Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(3); 2025 > Article
-
Original Article
Diagnostic yield of fine needle aspiration with simultaneous core needle biopsy for thyroid nodules -
Mohammad Ali Hasannia1,2
 , Ramin Pourghorban3,4
, Ramin Pourghorban3,4 , Hoda Asefi4
, Hoda Asefi4 , Amir Aria5
, Amir Aria5 , Elham Nazar6
, Elham Nazar6 , Hojat Ebrahiminik7
, Hojat Ebrahiminik7 , Alireza Mohamadian1,2
, Alireza Mohamadian1,2
-
Journal of Pathology and Translational Medicine 2025;59(3):180-187.
DOI: https://doi.org/10.4132/jptm.2025.03.04
Published online: March 28, 2025
1Department of Radiology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
2Advanced Diagnostic and Interventional Radiology Research Center (ADIR), Tehran University of Medical Sciences, Tehran, Iran
3Department of Medical Imaging, Nepean Hospital, Kingswood, New South Wales, Australia
4Department of Radiology, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
5Department of Internal Medicine, Alzahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
6Department of Pathology, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
7Department of Interventional Radiology and Radiation Sciences Research Center, Aja University of Medical Sciences, AJA Campus, Tehran, Iran
- Corresponding Author Alireza Mohamadian, MD, MPH Department of Radiology, School of Medicine, Tehran University of Medical Sciences, Sina Hospital, Imam Khomeini St., Tehran 11367-46911, Iran Tel: +98-937-323-3713, Fax: +98-21-8889-8532, E-mail: alirezamohamadian.md@gmail.com
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 10,206 Views
- 226 Download
Abstract
-
Background
- Fine needle aspiration (FNA) is a widely utilized technique for assessing thyroid nodules; however, its inherent non-diagnostic rate poses diagnostic challenges. The present study aimed to evaluate and compare the diagnostic efficacy of FNA, core needle biopsy (CNB), and their combined application in the assessment of thyroid nodules.
-
Methods
- A total of 56 nodules from 50 patients was analyzed using both FNA and simultaneous CNB. The ultrasound characteristics were categorized according to the American College of Radiology Thyroid Imaging Reporting and Data Systems classification system. The study compared the sensitivity, specificity, and accuracy of FNA, CNB, and the combination of the two techniques.
-
Results
- The concordance between FNA and CNB was notably high, with a kappa coefficient of 0.837. The sensitivity for detecting thyroid malignancy was found to be 25.0% for FNA, 66.7% for CNB, and 83.3% for the combined FNA/CNB approach, with corresponding specificities of 84.6%, 97.4%, and 97.4%. The accuracy of the FNA/CNB combination was the highest at 94.1%.
-
Conclusions
- The findings of this study indicate that both CNB and the FNA/CNB combination offer greater diagnostic accuracy for thyroid malignancy compared to FNA alone, with no significant complications reported. Integrating CNB with FNA findings may enhance management strategies and treatment outcomes for patients with thyroid nodules.
- Thyroid nodules are a common condition, with up to 76% of the population having them, and around 7%–15% of those nodules being malignant [1]. With advancements in manufacturing high-resolution ultrasound probes, more suspicious nodules are being diagnosed, which means there is an increasing need for accurate and efficient diagnostic methods [2-4].
- Fine needle aspiration (FNA) is the most cost-effective, safest, and quickest diagnostic method used in the initial evaluation of thyroid nodules. However, the Bethesda System classification used to diagnose thyroid nodules using FNA results can be unreliable, with unclear results reported in 10%–47% of cases, either non-diagnostic (Bethesda I) or atypia of undetermined significance (Bethesda III) [1,5,6]. This leads to the need for repeat FNA or core needle biopsy (CNB) procedures to obtain more conclusive results [7-9].
- CNB is a complementary diagnostic method that can overcome the limitations of FNA, including non-diagnostic or uncertain outcomes and the need for repeat procedures or unnecessary surgeries [8-13]. CNB is more sensitive and reliable than FNA due to the availability of tissue samples, which can be used for immunohistochemistry and molecular studies [2,7]. As a result, CNB is becoming increasingly competitive with surgical gold standards in terms of diagnostic value [3].
- Given the high prevalence of thyroid nodular diseases, it is crucial to select the most appropriate diagnostic method that provides optimal accuracy for assessing the nature of thyroid nodules. In particular, CNB may be a fruitful choice for diagnosing thyroid nodules, especially in cases with cytologic Bethesda I and III results. While many studies have reported that the FNA/CNB combination provides superior diagnostic accuracy [12-19], some suggest there is no significant difference between the two methods [20,21]. Therefore, this study aims to evaluate the diagnostic yield of FNA and CNB separately and in combination for the diagnosis of thyroid nodules.
INTRODUCTION
- Participants
- This study was conducted on 50 patients with a total of 56 nodules. The inclusion criteria were based on the American College of Radiology (ACR) Thyroid Imaging Reporting and Data System (TI-RADS) criteria [22,23], where patients had at least one TI-RADS 3 (TR3) thyroid nodule with a diameter of 25 mm or more, a TI-RADS 4 (TR4) thyroid nodule with a diameter of 15 mm or more, and a TI-RADS 5 (TR5) thyroid nodule with a diameter of 10 mm. Exclusion criteria included a history of thyroid cancer, coagulative disorders, recent antiplatelet or anticoagulant consumption, opioid use over the past 6 months, allergies to local anesthetics, and chronic pain syndrome.
- Procedures
- An experienced radiologist performed all procedures using an ultrasound machine (model WS80A, Samsung, Seoul, Korea). Prior to the aspiration and biopsy, an ultrasonographic evaluation was conducted to assess the nodule size, distance from the skin surface, type of nodule calcification, and ACR TI-RADS score. Local anesthesia was administered, and FNA was performed twice with a G23 needle and a 10 mL syringe from different sites of the nodule. If sampling was improper or insufficient, it was repeated with a larger diameter needle. Two samples were collected from the solid and suspicious parts of the nodules using a CNB sampling needle with a length of 10 cm and Gauge-18. FNA was always performed before CNB due to the destruction and disintegration of the nodule parenchyma after CNB. Patients were monitored for acute complications such as hematoma around the thyroid and voice changes or hoarseness. Follow-up was conducted for 18 months, and pathology reports of patients who underwent surgery were recorded as the gold standard.
- Pathologic analysis
- Samples obtained from FNA and CNB were classified into six categories based on the Bethesda 2023 system and the CNB sample reporting guideline [6,24]. The findings were considered equivalent one by one, and discrepancy between FNA and CNB results led to use of that with the higher probability of malignancy. The management approach for such cases involved either recommending surgery or a follow-up to assess any changes in their ultrasonographic features during 18 months.
- Statistical analysis
- The agreement between FNA and CNB methods in classifying nodules was assessed using the kappa coefficient, with values ranging from 0 to 1. Kappa values of 0–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.0 represent no agreement, slight agreement, fair agreement, moderate agreement, and substantial agreement, respectively [25]. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were calculated and reported for FNA, CNB, and FNA/CNB diagnostic parameters. Data were analyzed using SPSS software ver. 22 (IBM Corp., Armonk, NY, USA), and statistical significance was set at p < .05.
MATERIALS AND METHODS
- Between October 2020 and April 2021, 56 thyroid nodule samples were obtained at Sina Hospital, Tehran, which is affiliated with Tehran University of Medical Sciences. A convenient sampling method was employed and 50 patients were included in the study, comprising 11 males and 39 females. All nodules were sampled for the first time, and the participants were followed for 18 months to determine the outcome. The mean nodule diameter was 27 ± 12.3 mm, ranging from 12 to 67 mm. The nodules were classified according to the TI-RADS system, with 33.9% (n = 19) classified as TR3, 51.8% (n = 29) as TR4, and 14.3% (n = 8) as TR5. Calcification was observed in 46.4% (n = 26) of the nodules, with 21.4% (n = 12) showing punctate echogenic foci, 17.9% (n = 10) showing macrocalcification, 5.4% (n = 3) showing large comet-tail artifact, and 1.8% (n = 1) showing peripheral/rim calcification. In three-fourths of the malignant findings mentioned in the preoperative FNA cytologic reports, there was a suspicion of papillary thyroid carcinoma (PTC) (Tables 1 and 2).
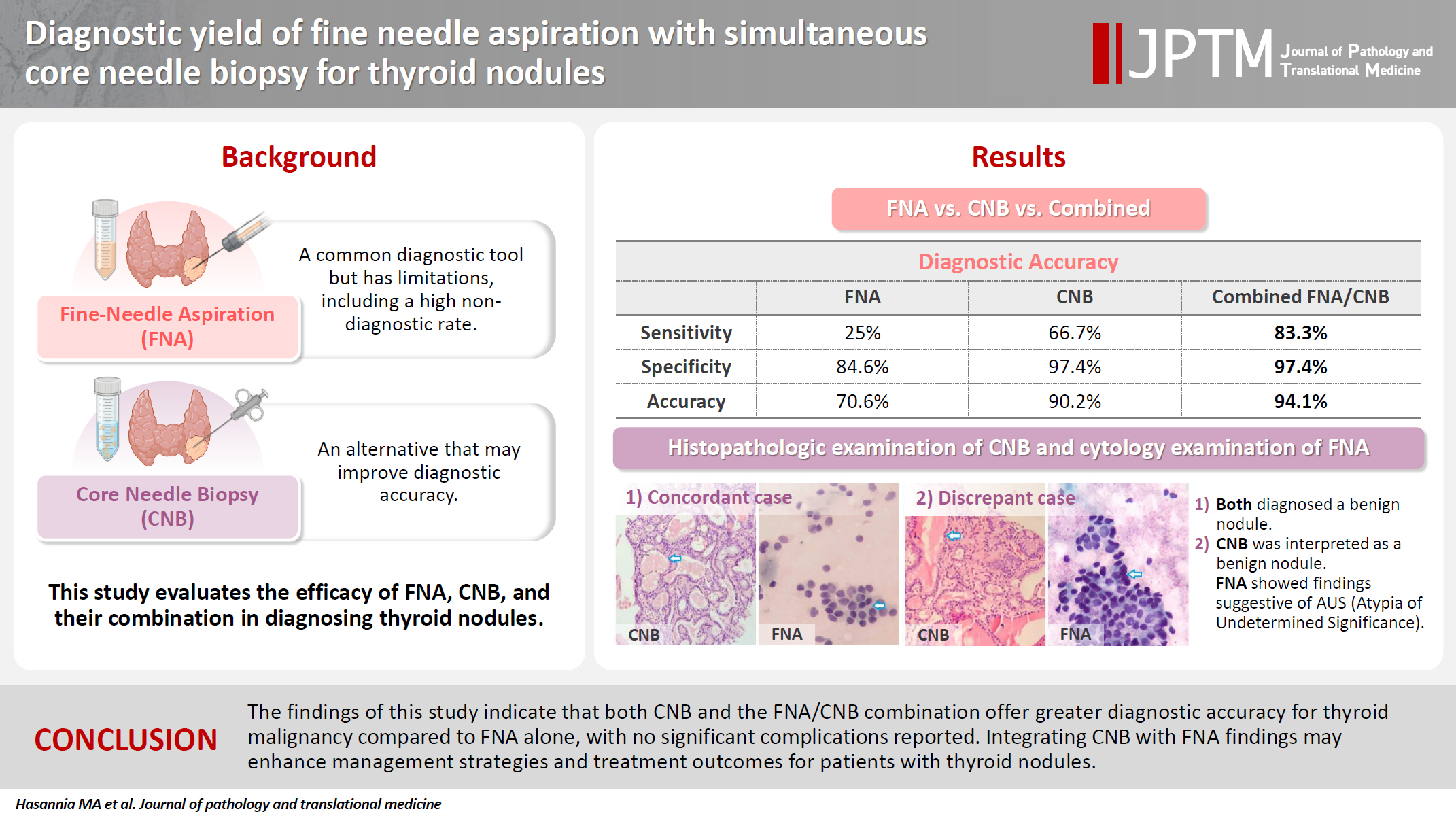
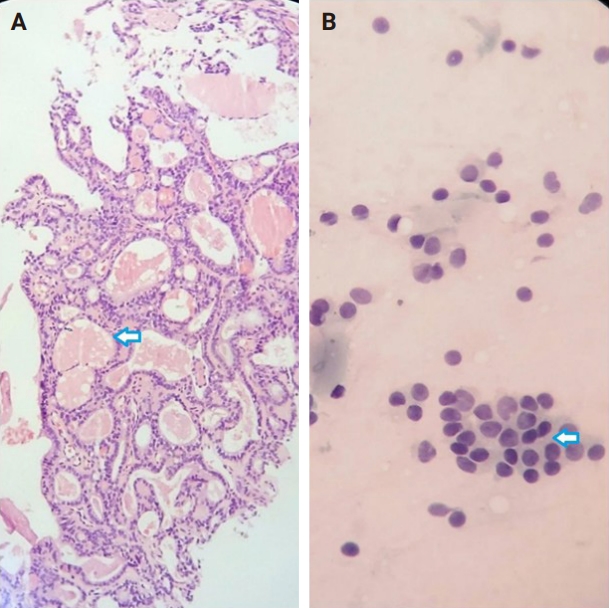
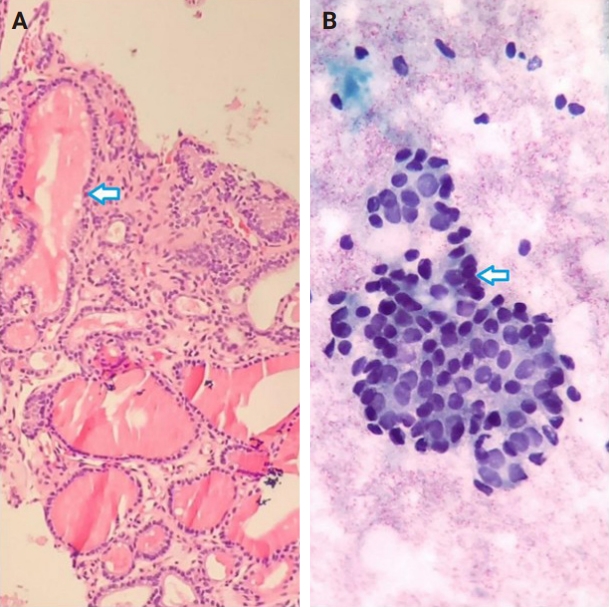
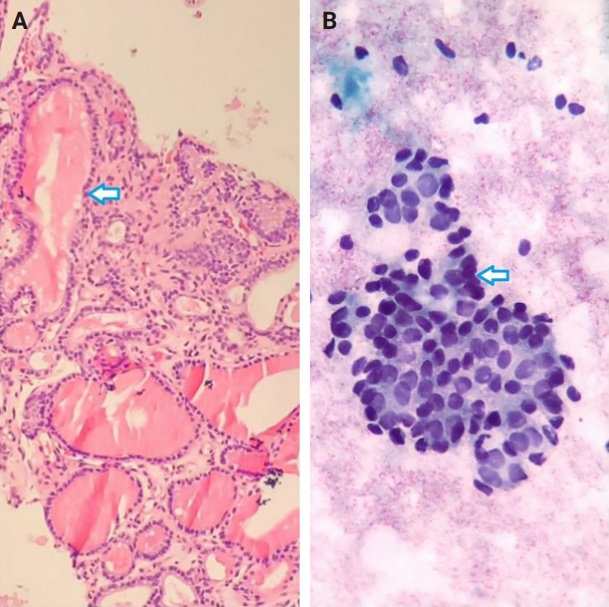
- Of the 56 thyroid nodules studied, 39 (76.5%) were benign and 12 (23.5%) were malignant. During the follow-up period, five patients with follicular neoplasm (FN) as the primary cytologic and CNB-based pathologic results were not operated on and were excluded from the final analysis due to the unavailability of postoperative pathologic tissue reports. Overall, the results indicate strong agreement between the FNA and CNB methods in the diagnosis of thyroid nodules, with a kappa coefficient of 0.837 (Fig. 1)
- Among the nodules diagnosed using the FNA method, 66.7% (n = 34) were benign (Bethesda II), 5.4% (n = 3) were suspicious for malignancy (Bethesda V), and 5.4% (n = 3) were malignant (Bethesda VI). The CNB method revealed 76.5% (n = 39) benign and 15.7% (n = 8) malignant nodules (category VI), with all FNA Bethesda V nodules being confirmed by the CNB method. One of the two indeterminate nodules marked by the CNB method was found to be benign, while the other was diagnosed as PTC in the postoperative pathology reports. Additionally, two FN nodules (Bethesda IV) were identified as Follicular Thyroid Carcinoma and Oncocytic carcinoma of the thyroid. Furthermore, all six non-diagnostic nodules identified by FNA were diagnosed as benign by CNB (Fig. 2).
- The combination of FNA and CNB methods resulted in 74.5% (n = 38) benign and 19.6% (n = 10) malignant (Bethesda VI) nodules. Following the FNA/CNB application, the number of non-diagnostic reports decreased, leaving only three nodules (5.8%) in the indeterminate category (category III).
- After an 18-month imaging follow-up, 38 nodules that were initially diagnosed as benign using the combined method remained unchanged. On the contrary, 13 individuals underwent thyroidectomy, with 12 of these cases classified as malignant. The post-surgery pathological evaluation revealed the following diagnoses: one case of oncocytic carcinoma of the thyroid, one case of follicular thyroid carcinoma, one case of metastatic lesion, and nine cases of PTC (Table 3).
- The results of Table 4 suggest that the CNB and FNA/CNB methods improve sensitivity and specificity in the diagnosis of malignancy compared to the FNA or CNB method alone. The FNA/CNB method showed a sensitivity of 83.3%, specificity of 97.4%, positive predictive value of 100%, negative predictive value of 100%, and a diagnostic accuracy of 94.1% for the malignancies. The CNB method had a sensitivity of 66.7%, specificity of 97.4%, positive predictive value of 100%, negative predictive value of 97.4%, and accuracy of 90.2% in diagnosis of malignant nodules. Overall, the combination of FNA and CNB methods resulted in a higher diagnostic accuracy in diagnosis of malignant thyroid lesions than either method alone. There were no cases of gross hematoma with compressive effect on the upper airways or changes in voice observed during the diagnostic procedures including FNA/CNB method (Table 4).
RESULTS
- The study demonstrated a strong agreement between the FNA and CNB methods for diagnosing benign and malignant thyroid nodules. The frequency of non-diagnostic results in FNA was dependent on the radiologist's skill and experience, as well as nodule characteristics. In this study, an experienced radiologist performed the FNA procedure and made an effort to obtain sufficient tissue samples with each needle application. Nonetheless, a considerable portion of FNA reports (10.7%) were non-diagnostic, whereas all non-diagnostic nodules were reported as benign with CNB.
- As expected, CNB had an advantage over FNA in eliminating non-diagnostic nodules. With precise penetration of the CNB needle, the results are less dependent on the operator's skill, and the obtained tissues have more cells, which reduces the frequency of non-diagnostic results. Consistent with our findings, previous studies have reported that CNB is more valuable than FNA for reducing the frequency of non-diagnostic results and providing more reliable diagnostic accuracy [7,26-28].
- The CNB method had a higher frequency of atypia of undetermined significance (AUS) and FN nodules compared to FNA, possibly due to larger tissue samples and additional pathologic findings. Sometimes, nodules with suspicious ultrasound features might have normal records in FNA, indicating the possibility of FN [29,30]. In Na et al. [7], the diagnostic rate of FN was higher with CNB than FNA.
- It is well-known that the rate of suspicious nodules for malignancy in FNA is higher than in CNB. Hahn et al. [26] reported that the definite diagnostic rate in CNB was significantly higher than in FNA, especially for nodules larger than 2 cm. Our results similarly showed that FNA had a lower diagnostic sensitivity than CNB.
- The diagnostic accuracy of FNA/CNB and CNB was significantly higher than that of FNA alone, as expected. The combination of CNB and FNA can reduce the need for repeated FNA and diagnostic surgeries in thyroid nodules, particularly by reducing non-diagnostic cases. Although some studies have reported a diagnostic advantage of CNB over FNA alone, other studies, including our own, have shown that the combination of CNB and FNA is more beneficial than CNB alone [12-15].
- While low-risk neoplasms such as non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) are classified as a surgical disease and cannot be definitively diagnosed through FNA, the cytological characteristics of this indolent tumor frequently result in its categorization as either AUS (Bethesda III), FN (Bethesda IV), or suspicious for malignancy (Bethesda V) on FNA [6]. In the cohort of 13 patients who underwent thyroidectomy in the present study, post-surgical histopathological examination revealed nine nodules diagnosed as PTC, one as follicular thyroid carcinoma, one as oncocytic carcinoma of the thyroid, and one as a metastatic lesion. Given our emphasis on diagnosing malignant lesions, we aimed to exclude any low-grade lesions (like NIFTP) from the malignant category; however, no low-grade lesions were identified in our study.
- In this study, the high rate of non-diagnostic cases in nodules examined with FNA reduced its diagnostic value compared to CNB. Among the 80% of malignant nodules not reported by FNA, most were PTC. Previous studies have reported that papillary cancer may be mistakenly classified as benign due to macrofollicular manifestations in the absence of cytological changes and atypia. Most of the false negatives in FNA were attributed to the follicular adenoma with papillary architecture [31,32].
- One nodule was reported as benign in FNA but as FN/suspicious for follicular neoplasm with CNB. Subsequently, it was diagnosed as oncocytic carcinoma of the thyroid during follow-up and after surgery. Yeh et al. [33] also encountered several patients with thyroid cancer in postoperative tissue diagnosis despite having FNA reports without malignant or suspected cells. False-negative rates in FNA could be due to poor cell aspiration or sampling error.
- Thyroid nodules containing calcified foci can reduce the diagnostic sensitivity of FNA. While peripheral calcification is rare among suspicious nodules and more common in benign lesions, in some cases, it may be necessary to sample from a calcified nodule. Macrocalcifications are not significantly associated with an increased risk of malignancy, while punctate echogenic foci are often linked to psammomatous calcification in papillary thyroid cancer and carry a high risk of malignancy. Peripheral calcification presents challenges for nodule sampling, including difficulties in penetrating the shell, visualizing the needle tip within the lesion, and unexpected complications [34-36].
- The results of the current study indicate that all AUS nodules, except for one malignant case, had calcified foci in their postoperative reports (five nodules with punctate echogenic foci and one nodule with macrocalcifications). In cases where there is clinical suspicion of malignancy in a lesion with macrocalcification, it may be reasonable to use CNB as a complementary diagnostic method to FNA.
- The current study encountered several limitations, particularly a reduction in sample size. Due to the infrequent use of CNB for thyroid nodules in Iran, as well as many other regions, this study was designed as a pilot project to establish a foundation for future research with larger cohorts. The coronavirus disease 2019 pandemic further exacerbated the situation, as many patients were hesitant to seek medical care, leading to the loss of numerous potentially eligible participants.
- While the study's setting and regional relevance are significant strengths, the limited follow-up period of 18 months and the potential impact of operator expertise on FNA results are other important limitations. The literature establishes that the TI-RADS criteria recommend a five-year follow-up for nodules scoring 3 or higher, with TR3 and TR4 lesions monitored at 1, 3, and 5 years, and TR5 lesions requiring annual assessments. This protocol is contingent upon the nodule size evaluated via FNA [23].
- In contrast, there is a lack of consensus regarding follow-up protocols for nodules subjected to CNB. The combination of CNB with FNA offers a significant advantage by potentially reducing follow-up intervals and enhancing decision-making in thyroid nodule management. Given that all nodules were subjected to the CNB, it is logical to propose a reduction in the follow-up period for patients compared to the conventional post-FNA follow-up duration outlined in TI-RADS. Therefore, the researchers established a maximum follow-up duration of 18 months for the patients involved in the study. However, extending follow-up for CNB-subjected nodules to 3 to 5 years could markedly improve diagnostic accuracy.
- Additionally, the diagnostic accuracy of FNA for thyroid nodules varies widely across studies, reported between 28% and 72% [8,16-18]. This variability arises from several factors, including lesion characteristics (solid and cystic components, calcifications), FNA technique, use of ultrasound guidance, quality of ultrasound equipment, experience of the technician, the pathologist expertise in cytological evaluation. Despite FNA’s affordability and accessibility, these limitations diminish its diagnostic accuracy [37-40]. Determining the specific contribution of each factor to FNA outcomes is challenging and may not be particularly beneficial. Therefore, employing CNB, which provides tissue samples, appears to be a reasonable strategy to overcome these limitations. Even with experienced technicians, optimal control over other influencing factors remains problematic [8,16,18,19,41].
- In conclusion, our study findings suggest that FNA/CNB is more effective than either FNA or CNB alone in nodules with a TI-RADS score ≥3, particularly when the initial FNA report is non-diagnostic. Therefore, we recommend using CNB in conjunction with FNA for nodules with a TI-RADS score ≥3 to decrease the need for further evaluations. This combined approach could also minimize unnecessary surgeries and enhance diagnostic accuracy.
DISCUSSION
Ethics Statement
All procedures were in compliance with the guidelines of the 1964 Helsinki Declaration and its later amendments and also were approved and monitored by the Medical Ethics Committee of Tehran University of Medical Sciences (reference number: IR.TUMS.SINAHOSPITAL.REC.1400.029). Patients who met the inclusion criteria were also provided a full explanation of the study procedure and were enrolled after signing a written informed consent.
Availability of Data and Material
All data generated or analyzed during the study are included in this published article (and its supplementary information files).
Code Availability
Not applicable.
Author Contributions
Conceptualization: RP, HA, HE. Data curation: MAH, AM. Formal analysis: EN. Funding acquisition: MAH. Investigation: MAH, AA. Methodology: RP, EN. Project administration: MAH. Resources: MAH, AM. Software: AA. Supervision: RP, HA, HE. Validation: RP. Visualization: EN, AM. Writing – original draft: MAH, AM. Writing – review & editing: RP, AA. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
The present study was supported by Tehran University of Medical Sciences.
Acknowledgments
This study formed a part of MAH's radiology specialty graduation thesis.
| Category | FNA | CNB | FNA/CNB |
|---|---|---|---|
| I | 6 (11.8) | 0 | 0 |
| II | 34 (66.7) | 39 (76.5) | 38 (74.5) |
| III | 4 (7.8) | 2 (3.9) | 3 (5.9) |
| IV | 1 (1.9) | 2 (3.9) | 0 |
| V | 3 (5.9) | 0 | 0 |
| VI | 3 (5.9) | 8 (15.7) | 10 (19.6) |
- 1. Chen H, Song A, Wang Y, et al. BRAF(V600E) mutation test on fine-needle aspiration specimens of thyroid nodules: Clinical correlations for 4600 patients. Cancer Med 2022; 11: 40-9. ArticlePubMedPMCPDF
- 2. Choi SH, Baek JH, Lee JH, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol 2014; 24: 2819-26. ArticlePubMedPDF
- 3. Strauss EB, Iovino A, Upender S. Simultaneous fine-needle aspiration and core biopsy of thyroid nodules and other superficial head and neck masses using sonographic guidance. AJR Am J Roentgenol 2008; 190: 1697-9. ArticlePubMed
- 4. Yoon JH, Kim EK, Kwak JY, Moon HJ. Effectiveness and limitations of core needle biopsy in the diagnosis of thyroid nodules: review of current literature. J Pathol Transl Med 2015; 49: 230-5. ArticlePubMedPMCPDF
- 5. Na DG, Kim JH, Sung JY, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid 2012; 22: 468-75. ArticlePubMed
- 6. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023; 33: 1039-44. ArticlePubMed
- 7. Na DG, Baek JH, Jung SL, et al. Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol 2017; 18: 217-37. ArticlePubMedPMCPDF
- 8. Su X, Yue C, Yang W, Ma B. A comparative analysis of core needle biopsy and repeat fine needle aspiration in patients with inconclusive initial cytology of thyroid nodules. Front Endocrinol (Lausanne) 2024; 15: 1309005.ArticlePubMedPMC
- 9. Pyo JS, Sohn JH, Kang G. Core needle biopsy is a more conclusive follow-up method than repeat fine needle aspiration for thyroid nodules with initially inconclusive results: a systematic review and meta-analysis. J Pathol Transl Med 2016; 50: 217-24. ArticlePubMedPMCPDF
- 10. Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute Thyroid fine needle aspiration state of the science conference: a summation. Cytojournal 2008; 5: 6.ArticlePubMedPMC
- 11. Park KT, Ahn SH, Mo JH, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck 2011; 33: 160-5. ArticlePubMed
- 12. Samir AE, Vij A, Seale MK, et al. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid 2012; 22: 461-7. ArticlePubMedPMC
- 13. Yi KS, Kim JH, Na DG, et al. Usefulness of core needle biopsy for thyroid nodules with macrocalcifications: comparison with fine-needle aspiration. Thyroid 2015; 25: 657-64. ArticlePubMedPMC
- 14. Renshaw AA, Pinnar N. Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol 2007; 128: 370-4. ArticlePubMed
- 15. Sung JY, Na DG, Kim KS, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol 2012; 22: 1564-72. ArticlePubMedPDF
- 16. Cortazar-Garcia R, Martin-Escalante MD, Robles-Cabeza L, Martinez-Santos C. Usefulness of ultrasound-guided core biopsy in thyroid nodules with inconclusive fine-needle aspiration biopsy findings. Radiologia (Engl Ed) 2022; 64: 195-205. ArticlePubMed
- 17. Ahn SH. Usage and diagnostic yield of fine-needle aspiration cytology and core needle biopsy in thyroid nodules: a systematic review and meta-analysis of literature published by Korean authors. Clin Exp Otorhinolaryngol 2021; 14: 116-30. ArticlePubMedPMCPDF
- 18. Kwon H, Lee J, Hong SW, Kwon HJ, Kwak JY, Yoon JH. Fine needle aspiration cytology vs. core needle biopsy for thyroid nodules: a prospective, experimental study using surgical specimen. Taehan Yongsang Uihakhoe Chi 2022; 83: 645-57. ArticlePubMedPMCPDF
- 19. Aysan E, Guler B, Kiran T, Idiz UO. Core needle biopsy in the diagnosis of thyroid nodules. Am Surg 2023; 89: 5170-4. ArticlePubMedPDF
- 20. Silverman JF, West RL, Finley JL, et al. Fine-needle aspiration versus large-needle biopsy or cutting biopsy in evaluation of thyroid nodules. Diagn Cytopathol 1986; 2: 25-30. ArticlePubMed
- 21. Stangierski A, Wolinski K, Martin K, Leitgeber O, Ruchala M. Core needle biopsy of thyroid nodules: evaluation of diagnostic utility and pain experience. Neuro Endocrinol Lett 2013; 34: 798-801. PubMed
- 22. Tessler FN, Middleton WD, Grant EG. Thyroid Imaging Reporting and Data System (TI-RADS): a user's guide. Radiology 2018; 287: 29-36. ArticlePubMed
- 23. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): white paper of the ACR TI-RADS Committee. J Am Coll Radiol 2017; 14: 587-95. PubMed
- 24. Jung CK, Baek JH, Na DG, Oh YL, Yi KH, Kang HC. 2019 Practice guidelines for thyroid core needle biopsy: a report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association. J Pathol Transl Med 2020; 54: 64-86. ArticlePubMedPMCPDF
- 25. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159-74. ArticlePubMed
- 26. Hahn SY, Shin JH, Oh YL, Park KW, Lim Y. Comparison between fine needle aspiration and core needle biopsy for the diagnosis of thyroid nodules: effective indications according to US findings. Sci Rep 2020; 10: 4969.ArticlePubMedPMCPDF
- 27. Trimboli P, Nasrollah N, Guidobaldi L, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol 2014; 12: 61.ArticlePubMedPMCPDF
- 28. Yeon JS, Baek JH, Lim HK, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology 2013; 268: 274-80. ArticlePubMed
- 29. Moon WJ, Baek JH, Jung SL, et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 2011; 12: 1-14. ArticlePubMedPMC
- 30. Seo HS, Lee DH, Park SH, Min HS, Na DG. Thyroid follicular neoplasms: can sonography distinguish between adenomas and carcinomas? J Clin Ultrasound 2009; 37: 493-500. ArticlePubMed
- 31. Mehanna R, Murphy M, McCarthy J, et al. False negatives in thyroid cytology: impact of large nodule size and follicular variant of papillary carcinoma. Laryngoscope 2013; 123: 1305-9. ArticlePubMedPDF
- 32. Jung CK, Bychkov A, Kakudo K. Update from the 2022 World Health Organization classification of thyroid tumors: a standardized diagnostic approach. Endocrinol Metab (Seoul) 2022; 37: 703-18. ArticlePubMedPMCPDF
- 33. Yeh MW, Demircan O, Ituarte P, Clark OH. False-negative fine-needle aspiration cytology results delay treatment and adversely affect outcome in patients with thyroid carcinoma. Thyroid 2004; 14: 207-15. ArticlePubMed
- 34. Erdem Toslak I, Martin B, Barkan GA, Kilic AI, Lim-Dunham JE. Patterns of sonographically detectable echogenic foci in pediatric thyroid carcinoma with corresponding histopathology: an observational study. AJNR Am J Neuroradiol 2018; 39: 156-61. ArticlePubMedPMC
- 35. Gwon HY, Na DG, Noh BJ, et al. Thyroid nodules with isolated macrocalcifications: malignancy risk of isolated macrocalcifications and postoperative risk stratification of malignant tumors manifesting as isolated macrocalcifications. Korean J Radiol 2020; 21: 605-13. ArticlePubMedPMCPDF
- 36. Nabahati M, Ghaemian N, Moazezi Z, Mehraeen R. Different sonographic features of peripheral thyroid nodule calcification and risk of malignancy: a prospective observational study. Pol J Radiol 2021; 86: e366-71. ArticlePubMedPMCPDF
- 37. Choi SH, Han KH, Yoon JH, et al. Factors affecting inadequate sampling of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Clin Endocrinol (Oxf) 2011; 74: 776-82. ArticlePubMed
- 38. Garcia Pascual L, Surralles ML, Morlius X, Gonzalez Minguez C, Viscasillas G, Lao X. Ultrasound-guided fine needle aspiration of thyroid nodules with on-site cytological examination: Diagnostic efficacy, prevalence, and factors predicting for Bethesda category I results. Endocrinol Diabetes Nutr (Engl Ed) 2019; 66: 495-501. ArticlePubMed
- 39. Wang CY, Zhou Y, Ren YY, Luan YS, Jiang ZC, Wang ZX. Analysis of the influencing factors on fine-needle aspiration biopsy results of the thyroid. Front Surg 2022; 9: 907086.ArticlePubMedPMC
- 40. Fu Y, Sun Y, Pei Q, et al. Factors influencing the sample adequacy of ultrasound-guided fine-needle aspiration from solid thyroid nodules for liquid-based cytology: a demographic, sonographic, and technical perspective. Medicina (Kaunas) 2022; 58: 1639.ArticlePubMedPMC
- 41. Pantanowitz L, Thompson LDR, Jing X, Rossi ED. Is thyroid core needle biopsy a valid compliment to fine-needle aspiration? J Am Soc Cytopathol 2020; 9: 383-8. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
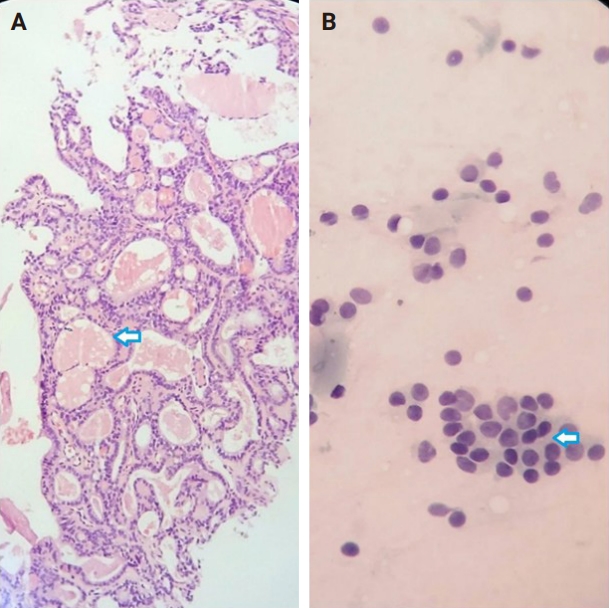
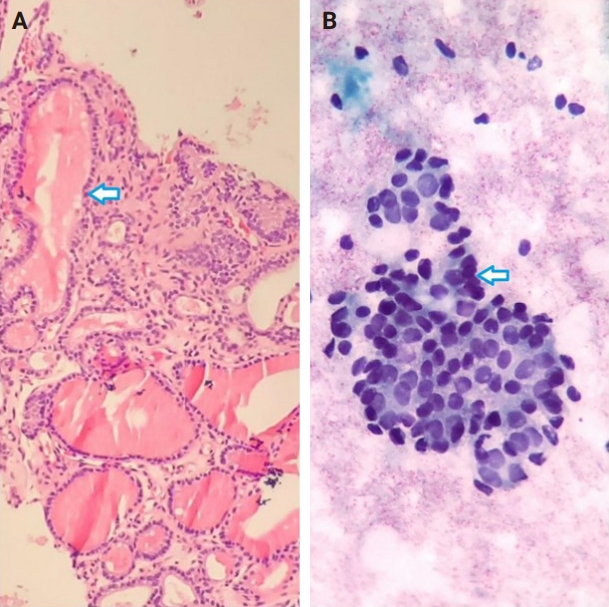
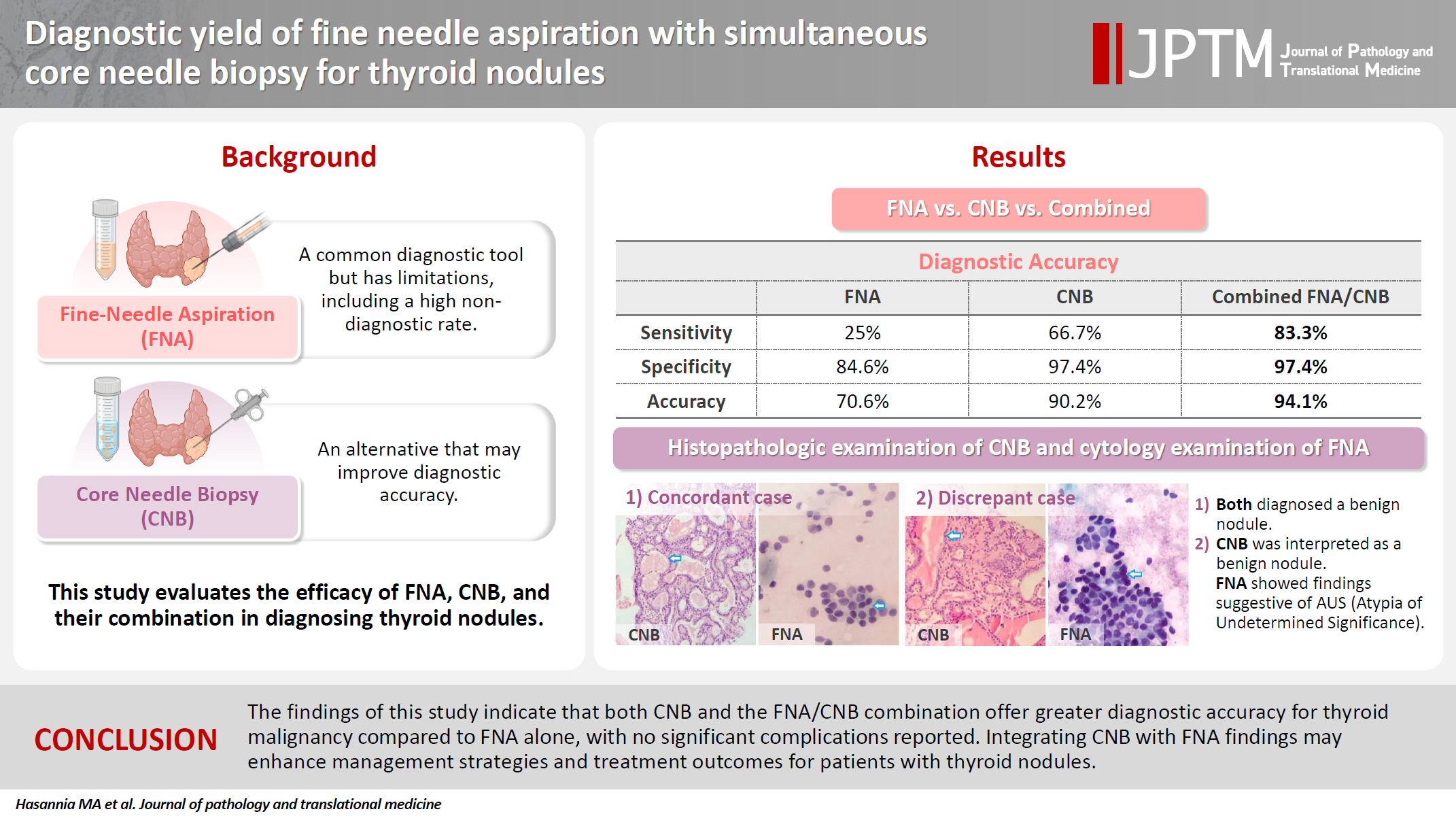
Fig. 1.
Fig. 2.
Graphical abstract
| Variable | Value |
|---|---|
| Age (y/o) | 54.23 ± 8.63 |
| Size of nodule (mm) | 27.0 ± 12.3 |
| Distance from skin (mm) | 12.2 ± 4.7 |
| TI-RADS | |
| 3 | 19 (33.9) |
| 4 | 29 (51.8) |
| 5 | 8 (14.3) |
| Calcification type | |
| None | 30 (53.6) |
| Large comet-tail artifact | 3 (5.4) |
| Macrocalcification | 10 (17.9) |
| Peripheral/rim calcification | 1 (1.8) |
| Punctate echogenic foci | 12 (21.4) |
| Category | FNA | CNB | FNA/CNB |
|---|---|---|---|
| I | 6 (11.8) | 0 | 0 |
| II | 34 (66.7) | 39 (76.5) | 38 (74.5) |
| III | 4 (7.8) | 2 (3.9) | 3 (5.9) |
| IV | 1 (1.9) | 2 (3.9) | 0 |
| V | 3 (5.9) | 0 | 0 |
| VI | 3 (5.9) | 8 (15.7) | 10 (19.6) |
| Category | FNA |
CNB |
FNA/CNB |
|||
|---|---|---|---|---|---|---|
| Benign (n = 39) | Malignant (n = 12) | Benign (n = 39) | Malignant (n = 12) | Benign (n = 39) | Malignant (n = 12) | |
| I | 6 (15.4) | 0 | 0 | 0 | 0 | 0 |
| II | 33 (84.6) | 1 (8.3) | 38 (97.4) | 1 (8.3) | 38 (97.4) | 0 |
| III | 0 | 4 (33.3) | 1 (2.6) | 1 (8.3) | 1 (2.6) | 2 (16.7) |
| IV | 0 | 1 (8.3) | 0 | 2 (16.7) | 0 | 0 |
| V | 0 | 3 (25) | 0 | 0 | 0 | 0 |
| VI | 0 | 3 (25) | 0 | 8 (66.7) | 0 | 10 (83.3) |
| Diagnostic values | FNA | CNB | FNA/CNB |
|---|---|---|---|
| Sensitivity (%) | 25 | 66.7 | 83.3 |
| Specificity (%) | 84.6 | 97.4 | 97.4 |
| Positive predictive value (%) | 100 | 100 | 100 |
| Negative predictive value (%) | 97.1 | 97.4 | 100 |
| Accuracy | 70.6 | 90.2 | 94.1 |
Values are presented as mean ± SD or number (%). SD, standard deviation; TI-RADS, Thyroid Imaging Reporting and Data System.
Values are presented as number (%). CNB, core needle biopsy; FNA, fine needle aspiration.
Values are presented as number (%). FNA, fine needle aspiration; CNB, core needle biopsy.
FNA, fine needle aspiration; CNB, core needle biopsy.

 E-submission
E-submission