disease: a comprehensive review of the molecular mechanisms. Int J Mol Sci 2015; 16: 6419-31. ArticlePubMedPMC
- 2. Catana AM, Medici V. Liver transplantation for Wilson disease. World J Hepatol 2012; 4: 5-10. ArticlePubMedPMC
- 3. Fromme M, Schneider CV, Trautwein C, Brunetti-Pierri N, Strnad P. Alpha-1 antitrypsin deficiency: a re-surfacing adult liver disorder. J Hepatol 2022; 76: 946-58. ArticlePubMed
- 4. Mitchell EL, Khan Z. Liver disease in alpha-1 antitrypsin deficiency: current approaches and future directions. Curr Pathobiol Rep 2017; 5: 243-52. ArticlePubMedPMCPDF
- 5. Dobrindt EM, Keshi E, Neulichedl J, et al. Long-term outcome of orthotopic liver transplantation in patients with hemochromatosis: a summary of a 30-year transplant program. Transplant Direct 2020; 6: e560. ArticlePubMedPMC
- 6. Shubin AD, De Gregorio L, Hwang C, MacConmara M. Combined heart-liver transplantation in a case of haemochromatosis. BMJ Case Rep 2021; 14: e241508. ArticlePubMedPMC
- 7. Schreibman I, Regev A. Recurrent primary biliary cirrhosis after liver transplantation: the disease and its management. MedGenMed 2006; 8: 30.
- 8. Visseren T, Erler NS, Polak WG, et al. Recurrence of primary sclerosing cholangitis after liver transplantation: analysing the European Liver Transplant Registry and beyond. Transpl Int 2021; 34: 1455-67. PubMed
- 9. Harputluoglu M, Caliskan AR, Akbulut S. Autoimmune hepatitis and liver transplantation: Indications, and recurrent and de novo autoimmune hepatitis. World J Transplant 2022; 12: 59-64. ArticlePubMedPMC
- 10. Faisal N, Renner EL. Recurrence of autoimmune liver diseases after liver transplantation. World J Hepatol 2015; 7: 2896-905. ArticlePubMedPMC
- 11. Shetty A, Giron F, Divatia MK, Ahmad MI, Kodali S, Victor D. Nonalcoholic fatty liver disease after liver transplant. J Clin Transl Hepatol 2021; 9: 428-35. PubMedPMC
- 12. Jothimani D, Venugopal R, Vij M, Rela M. Post liver transplant recurrent and de novo viral infections. Best Pract Res Clin Gastroenterol 2020; 46-47: 101689.ArticlePubMedPMC
- 13. Salomao MA. Pathology of hepatic iron overload. Clin Liver Dis (Hoboken) 2021; 17: 232-7. ArticlePubMedPMCPDF
- 14. Mazhar A, Piper MS. Updates on Wilson disease. Clin Liver Dis (Hoboken) 2023; 22: 117-21. ArticlePubMedPMC
- 15. Choudhary NS, Saigal S, Bansal RK, Saraf N, Gautam D, Soin AS. Acute and chronic rejection after liver transplantation: what a clinician needs to know. J Clin Exp Hepatol 2017; 7: 358-66. ArticlePubMedPMC
- 16. Demetris AJ, Bellamy C, Hubscher SG, et al. 2016 Comprehensive update of the Banff Working Group on Liver Allograft Pathology: introduction of antibody-mediated rejection. Am J Transplant 2016; 16: 2816-35. PubMed
- 17. Lee BT, Fiel MI, Schiano TD. Antibody-mediated rejection of the liver allograft: an update and a clinico-pathological perspective. J Hepatol 2021; 75: 1203-16. ArticlePubMed
- 18. Rampes S, Ma D. Hepatic ischemia-reperfusion injury in liver transplant setting: mechanisms and protective strategies. J Biomed Res 2019; 33: 221-34. ArticlePubMedPMC
- 19. Lee M, Agostini-Vulaj D, Gonzalez RS. Evaluation of histologic changes in the livers of patients with early and late hepatic artery thrombosis. Hum Pathol 2019; 90: 8-13. ArticlePubMed
- 20. Gupta NB. Liver biopsy made easy. New Delhi: Jaypee Brothers Medical Publishers, 2017.
- 21. Nakhleh RE. Pathology and differential diagnosis of hepatic venous outflow impairment. Clin Liver Dis (Hoboken) 2017; 10: 49-52. ArticlePubMedPMCPDF
- 22. Terada T, Hoso M, Nakanuma Y. Microvasculature in the small portal tracts in idiopathic portal hypertension: a morphological comparison with other hepatic diseases. Virchows Arch A Pathol Anat Histopathol 1989; 415: 61-7. ArticlePubMed
- 23. Geramizadeh B, Hassani M, Kazemi K, Shamsaifar AR, Malek-Hosseini SA. Value of histopathologic findings of post-reperfusion liver needle biopsies. Int J Organ Transplant Med 2018; 9: 168-72. PubMedPMC
- 24. Dancygier H. Viral infections by nonhepatotropic viruses. In: Dancygier H, ed. Clinical hepatology. Berlin: Springer, 2010; 823-30.
- 25. Zheng N, Wang Y, Rong H, Wang K, Huang X. Human adenovirus associated hepatic injury. Front Public Health 2022; 10: 878161.ArticlePubMedPMC
- 26. Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19: 3-26. ArticlePubMedPDF
- 27. Livertox: clinical and research information on drug-induced liver injury [Internet]. Bethesda: National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [cited 2024 Jul 31]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK547852/.
- 28. Fischer AK, Baba H, Lainka E, et al. Rapid development of pseudo-ground-glass bodies in liver transplants. Histopathology 2024; 85: 190-2. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Histological and Molecular Evaluation of Liver Biopsies: A Practical and Updated Review
Joon Hyuk Choi
International Journal of Molecular Sciences.2025; 26(16): 7729. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

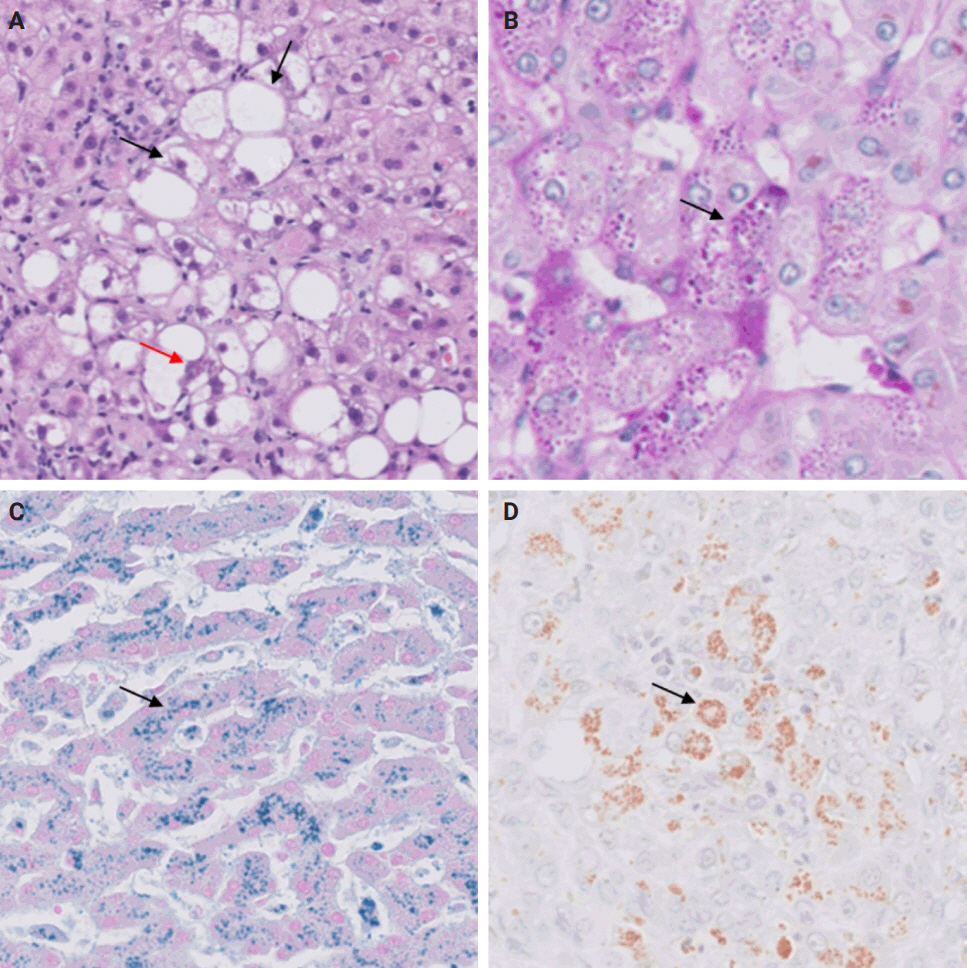
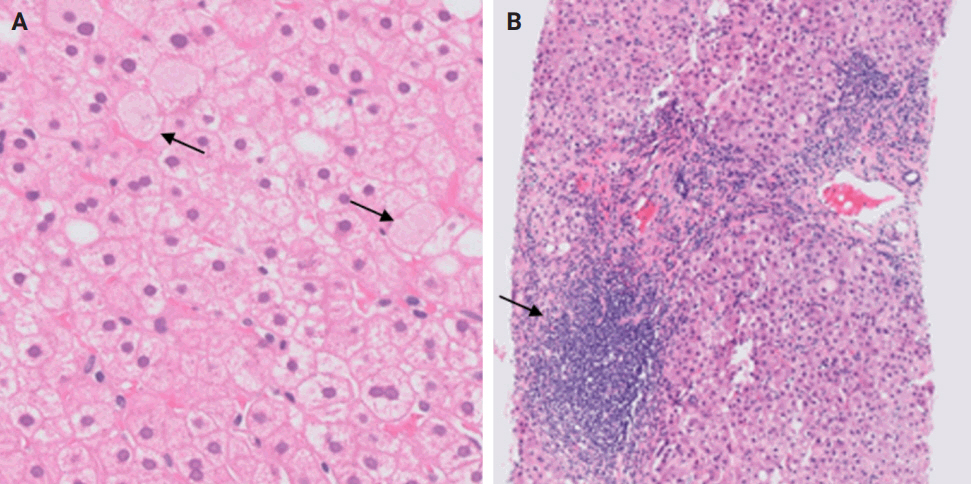
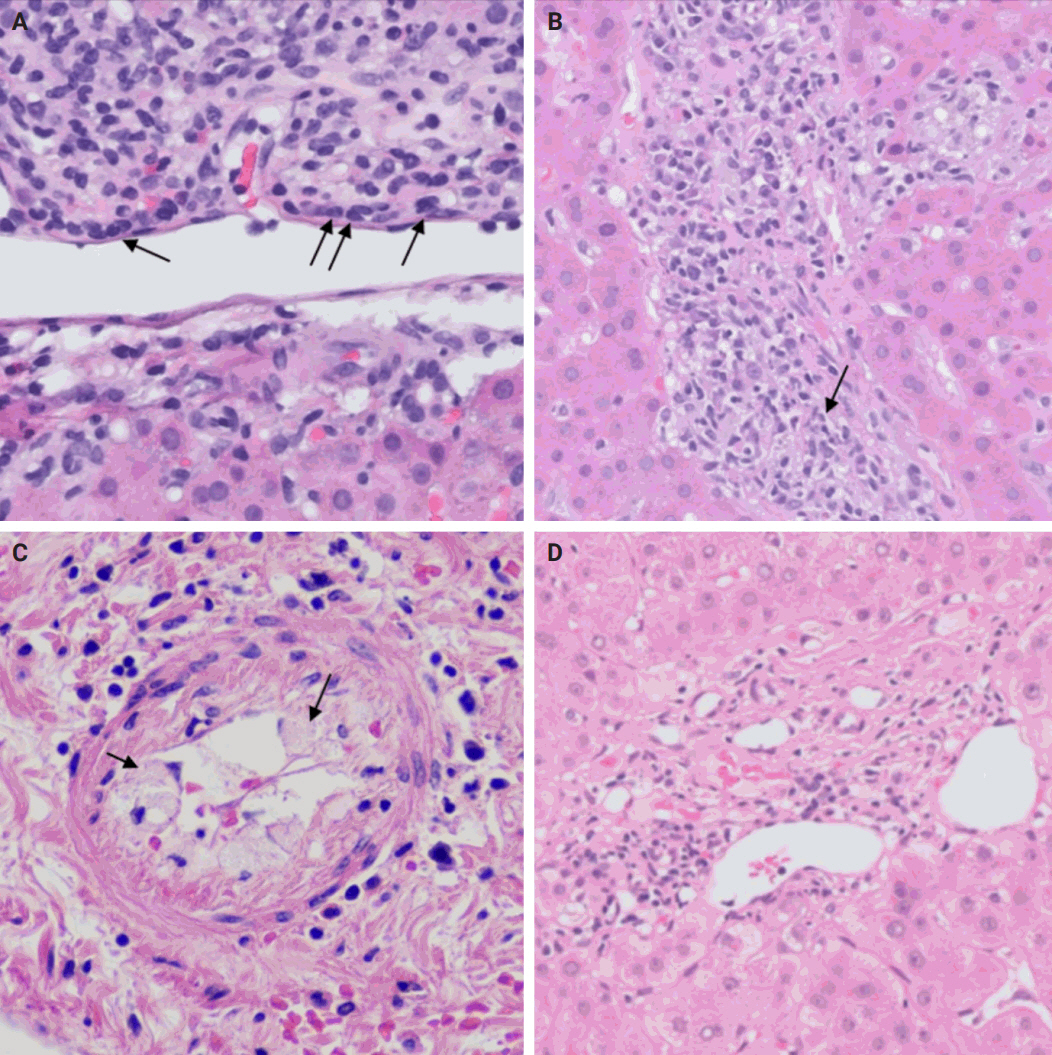
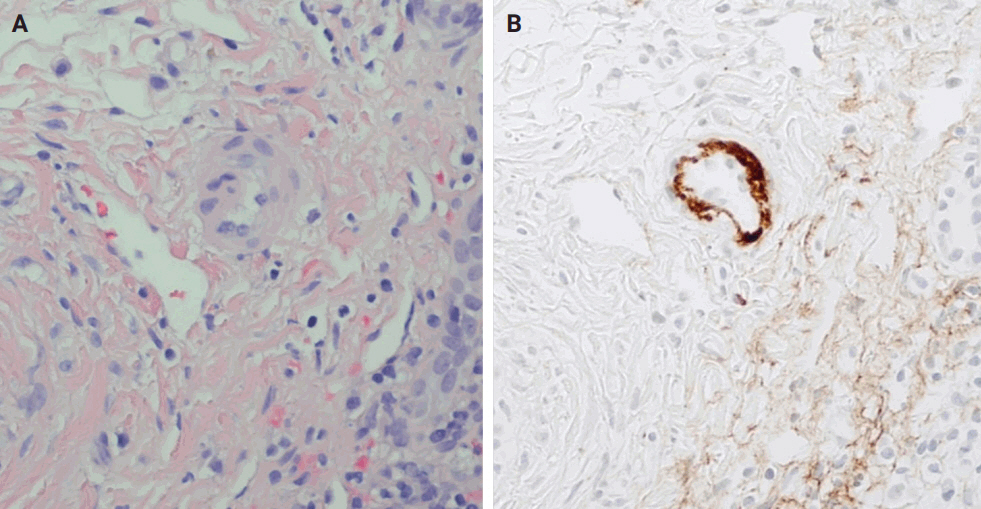
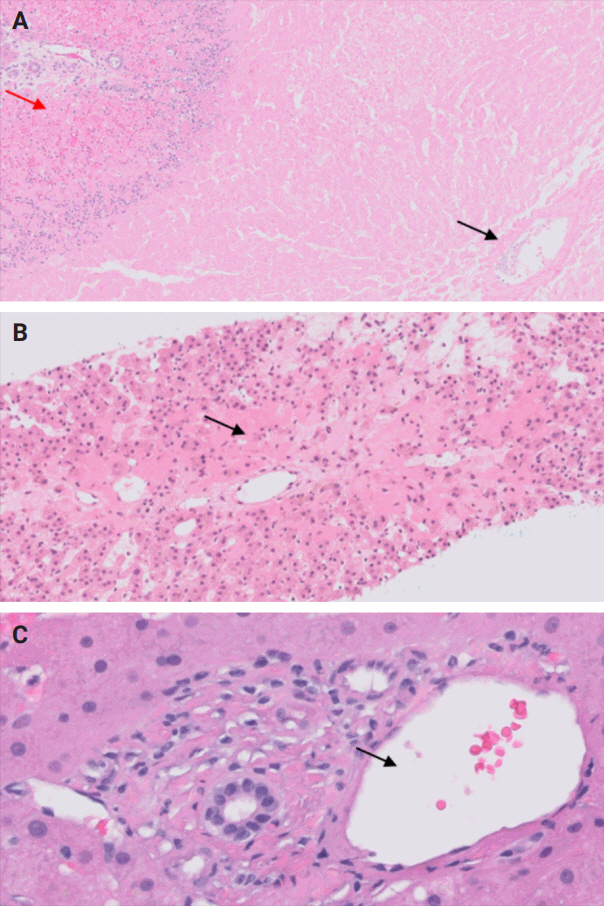
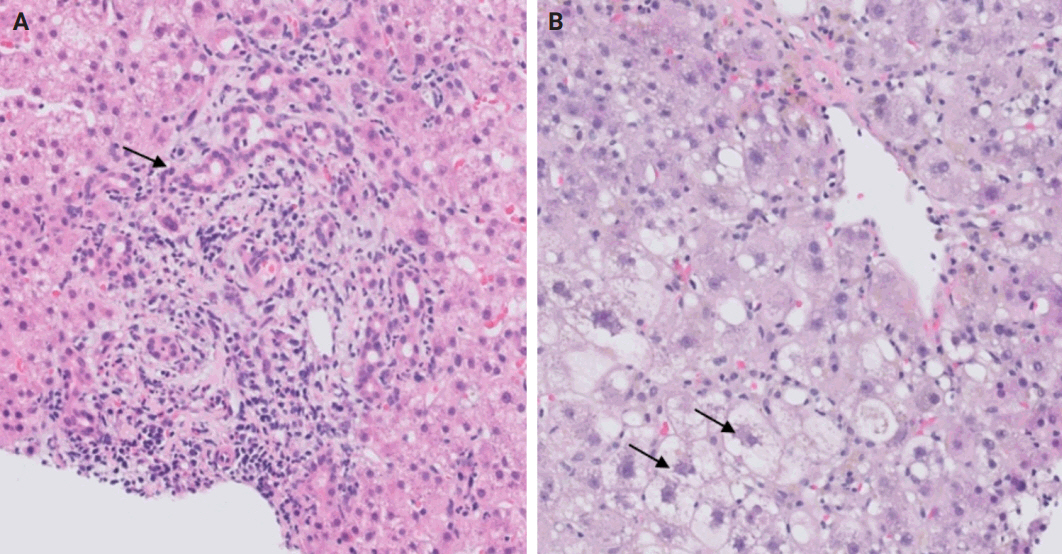
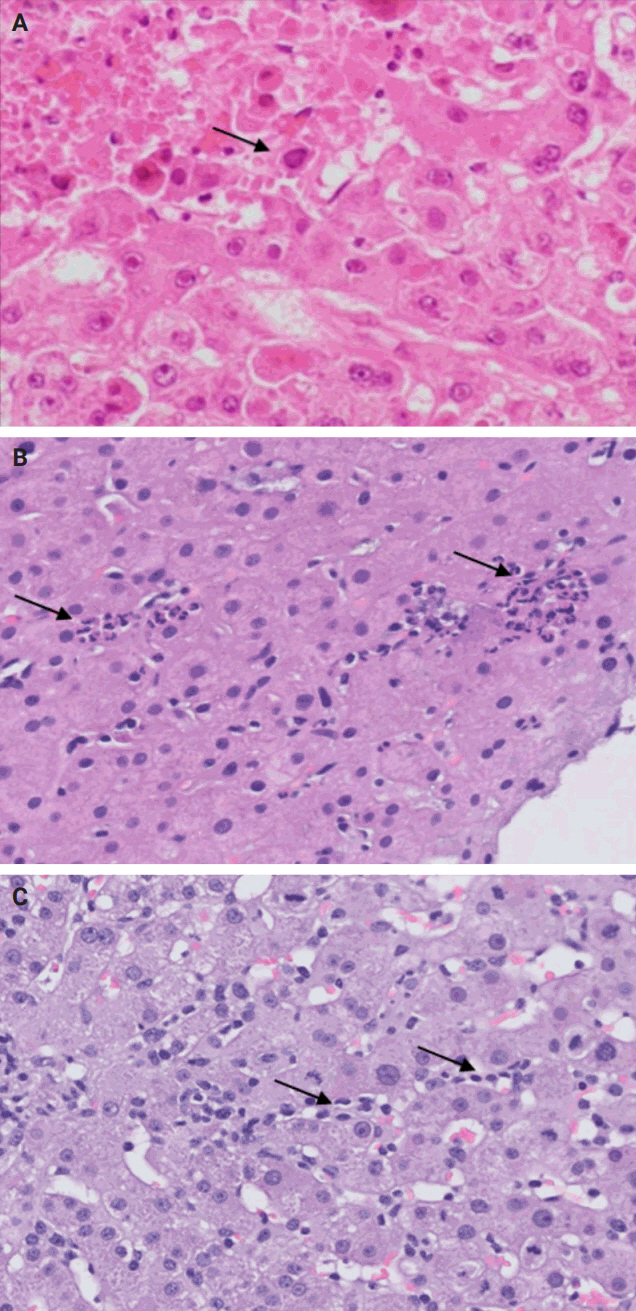
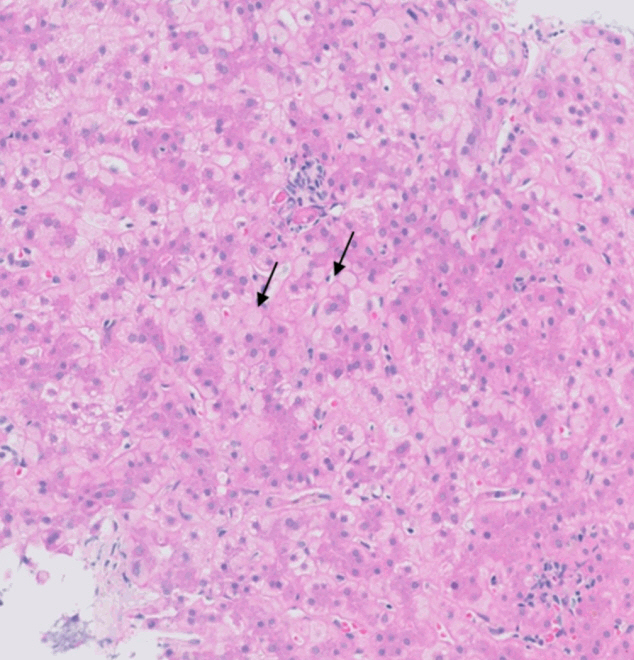
Fig. 1.
Fig. 2.
Fig. 3.
Fig. 4.
Fig. 5.
Fig. 6.
Fig. 7.
Fig. 8.
Fig. 9.
| Disease | Characteristic histologic findings |
|---|---|
| Autoimmune hepatitis | Plasma cell-rich portal inflammation, interface activity, apoptotic hepatocytes, and lobular hepatitis [10] |
| Primary biliary cholangitis | Lymphocytes-predominant portal inflammation, damaged bile ducts with intraepithelial lymphocytes and epithelioid granulomas [7,10] |
| Primary sclerosing cholangitis | Edematous portal tracts with bile ductular reaction and neutrophils (early stages) fibrous cholangitis (late stages) [10] |
| Alcoholic and non-alcoholic steatohepatitis | Steatosis with lobular inflammation and ballooning degeneration with Mallory-Denk bodies [11] |
| Alpha-1 antitrypsin deficiency | Presence of intrahepatocytic alpha-1 antitrypsin globules visible on PAS-D stain [4] |
| Hereditary hemochromatosis | Panacinar intrahepatocytic deposition of iron visible on Prussian blue stain [13] |
| Wilson’s disease | Panacinar intrahepatocytic deposition of copper visible on Rhodanin stain [14] |
| Hepatitis B virus hepatitis | Lymphocytes-predominant portal inflammation, interface activity, and ground glass hepatocytes inclusion [12] |
| Hepatitis C virus hepatitis | Lymphocytes-predominant portal inflammation, interface activity, and lymphoid follicles formation [12] |
| Type of rejection | Characteristic histologic findings |
|---|---|
| Acute cellular rejection | Lymphocytes-rich portal inflammation, bile duct injury, and venous endothelial inflammation (endotheliitis) [15,16] |
| Chronic ductopenic rejection | Ductopenia (more than half of the portal tracts without native bile ducts), bile ductular reaction, and metaplastic hepatocytes (best seen on CK7 immunostain) [15,16] |
| Chronic rejection with foam cell arteriopathy | Arteriolar damage with accumulation of foam cells within the vessel walls [15,16] |
| Acute antibody-mediated rejection | Portal microvascular injury including microvascular endothelial cell enlargement, microvasculitis/capillaritis, capillary dilatation, and microvascular disruption [15,16] |
| Chronic active antibody-mediated rejection | Mononuclear portal or perivenular inflammation, with interface or perivenular necroinflammatory activity and at least moderate portal/periportal, sinusoidal or perivenular fibrosis [15,16] |
| Plasma cell-rich rejection | Plasma cell-rich (>30%) portal infiltrate or central infiltrate, with interface or perivenular necroinflammatory activity in patients without history of autoimmune hepatitis [15,16] |
| Complication | Characteristic histologic findings |
|---|---|
| Hepatic artery thrombosis | Centrilobular (zone 3) necrosis and/or localized coagulative necrosis (could involve zone 1 and zone 2) and possible subsequent ischemic cholangiopathy [19,20] |
| Hepatic vein thrombosis | Centrilobular (zone 3) congestion and/or necrosis [21] |
| Portal vein thrombosis | Sinusoidal dilatation, portal veins dystrophy (dilatation, occlusion, and herniation), and possible centrilobular (zone 3) necrosis [22] |
| Bile duct obstruction | Portal tracts edema with bile ductular reaction and neutrophils [20] |
| Reperfusion injury | Centrilobular (zone 3) hepatocytes ballooning, cholestasis, and neutrophilic infiltrate with possible subsequent centrilobular (zone 3) hepatocyte necrosis [23] |
| Virus | Characteristic histologic findings |
|---|---|
| Cytomegalovirus | Scattered foci of apoptotic hepatocytes with neutrophilic microabscesses and rare nuclear inclusions [24] |
| Human simplex virus | Non-zonal necrosis of the hepatocytes with viral cytopathic effect in the remaining hepatocytes [24] |
| Epstein-Barr virus | Prominent sinusoidal mildly atypical lymphocytic infiltrate with rare hepatocytic/parenchymal necrosis [24] |
| Adenovirus | Non-zonal necrosis with smudge cells (cells with basophilic nuclei and indistinct chromatin) [25] |
| Medication | Likelihood score |
|---|---|
| Immunosuppressants | |
| Glucocorticoids | A |
| Calcineurin inhibitors | C |
| Everolimus | E |
| Mycophenolate | D |
| Sirolimus | C |
| Azathioprine | A |
| Anti-thymocyte globulin | D |
| Basiliximab | E |
| Antimicrobial prophylaxis | |
| Valganciclovir | C |
| Valacyclovir | D |
| Trimethoprim-sulfamethoxazole | A |
| Atovaquone | D |
| Dapsone | A |
| Clotrimazole | E |
| Fluconazole | B |
| Itraconazole | B |
| Isoniazid | A |
PAS-D, periodic acid–Schiff–diastase.
CK7, cytokeratin 7.
DILI, drug-induced liver injury; NIH, National Institutes of Health. aLikelihood scoring: A, well established cause of liver injury; B, highly likely case of liver injury; C, probable rare cause of clinically apparent liver injury; D, possible rare cause of clinically apparent liver injury; E, unproven and also unlikely cause of clinically apparent liver injury.

 E-submission
E-submission