Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(3); 2025 > Article
-
Case Study
Histopathological characteristics of Epstein-Barr virus (EBV)–associated encephalitis and colitis in chronic active EBV infection -
Betty A Kasimo1
 , James J Yahaya2
, James J Yahaya2 , Sun Och Yoon3
, Sun Och Yoon3 , Se Hoon Kim3
, Se Hoon Kim3 , Minsun Jung3
, Minsun Jung3
-
Journal of Pathology and Translational Medicine 2025;59(3):188-194.
DOI: https://doi.org/10.4132/jptm.2025.02.21
Published online: April 16, 2025
1Department of Pathology, Faculty of Health Sciences, Busitema University, Mbale, Uganda
2Department of Pathology, School of Health Sciences, Soroti University, Soroti, Uganda
3Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- Corresponding Author: Minsun Jung, MD, PhD Department of Pathology, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea Tel: +82-2-2228-1771, Fax: +82-2-362-0860, E-mail: jjunglammy@gmail.com
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 3,990 Views
- 164 Download
Abstract
- Chronic active Epstein-Barr virus (CAEBV) can induce complications in various organs, including the brain and gastrointestinal tract. A 3-year-old boy was referred to the hospital with a history of fever and seizures for 15 days. A diagnosis of encephalitis based on computed tomography (CT) and magnetic resonance imaging findings and clinical correlation was made. Laboratory tests showed positive serology for Epstein-Barr virus (EBV) and negative for Rotavirus antigen and IgG and IgM antibodies for cytomegalovirus, herpes simplex virus, and varicella zoster virus, respectively. Abdominal CT showed diffuse wall thickening with fluid distension of small bowel loops, lower abdomen wall thickening, and a small amount of ascites. The biopsy demonstrated positive Epstein-Barr encoding region in situ hybridization in cells within the crypts and lamina propria. The patient was managed with steroids and hematopoietic stem cell transplantation (HSCT). This case showed histopathological characteristics of concurrent EBV-associated encephalitis and colitis in CAEBV infection. The three-step strategy of immunosuppressive therapy, chemotherapy, and allogeneic HSCT should be always be considered for prevention of disease progression.
- Epstein-Barr virus (EBV), a double-stranded DNA virus categorized within the Herpesviridae family, causes a ubiquitous infection in more than 90% of the world’s population [1,2]. EBV is usually acquired during childhood or adolescence and then establishes a permanent latent infection in B lymphocytes in immunocompetent patients [3-5]. Chronic active EBV (CAEBV) infection is a rare lymphoproliferative disorder characterized by persistent infectious mononucleosis-like symptoms for more than 3 months, increased EBV DNA (>102.5 copies/mg) in peripheral blood, and organ involvement in an immunocompetent patient [6-8]. Various organs can be affected in CAEBV infection and they show a spectrum of manifestations. The ill-defined, diverse clinicopathological characteristics of CAEBV infection often delay the diagnosis and treatment [1,9,10]. In this study, we report a 3-year-old patient with CAEBV infection, who was confirmed to have both EBV encephalitis and EBV colitis through pathological examination. The diagnosis of EBV encephalitis and EBV colitis can be a challenge in pathological practice because of the deceptive morphologies. Using microscopic examination and in situ hybridization technique, we diagnosed EBV infection in both the brain and colon tissue, resulting in successful diagnosis and treatment.
INTRODUCTION
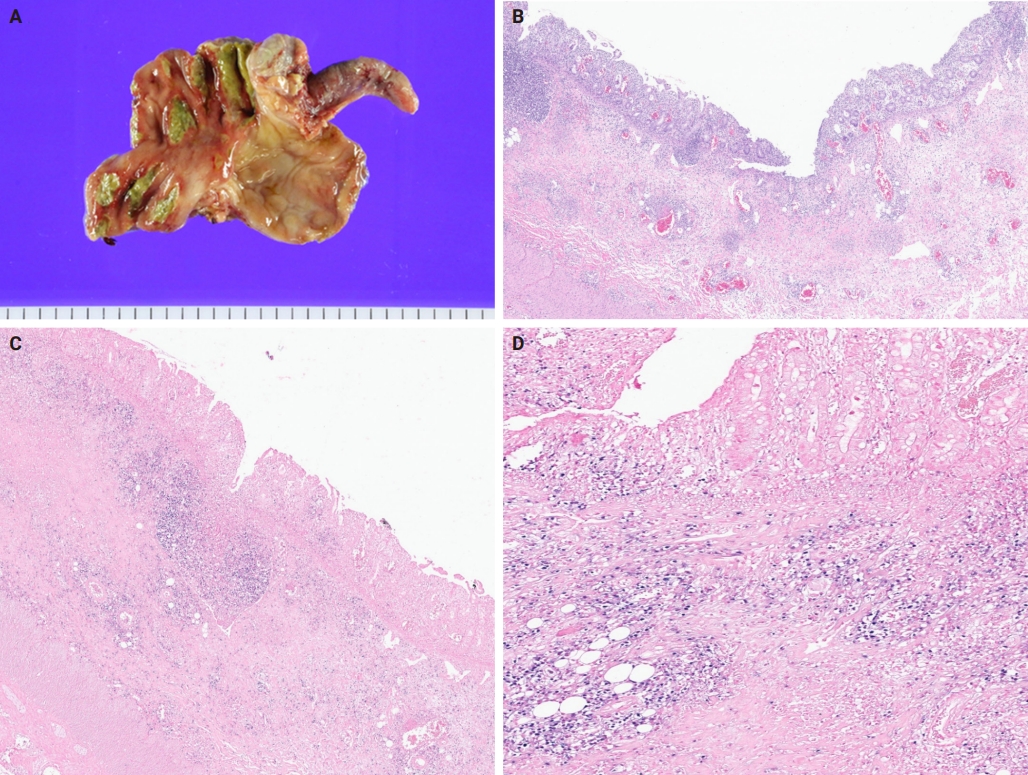
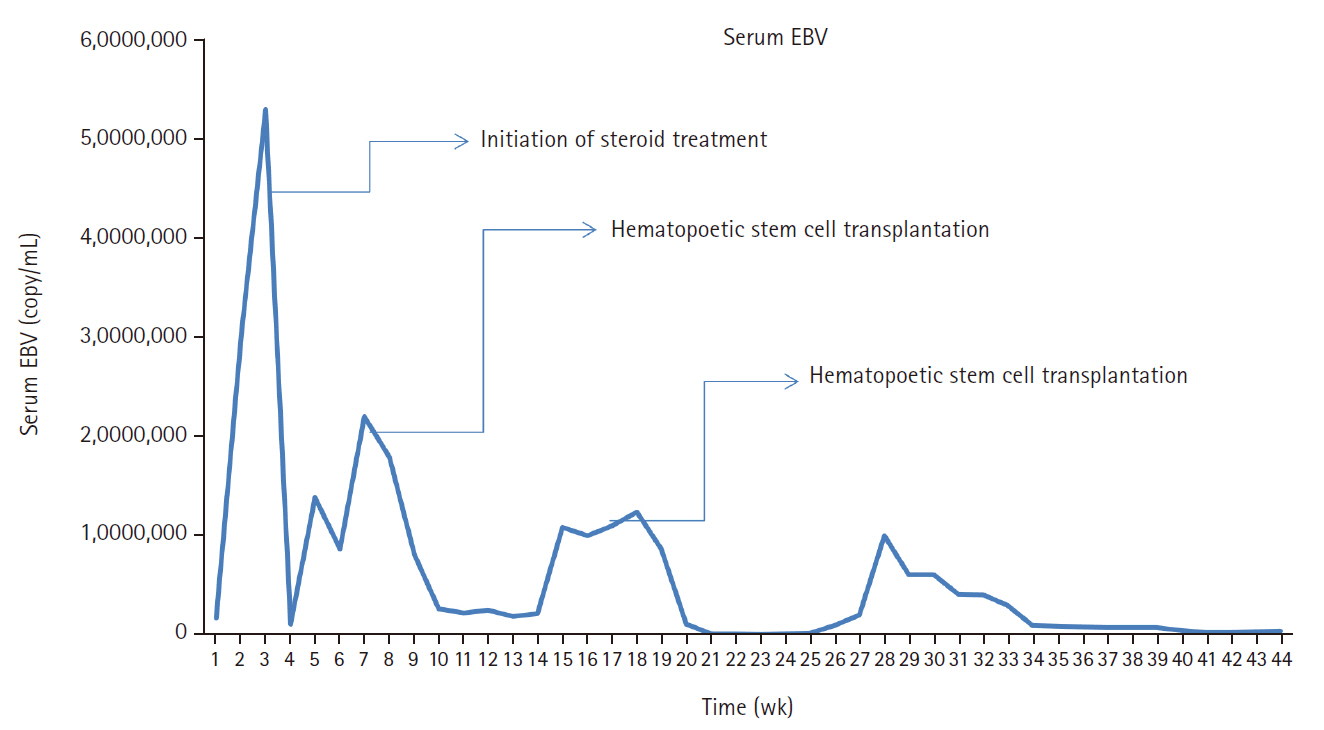
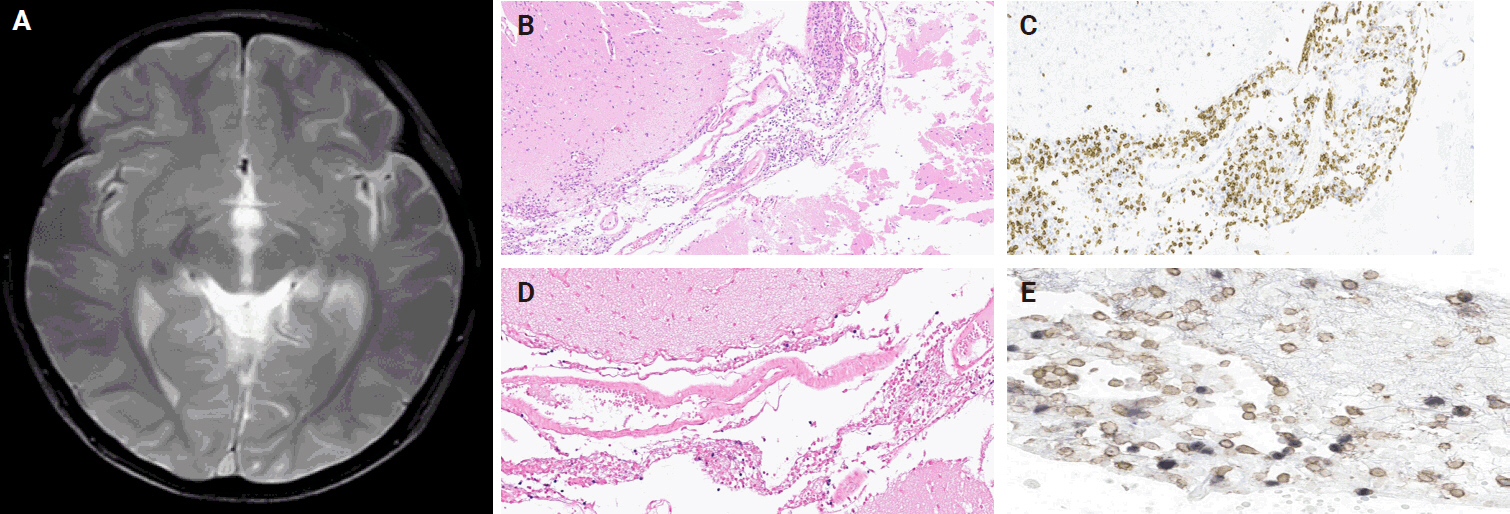
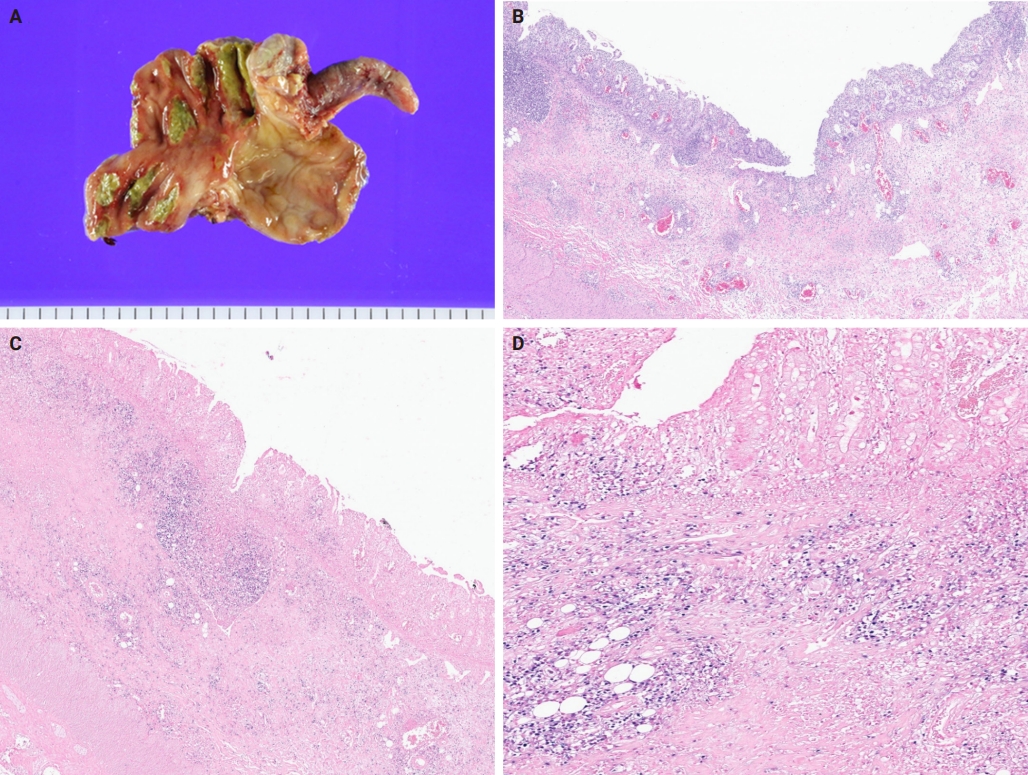
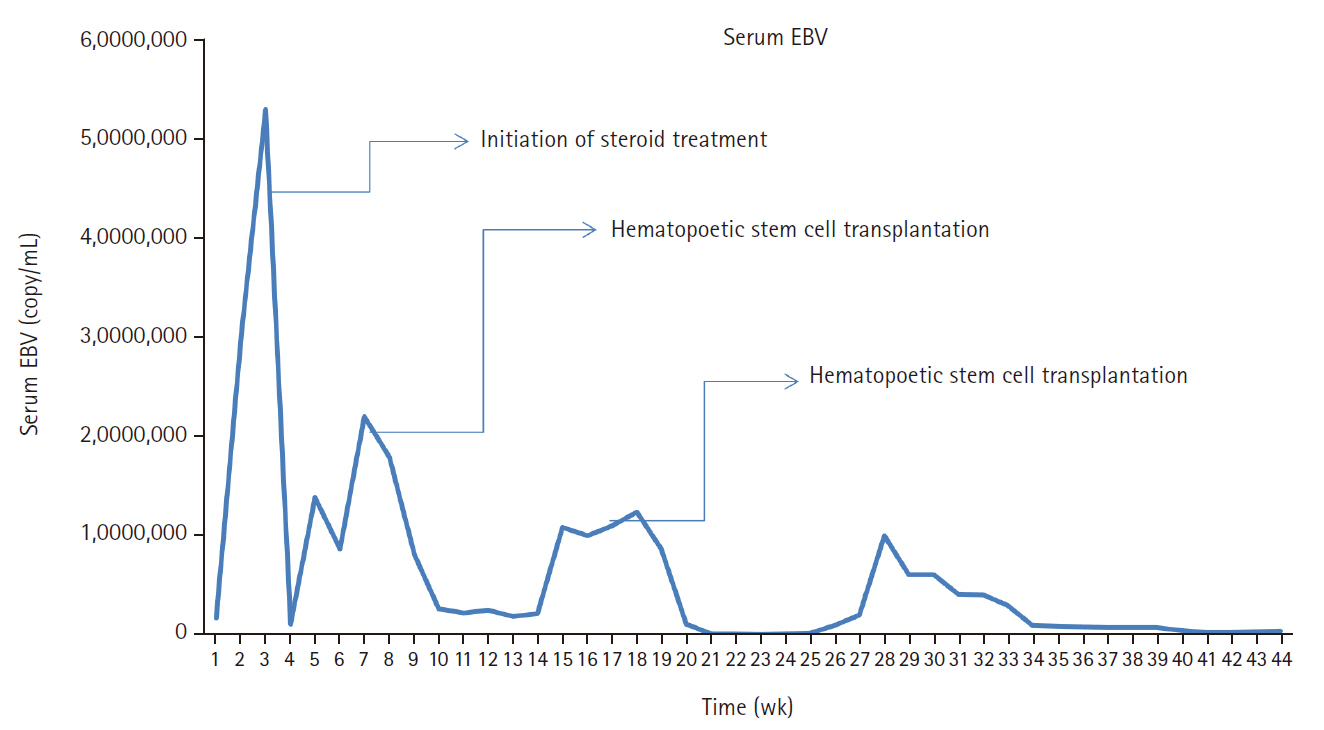
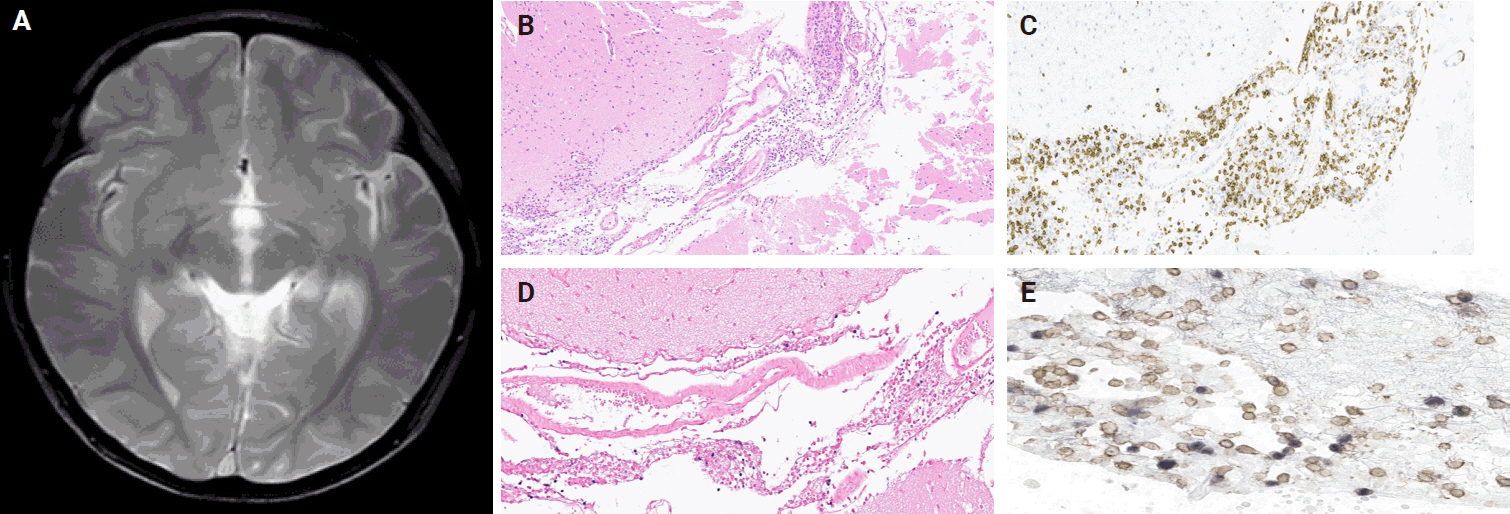
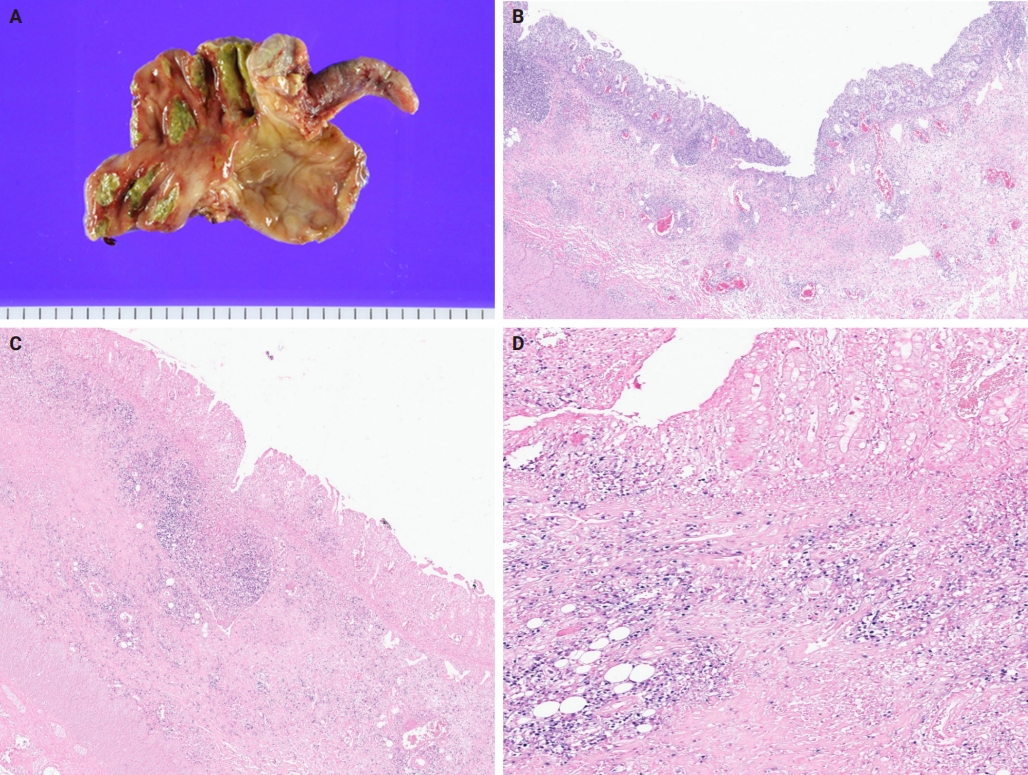
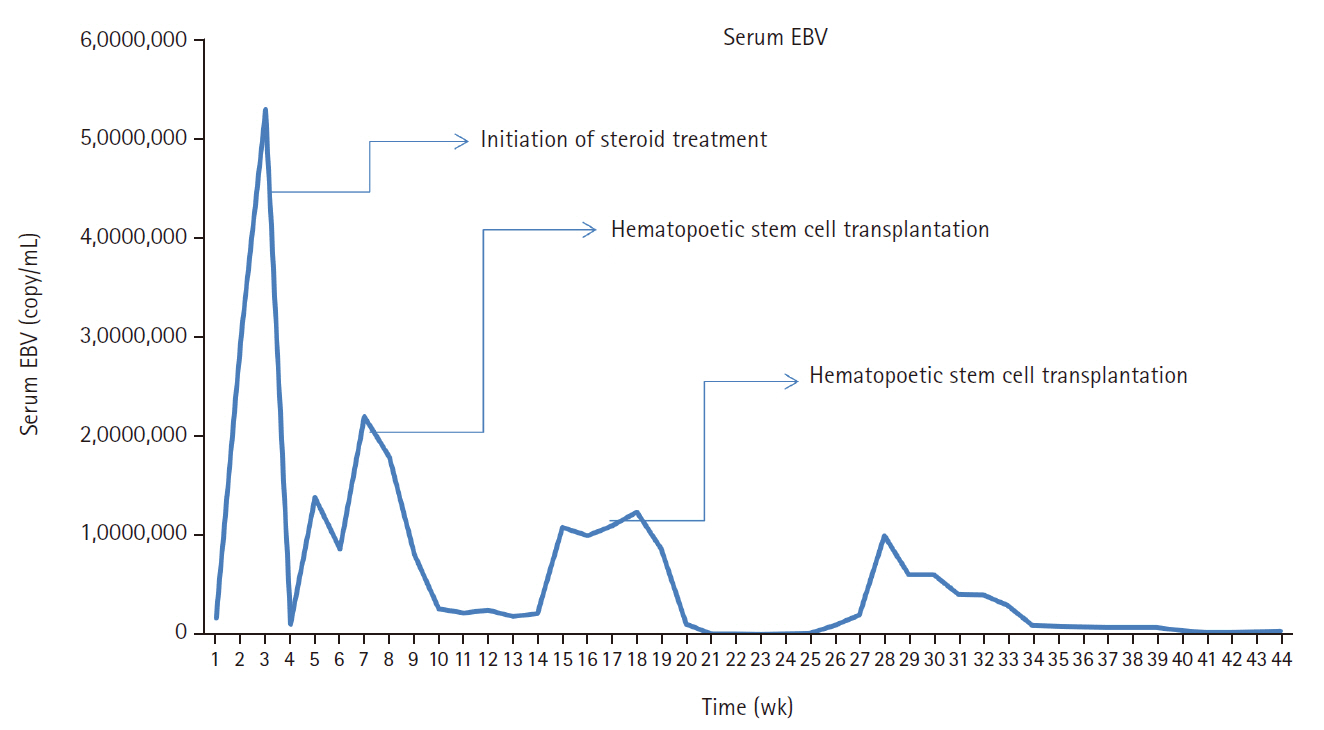
- A 3-year-old boy was admitted to the Severance Hospital (Seoul, Korea) with a history of fever and seizures for 15 days. The boy did not have any history of recurrent infections or family history of immune disease. Computed tomography (CT) and magnetic resonance imaging (MRI) showed swelling in the basal ganglia, thalamus, midbrain, pons, cerebellum, and left hypothalamus (Fig. 1A). The initial EBV level was elevated to 1,685,506 U/mL in the serum and 103,285 U/mL in the cerebrospinal fluid. In serology, anti–viral capsid antigen (VCA) IgM was undetected while anti-VCA IgG was positive which indicated chronic response to EBV. Although the patient was managed empirically with steroids, his mental status remained unstable. To confirm the diagnosis, brain biopsy was performed. The brain tissue sections showed focal perivascular and subarachnoid lymphocyte infiltration and focal microglial cell proliferation (Fig. 1B). However, CD3-positive T lymphocytes infiltrated the perivascular area (Fig. 1C) and these cells were positive to EBV in situ hybridization (Fig. 1D). Further testing revealed that CD3-positive cells expressed CD8 (Fig. 1E), but not CD4. Moreover, molecular analysis showed no evidence of T-cell receptor (TCR) beta or TCR gamma gene rearrangement. A week later, the patient developed perianal ulceration, diarrhea, and abdominal pain. Abdominal CT showed diffuse wall thickening and distension in the small bowel loops indicating high risk of perforation. The patient underwent ileocecectomy (Fig. 2A). The resected colon showed dense infiltration of lymphocytes, neutrophils, and plasma cells in the lamina propria and widespread neutrophilic cryptitis, crypt abscess, and distortion. The lymphocytes showed minimal atypia. Multifocal submucosal edema with serositis was prominent (Fig. 2B). Diagnosis of EBV-associated colitis was made based on Epstein-Barr encoding region (EBER) in situ hybridization of positive CD3 T lymphocytes that infiltrated the mucosa, submucosa, and intestinal walls (Fig. 2C, D). Immunohistochemical staining for CD56 showed a few scattered natural killer (NK) cells. The diagnosis of CAEBV also befits the patient. Germline testing using next-generation sequencing analysis revealed no pathogenic gene mutations associated with immune dysregulation. The human leukocyte antigen (HLA) typing results were as follows: HLA-A*02:06/02:06, -B*15:18/51:01, -C*08:01/14:02, -DRB1*04:05/14:05, -DQA1*01:04/03:03, -DQB1*04:01/05:03, -DPA1*01:03/02:02, and -DPB1*02:01/05:01. Full matched allogenic hematopoietic stem cell transplantation (HSCT) was performed 6 months after the initial diagnosis. EBV level decreased to 34,262 copies/mL in serum 12 months after treatment (Fig. 3).
CASE REPORT
- We herein report a patient with CAEBV infection showing both EBV encephalitis and colitis. It is an infection induced by infiltration of EBV-infected lymphocytes into the affected tissues [11,12]. EBV infects NK and T cells in CAEBV; T-cell CAEBV is further classified into CD4- and CD8-positive types [12,13].
- Comprehensive assessment of multiple organs with high suspicion and correct recognition of EBV-associated histopathological features are important for the diagnosis of CAEBV infection [14]. It was difficult to diagnose EBV encephalitis and colitis in our patient owing to the subtle and deceptive morphologies. A brain biopsy performed after steroid treatment demonstrated mild lymphocytic infiltration and subtle microglial proliferation. The colon showed inflammatory bowel disease (IBD)–like microscopic changes, including marked glandular distortion and transmural chronic inflammation with cryptitis and crypt abscess. The detection of EBV in tissue samples using in situ hybridization demonstrated an EBV-induced pathological process, highlighting the critical role of EBV assay in the diagnosis of CAEBV infection [4].
- EBV can lead to various central nervous system complications [15]. Some authors have suggested that there has been an increase in the occurrence of neurological complications of EBV infection [16,17]. A study examining pediatric EBV-associated encephalitis found that EBV infection was present in 9.7% of the children hospitalized with neurological complications [18]. EBV encephalitis is rare in children but can have severe neurological complications; usually, the positive MRI findings occur in the brain stem, basal ganglia, cerebellum, and thalamus [19-21]. This was somewhat similar to MRI findings of the present case that showed hyperintense T2 lesion in the thalamus, basal ganglia, and brain stem.
- EBV encephalitis can show various clinical and histological characteristics [16,22]. Establishing a diagnosis of EBV encephalitis is difficult; consequently, molecular, serological, and imaging techniques should be used when investigating children with encephalitis [15]. Biopsy is important to confirm general pediatric encephalitis because it may reveal the underlying infective process, chronic inflammatory change, or neoplastic disease [23]. Diagnosing EBV encephalitis requires demonstration of EBV-infected cells. Our case showed EBV-infected T lymphocytes infiltrating the perivascular area; this is consistent with the previous reports [16,22,24]. The EBV-infected T lymphocytes in our case were CD8-positive, thereby confirming EBV encephalitis involving CD8-positive T lymphocytes. Another study conducted on EBV encephalitis reported features of cortical infiltration of T lymphocytes with perivascular and perineuronal clusters, while the B lymphocytes were frequently seen in the perivascular cuffs [25]. The prognosis associated with EBV encephalitis is controversial. Some reports have suggested it to be a relatively benign, self-limited disease with an almost full recovery; however, others have documented the occurrence of various neurologic sequelae in a substantial number of cases [15,18,26].
- Gastrointestinal involvement is very rare and few cases of immunocompetent hosts have been reported [27-29]. Among these, the lymphoid cells that were involved were of the B-cell lineage. However, our case had involvement of the T-cell lineage. EBV colitis is rare in children, and few cases have been reported [30]. EBV colitis is difficult to differentiate from IBD owing to overlapping symptoms and endoscopic findings, and discerning whether the severity of symptoms is attributable to CAEBV or the exacerbation of IBD is challenging, thereby making diagnosis of EBV colitis and IBD difficult [9,31,32]. Our case demonstrated findings that were consistent with the pathological findings observed by other authors in their studies [9,33,34]. Despite the similarity between IBD and EBV colitis, it was noted that atypical infiltrates were more frequently observed in patients with EBV positivity. Consequently, every patient with IBD should undergo the EBV test [35]. Although further studies are warranted to clarify the role of EBV in inflammatory gut disorders, it was proposed that EBV may induce immune alterations in the colon, thereby aiding the pathogenesis of EBV colitis [36]. The molecular detection of EBV-encoded RNA transcripts by in situ hybridization remains the gold standard in the identification of EBV in biopsies [35].
- Our patient’s viral load was initially high on admission, after the initiation of steroids and immunosuppressive agents; it reduced gradually with significant improvement after HSCT. The effective treatment strategy for eradicating EBV-infected Tor NK-positive cells is HSCT, if initiated before deterioration of the patient’s condition. HSCT is, by far, considered the most effective treatment for CAEBV by revitalizing the hematopoietic system. However, because not all patients with CAEBV may undergo HSCT, immunosuppression and chemotherapy can also be considered with or without HSCT [37]. Although the outcome of patients with active disease accompanied by fever, liver dysfunction among others may be worse [1,38]. Studies have proved that manifestation of signs and symptoms is subject to the host immune responses [9,39]. Usually, patients with EBV infection have serious clinical abnormalities that may persist for 6 months or more with high antibody titers against EBV but not against EBV nuclear antigen [9,39]. Thus, multiple organ involvement in CAEBV infection often results in poor prognosis [14,40]. The present case may be slightly different owing to early recognition; awareness of CAEBV, especially regarding the histological changes, EBER, and EBV DNA, are crucial for patients with EBV, similar to what was observed in another study [34]. CAEBV disease and post-HSCT lymphoproliferative disorders share similarities. However, although both post-HSCT posttransplant lymphoproliferative disorders (PTLD) and CAEBV disease involve EBV reactivation and immune dysfunction, distinct differences exist between conditions [3,6]. PTLD is predominantly of B-cell origin, compared to CAEBV being of T-cell origin. Although monomorphic PTLD of T-cell origin exist, it is typically EBV-negative [41].
- Several genetic factors of CAEBV have been found to increase genetic susceptibility of the hosts. For example, candidate gene studies of transplant HLA have reported that transplant recipients have haplotypes, such as HLA-A26 and B36, that are associated with a higher risk of developing EBV-positive B-cell origin PTLD [42]. The HLA result of our patient indicated no increased risk to CAEBV disease.
- This case showed histopathological characteristics of concurrent EBV-associated encephalitis and colitis in CAEBV infection. CAEBV is an extremely rare and severe complication that can arise from EBV infection. It is a life-threatening medical condition that is more likely to occur as a result of primary infection, reactivation, and immunosuppression. Histopathological features will help the discrimination, serum EBV DNA and in situ hybridization for EBV-encoded RNA are recommended to exclude. This condition is more likely to pose treatment challenges, especially when the treatment is initiated at a late stage. HSCT should be considered a crucial therapeutic option for preventing the progression of disease.
DISCUSSION
Ethics Statement
All procedures performed in the current study were approved by the institutional review board (IRB) of Severance Hospital (reference # 4-2024-0533 dated 19th June 2024) in accordance with the 1964 Helsinki Declaration and its later amendments. Patient consent waiver was obtained for this study.
Availability of Data and Material
All relevant data and information pertaining to the patient presented in this case report are included in the manuscript.
Code Availability
Not applicable.
Author Contributions
Conceptualization: MJ. Data curation: BKA, MJ. Formal analysis: SOY, SHK, MJ. Funding acquisition: MJ. Investigation: BKA, MJ. Methodology: MJ. Project administration: MJ. Resources: SOY, SHK, MJ. Software: BKA. Supervision: JJY, MJ. Validation: MJ. Visualization: BKA. Writing—original draft: BKA, MJ. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
S.H.K., a contributing editor of the Journal of Pathology and Translational Medicine, was not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2024-00341570).
- 1. Arai A. Advances in the study of chronic active Epstein-Barr virus infection: clinical features under the 2016 WHO classification and mechanisms of development. Front Pediatr 2019; 7: 14.ArticlePubMedPMC
- 2. Zhang T, Fu Q, Gao D, Ge L, Sun L, Zhai Q. EBV associated lymphomas in 2008 WHO classification. Pathol Res Pract 2014; 210: 69-73. ArticlePubMed
- 3. Kimura H. Pathogenesis of chronic active Epstein-Barr virus infection: is this an infectious disease, lymphoproliferative disorder, or immunodeficiency? Rev Med Virol 2006; 16: 251-61. ArticlePubMed
- 4. Cohen JI, Jaffe ES, Dale JK, et al. Characterization and treatment of chronic active Epstein-Barr virus disease: a 28-year experience in the United States. Blood 2011; 117: 5835-49. ArticlePubMedPMCPDF
- 5. Kimura H, Hoshino Y, Hara S, et al. Differences between T cell-type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis 2005; 191: 531-9. ArticlePubMed
- 6. Kimura H, Cohen JI. Chronic active Epstein-Barr virus disease. Front Immunol 2017; 8: 1867.ArticlePubMedPMC
- 7. Ambinder RF. Epstein-Barr virus-associated lymphoproliferative disorders. Rev Clin Exp Hematol 2003; 7: 362-74. PubMed
- 8. Hong M, Ko YH, Yoo KH, et al. EBV-positive T/NK-Cell lymphoproliferative disease of childhood. Korean J Pathol 2013; 47: 137-47. ArticlePubMedPMC
- 9. Xu W, Jiang X, Chen J, et al. Chronic active Epstein-Barr virus infection involving gastrointestinal tract mimicking inflammatory bowel disease. BMC Gastroenterol 2020; 20: 257.ArticlePubMedPMCPDF
- 10. Quintanilla-Martinez L, Ko YH, Kimura H, Jaffe ES. EBV-positive T-cell and NK-cell lymphoproliferative diseases of childhood. In: Swerdlow SH, Campo E, Harris N, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2017; 355-60.
- 11. Kimura H, Morishima T, Kanegane H, et al. Prognostic factors for chronic active Epstein-Barr virus infection. J Infect Dis 2003; 187: 527-33. ArticlePubMed
- 12. Kimura H, Hoshino Y, Kanegane H, et al. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood 2001; 98: 280-6. ArticlePubMedPDF
- 13. Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood 2012; 119: 673-86. ArticlePubMedPDF
- 14. Liu R, Wang M, Zhang L, et al. The clinicopathologic features of chronic active Epstein-Barr virus infective enteritis. Mod Pathol 2019; 32: 387-95. ArticlePubMedPDF
- 15. Hashemian S, Ashrafzadeh F, Akhondian J, Beiraghi Toosi M. Epstein-barr virus encephalitis: a case report. Iran J Child Neurol 2015; 9: 107-10. PubMedPMC
- 16. Hausler M, Ramaekers VT, Doenges M, Schweizer K, Ritter K, Schaade L. Neurological complications of acute and persistent Epstein-Barr virus infection in paediatric patients. J Med Virol 2002; 68: 253-63. ArticlePubMed
- 17. Jang YY, Lee KH. Transient asymptomatic white matter lesions following Epstein-Barr virus encephalitis. Korean J Pediatr 2011; 54: 389-93. ArticlePubMedPMC
- 18. Doja A, Bitnun A, Ford Jones EL, et al. Pediatric Epstein-Barr virus-associated encephalitis: 10-year review. J Child Neurol 2006; 21: 384-91. ArticlePubMedPDF
- 19. Phowthongkum P, Phantumchinda K, Jutivorakool K, Suankratay C. Basal ganglia and brainstem encephalitis, optic neuritis, and radiculomyelitis in Epstein-Barr virus infection. J Infect 2007; 54: e141-4. ArticlePubMed
- 20. Shian WJ, Chi CS. Fatal brainstem encephalitis caused by Epstein-Barr virus. Pediatr Radiol 1994; 24: 596-7. ArticlePubMedPDF
- 21. Johkura K, Momoo T, Kuroiwa Y. Thalamic involvement of Epstein-Barr virus encephalitis demonstrated by MRI. J Neurol 2003; 250: 357-8. ArticlePubMedPDF
- 22. Francisci D, Sensini A, Fratini D, et al. Acute fatal necrotizing hemorrhagic encephalitis caused by Epstein-Barr virus in a young adult immunocompetent man. J Neurovirol 2004; 10: 414-7. ArticlePubMed
- 23. Layard Horsfall H, Toescu SM, Grover PJ, et al. The utility of brain biopsy in pediatric cryptogenic neurological disease. J Neurosurg Pediatr 2020; 26: 431-8. ArticlePubMed
- 24. Kano K, Katayama T, Takeguchi S, et al. Biopsy-proven case of Epstein-Barr virus (EBV)-associated vasculitis of the central nervous system. Neuropathology 2017; 37: 259-64. ArticlePubMedPDF
- 25. Hart M. Greenfield's neuropathology. J Neuropathol Exp Neurol 2008; 67: 828.Article
- 26. Domachowske JB, Cunningham CK, Cummings DL, Crosley CJ, Hannan WP, Weiner LB. Acute manifestations and neurologic sequelae of Epstein-Barr virus encephalitis in children. Pediatr Infect Dis J 1996; 15: 871-5. ArticlePubMed
- 27. Clayton RA, Malcomson RD, Gilmour HM, Crawford DH, Parks RW. Profuse gastrointestinal haemorrhage due to delayed primary Epstein-Barr virus infection in an immunocompetent adult. Histopathology 2005; 47: 439-41. ArticlePubMed
- 28. Na HK, Ye BD, Yang SK, et al. EBV-associated lymphoproliferative disorders misdiagnosed as Crohn's disease. J Crohns Colitis 2013; 7: 649-52. ArticlePubMed
- 29. Karlitz JJ, Li ST, Holman RP, Rice MC. EBV-associated colitis mimicking IBD in an immunocompetent individual. Nat Rev Gastroenterol Hepatol 2011; 8: 50-4. ArticlePubMedPDF
- 30. Tseng YJ, Ding WQ, Zhong L, Chen J, Luo ZG. Chronic active Epstein-Barr virus (CAEBV) enteritis. Int J Infect Dis 2019; 82: 15-7. ArticlePubMed
- 31. Wakefield AJ, Fox JD, Sawyerr AM, et al. Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn's disease using the nested polymerase chain reaction. J Med Virol 1992; 38: 183-90. ArticlePubMed
- 32. Zhang B, Wang X, Tian X, Cai Y, Wu X. Chronic active Epstein-Barr virus-associated enteritis: CT findings and clinical manifestation. Biomed Res Int 2020; 2020: 2978410.ArticlePubMedPMCPDF
- 33. Wang Y, Li Y, Meng X, et al. Epstein-Barr virus-associated T-cell lymphoproliferative disorder presenting as chronic diarrhea and intestinal bleeding: a case report. Front Immunol 2018; 9: 2583.ArticlePubMedPMC
- 34. Tian S, Westbrook LM, Xiao SY, Zhang Y, Huang Y, Wang HL. The morphologic features of primary Epstein-Barr virus infection in the gastrointestinal tract: an approach to correct diagnosis. Am J Surg Pathol 2019; 43: 1253-63. ArticlePubMed
- 35. Nissen LH, Nagtegaal ID, de Jong DJ, et al. Epstein-Barr virus in inflammatory bowel disease: the spectrum of intestinal lymphoproliferative disorders. J Crohns Colitis 2015; 9: 398-403. ArticlePubMed
- 36. Ryan JL, Shen YJ, Morgan DR, et al. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci 2012; 57: 1887-98. ArticlePubMedPMCPDF
- 37. Sawada A, Inoue M, Kawa K. How we treat chronic active Epstein-Barr virus infection. Int J Hematol 2017; 105: 406-18. ArticlePDF
- 38. Okamura T, Hatsukawa Y, Arai H, Inoue M, Kawa K. Blood stem-cell transplantation for chronic active Epstein-Barr virus with lymphoproliferation. Lancet 2000; 356: 223-4. ArticlePubMed
- 39. Yamashita S, Murakami C, Izumi Y, et al. Severe chronic active Epstein-Barr virus infection accompanied by virus-associated hemophagocytic syndrome, cerebellar ataxia and encephalitis. Psychiatry Clin Neurosci 1998; 52: 449-52. ArticlePubMed
- 40. Huang L, Zhang X, Fang X. Case report: Epstein-Barr virus encephalitis complicated with brain stem hemorrhage in an immune-competent adult. Front Immunol 2021; 12: 618830.ArticlePubMedPMC
- 41. Ok CY, Li L, Young KH. EBV-driven B-cell lymphoproliferative disorders: from biology, classification and differential diagnosis to clinical management. Exp Mol Med 2015; 47: e132. ArticlePubMedPMCPDF
- 42. Reshef R, Luskin MR, Kamoun M, et al. Association of HLA polymorphisms with post-transplant lymphoproliferative disorder in solid-organ transplant recipients. Am J Transplant 2011; 11: 817-25. ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure

 E-submission
E-submission