Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 59(4); 2025 > Article
-
Review Article
Multiple sclerosis: a practical review for pathologists -
Rachel A. Multz, Pouya Jamshidi
 , Jared T. Ahrendsen
, Jared T. Ahrendsen
-
Journal of Pathology and Translational Medicine 2025;59(4):203-213.
DOI: https://doi.org/10.4132/jptm.2025.05.20
Published online: June 27, 2025
Department of Pathology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
- Corresponding Author Jared T. Ahrendsen, MD, PhD, Department of Pathology, Northwestern University Feinberg School of Medicine, 710 N. Fairbanks Court, Olson 2-462, Chicago, IL 60611, USA Tel: +1-312-695-0416 Fax: +1-312-503-8249 E-mail: Jared.Ahrendsen@nm.org
- *This invited review is a featured collaboration with PathologyOutlines.com.
© The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Multiple sclerosis (MS) is an immune-mediated demyelinating disorder of the central nervous system. It is a chronic disorder resulting in neurologic dysfunction that is disseminated both in time (multiple discrete episodes) and space (involving multiple sites). Histologically, MS is characterized by localized loss of myelin with relative preservation of axons. This review will discuss the epidemiology, clinical, laboratory, radiologic, and pathologic features of multiple sclerosis, as well as briefly touch on the differential diagnosis, treatment, and prognosis of the disease, especially as they relate to the pathologic interpretation of tissue specimens.
- Multiple sclerosis (MS) is a primary immune-mediated demyelinating disorder of the central nervous system (CNS), notable for significant clinical and pathologic heterogeneity, and results in substantial morbidity amongst affected patients. MS is a chronic disorder resulting in neurologic dysfunction that is disseminated in time, in the form of multiple discrete episodes, as well as in space, resulting in multiple discreet lesions. While the pathogenesis of MS remains elusive, substantial efforts in recent years has led to new treatment options and dramatically improved patient outcomes. The pathology is heterogenous but key histopathologic features of MS include localized loss of myelin with relative preservation of axons and eventual glial scar formation, though several unique pathologic patterns exist. In this review, we provide a succinct discussion of the epidemiology, clinical, laboratory, radiologic, and pathologic features of multiple sclerosis, as well as briefly touch on the differential diagnosis, treatment, and prognosis of the disease.
INTRODUCTION
- The etiology of MS is not well understood. However, it is favored to be due to a combination of genetic and environmental risk factors that lead to dysfunction of immune cells within the CNS. T lymphocytes, B lymphocytes, and macrophages/microglia have all been implicated in the pathogenesis of MS, though a specific etiologic agent has not been identified [1]. Epstein-Barr virus (EBV) infection has been posited as a causative agent for the immune dysfunction seen in MS. While there is a strong association between EBV infection and development of MS (one study cited a 32-fold increase in risk for the development of MS), the evidence of causality is inconclusive [2]. Similarly, genome-wide association studies have discerned that the HLA-DRB1*1501 haplotype confers an increased risk for the development of MS [1].
- There are several other environmental and lifestyle risk factors that have been associated with MS. Cigarette smoking and exposure to secondhand smoke are associated with an increased risk for developing MS [3]. Moreover, smoking has been shown to increase the risk of developing antibodies against drugs used to treat MS [4]. Interestingly, the use of oral/chewing tobacco or finely ground smokeless tobacco (‘snuff’) has been shown to have protective effects against the development of MS, implying that factors other than direct nicotine exposure confers the increased risk of MS in cigarette smokers [5]. Geographic location (i.e., living at higher latitude), obesity, decreased sun exposure, and low vitamin D levels also correlate with an increased risk of developing MS [3].
- Epidemiologically, MS is the most common cause of non-traumatic neurologic disability in young adults [6]. There is a female predominance, particularly in the relapsing-remitting type, with a female to male ratio ranging from 2:1 to 3:1 [7] and an average age of onset between 28 and 31 years. MS is most common among individuals from North America, Western Europe, and Australasia, and least common in sub-Saharan Africa and Oceania [8].
ETIOLOGY AND EPIDEMIOLOGY
- There are several clinical subtypes of MS. While each has a unique clinical course, they are all characterized by one or more episodes of neurologic dysfunction with distinct imaging findings. Presenting symptoms vary based on the anatomic location affected. Common presenting signs and symptoms include optic neuritis, diplopia, dysarthria, weakness, paresthesia, and bowel/bladder incontinence. Symptom onset is typically gradual, worsening over the course of hours to days, and then subsiding after 2–4 weeks [9]. The most common clinical subtype of MS is relapsing-remitting MS (RRMS), which involves episodic neurologic deficits that may partially or fully resolve over time but are followed by subsequent relapses. At the point when the neurologic dysfunction transitions from being episodic in nature to a steady decline in neurologic functions, it is known as secondary progressive MS (SPMS). In primary progressive MS (PPMS), patients will show gradual and progressive neurologic decline from the initial onset of symptoms without intervening symptomatic improvement [10].
- Occasionally, patients present acutely with fulminant demyelinating disease. One form of this presentation is the Marburg variant of MS, a rare subtype with a rapid clinical course, usually resulting in death within one year of symptom onset [11]. Acute/fulminant MS may also present as a pseudotumor (‘tumefactive MS’), appearing mass-like on imaging and causing cerebral edema [12]. Both the Marburg variant and tumefactive MS tend to occur in younger individuals (20s–30s) and are the most likely variants of MS to undergo neurosurgical biopsy and sent for intraoperative consultation and histopathologic assessment.
- MS is a diagnosis that is made using a combination of clinical, imaging, and laboratory information. The McDonald criteria, outlining what defines dissemination in time and dissemination in space, can be used in cases where there is clinical suspicion for MS. The criteria are based primarily on the number of episodes of neurologic dysfunction the patient has experienced and how many lesions are seen on neuroimaging studies [13]. In addition to clinical and imaging findings, the presence of oligoclonal bands in the cerebrospinal fluid (CSF) can support the diagnosis of MS when there is insufficient data to demonstrate dissemination in time, but dissemination in space is present [14]. However, lumbar puncture with CSF analysis is not required for the diagnosis of MS and the presence of oligoclonal bands is not specific for MS.
CLINICAL AND LABORATORY FEATURES
- Imaging studies are crucial to the diagnosis and monitoring of patients with MS. Magnetic resonance imaging (MRI) is the preferred modality, as findings on computed tomography are often nonspecific. MS can involve any site in the CNS, typically involving the white matter but can also extend into the gray matter. The most common sites of involvement are periventricular and subcortical white matter, corpus callosum, white matter of the brainstem and cerebellum, and white matter tracts of the spinal cord (preferentially affecting the cervical spine) [15]. There is nearly always involvement of the optic nerve/chiasm.
- The typical MRI appearance of an acute MS lesion is a T2- or FLAIR-hyperintense lesion that is ovoid in shape and measures at least 3 mm, sometimes measuring up to 1 or 2 cm (Fig. 1A) [15]. There may or may not be a corresponding hypointense lesion on T1 weighted sequences (‘black hole’) [16]. A characteristic finding in periventricular plaques is the radial orientation perpendicular to the lateral ventricles, a pattern known as “Dawson’s fingers” (Fig. 1B) [17]. Chronic MS plaques may persist as T1 hypointensities, reflecting the extent of demyelination and associated tissue damage. Healing or fully healed plaques will convert from being hypointense to isointense [16].
- Tumefactive MS and other atypical variants (Marburg) have imaging findings distinct from classic MS plaques. These can appear as solitary or multiple lesions (>2 cm) which may show incomplete ring-like enhancement, mimicking a broad range of differential diagnoses. There is typically also mass effect and edema of the adjacent brain parenchyma. A particularly unique pattern of MS (both radiologically and histologically) is Baló concentric sclerosis, which is characterized by alternating layers of demyelination and preserved myelination that will appear mass-like on imaging. Patients with Baló concentric sclerosis demonstrate a subacute onset of symptoms, resulting in severe and often fatal neurologic deterioration [12].
RADIOLOGY
- Gross examination of the brains from MS patients can be performed at autopsy. Chronic MS plaques will appear tan-gray in color and can be variably sized. There will be a sharp border between the plaque and the surrounding brain parenchyma (Fig. 1C). In the spinal cord, plaques will have a fanned-out appearance and be similarly well-demarcated from the uninvolved parenchyma. Acute/subacute lesions in both the brain and the spinal cord will be tan-pink to tan-yellow with more ill-defined borders. Although the lesions are well-demarcated, they are not strictly bound to geographic areas of the brain and spinal cord and may involve multiple regions throughout the neuraxis [18]. In the spinal cord, this asymmetric pattern of demyelination helps to distinguish MS from other diseases that can affect white matter tracts in the spinal cord such as amyotrophic lateral sclerosis, subacute combined degeneration, and tabes dorsalis.
- The microscopic appearance of MS can vary based on the stage of the plaque. Active MS plaques are hypercellular lesions with numerous parenchymal and perivascular macrophages. There is also prominent perivascular lymphocytic inflammation consisting predominantly of CD8+ T lymphocytes; however, this finding is less specific for active demyelination than macrophages (Fig. 2A). Luxol fast blue (LFB) stain can highlight the loss of myelin and the presence of myelin globules within macrophages (Fig. 2A, inset). The involved brain parenchyma in an active plaque will show reactive gliosis with relative preservation of axons, sometimes with characteristic axonal swellings (‘spheroids’), best highlighted by a neurofilament immunohistochemistry (IHC) or silver stains. At the periphery of an active plaque there may be a narrow rim of partial remyelination with thinner myelin sheaths along the axons, known as a ‘shadow plaque’ (Fig. 2B, white arrow) [19,20].
- Chronic or inactive MS plaques are more hypocellular than their active counterparts. There will be near complete loss of myelin with associated reactive gliosis and only rare lymphocytes and macrophages (Fig. 2B–E). Areas of demyelination generally show paler staining with eosin on hematoxylin and eosin sections (Fig. 2C). The presence of rare macrophages indicates that the lesion is post-demyelination and may be termed a mixed active/inactive plaque [19]. Similar to active MS plaques, there will be background gliosis. LFB stain will highlight the sharply demarcated plaque with complete loss of myelin (Fig. 2B, D, E), while neurofilament IHC will demonstrate the relative preservation of axons.
- The rare variants of MS have distinct histologic findings. Marburg MS will show multiple poorly defined active plaques. There may even be areas of edema and/or necrosis. The typical features of active demyelination will also be present including numerous macrophages, perivascular lymphocytic cuffs, and background reactive gliosis [21]. Baló concentric sclerosis shows a unique histology of multiple concentric layers with alternating areas of demyelination and myelin preservation, corresponding to the similarly distinct MRI findings. The concentric pattern can be demonstrated with LFB and other myelin stains [22].
- IHC and other special stains can be useful to highlight the extent and temporal phase of disease. As previously mentioned, an LFB special stain will highlight the well-demarcated area of demyelination as well as myelin globules within macrophages in an active lesion (Fig. 2A). LFB can also highlight areas of partial remyelination/shadow plaque formation (Fig. 2B). In a chronic plaque, the border between the area of demyelination and normal brain parenchyma will be even sharper (Fig. 2D, E). Macrophage markers such as CD68 or CD163 will highlight abundant cells in areas of active demyelination. Axonal markers, such as neurofilament IHC or silver stains, will highlight the relative preservation of the axons (see discussion below). The combination of LFB and neurofilament IHC are useful to distinguish demyelination from a subacute infarct. Both entities will show numerous macrophages and myelin loss. However, subacute infarcts will also show loss/disruption of neurofilament-positive axons.
- Other stains that may be used but are not necessary for diagnosis include glial fibrillary acidic protein (GFAP) to highlight background reactive astrogliosis, CD3 to highlight the T cell infiltrates in an active plaque, and a Ki-67 which may be slightly elevated in the area of active demyelination but should not be misinterpreted as evidence of a neoplastic process. Stains that should be negative include viral markers such as SV40, herpes simplex virus, and human immunodeficiency virus; stains for other infectious organisms such as bacteria and fungi; and markers of glioma such as IDH1 (isocitrate dehydrogenase 1) R132H, p53, and ATRX (alpha-thalassemia/mental retardation syndrome X-linked). There should not be a significant CD20-positive B cell population [19].
- While electron microscopy is no longer used in routine clinical practice, there are characteristic ultrastructural findings in MS plaques [23]. Active plaques will show lymphocytes within the endothelium of blood vessels, axonal spheroids full of mitochondria and neurofilaments, and myelin globules within macrophages. There will also be a network of degenerated myelin surrounding the preserved axons. Chronic plaques will show axons with either thinner than expected myelin sheaths or no myelin whatsoever [23].
- Tissue biopsy is performed on rare occasions, where an intraoperative frozen section may be sent for either suspected demyelinating disease or demyelinating disease mimicking a mass lesion (Fig. 3A). On smear/frozen and permanent preparations, there will be sheets of foamy macrophages in a background of reactive-appearing astrocytes (Fig. 3B, C). The overall cell population is more heterogeneous than those seen in neoplastic processes. However, there may be striking reactive atypia, and caution should be exercised before diagnosing a macrophage-rich smear as a neoplasm. The picture may become clearer on permanent sections, as the sharp demarcation between the macrophage-rich area and background brain becomes apparent (Fig. 3C) [19]. A unique type of reactive astrocyte is often encountered in demyelinating diseases, called the Creutzfeldt-Peters cell. These astrocytes demonstrate abundant eosinophilic cytoplasm with multiple fragmented nuclei or condensed chromatin forming atypical starburst-like mitotic figures (Fig. 3C, inset). Stains can be helpful to confirm demyelination (Fig. 3D–F), as discussed above. In such cases, it is important to rule out malignancy and infection with the appropriate studies. On a practical note, it is often impossible to distinguish MS from other inflammatory demyelinating conditions based on the histopathologic findings in a small biopsy specimen. Clinical correlation and a descriptive diagnosis is often prudent, with a comment discussing the differential diagnoses.
PATHOLOGIC FINDINGS
- The histopathologic differential diagnosis of MS consists predominantly of other macrophage-rich and demyelinating lesions. In this section, we will cover a subset of the more common differential considerations, which are summarized in Table 1.
- Cerebral infarct
- A cerebral infarct can have a similar histologic appearance to MS in that it shows myelin loss with numerous macrophages. However, unlike MS, the lesions are usually distributed along vascular territories and show both axonal and myelin loss. Other features of ischemic injury will also be present such as acute neuronal necrosis and reactive endothelial hypertrophy. Neurofilament IHC will demonstrate loss/disruption of the axonal network in a cerebral infarct.
- Neuromyelitis optica
- Neuromyelitis optica (NMO) was once considered a variant of MS (Devic’s disease), but it is now a distinct pathologic entity. In particular, the serologic presence of anti-Aquaporin 4 (AQP4) IgG antibodies is a diagnostic feature of NMO that is not seen in patients with MS [24]. Optic neuritis seen in NMO is bilateral and severe while in MS it is usually unilateral [11]. Other laboratory findings in NMO that distinguish it from MS are pleocytosis of the CSF with the presence of neutrophils and eosinophils and elevated GFAP levels [25]. These features should be absent in MS patients. The imaging abnormalities of NMO in the spinal cord characteristically involve three or more vertebral levels, whereas MS spinal cord lesions are typically smaller. The lesions in the optic nerve/chiasm and cerebrum are also larger than those of MS. There can also be extensive (>70%) involvement of the gray matter, which is not typically seen in MS [25]. Patients with NMO are slightly older at the onset of symptoms than MS patients. As a confounding factor, some patients with NMO are negative for AQP4-IgG, and distinguishing these patients from MS patients can be challenging [25].
- Acute disseminated encephalomyelitis
- Acute disseminated encephalomyelitis (ADEM) is a monophasic demyelinating disease. It is most commonly seen in children, with an average age of onset ranging from 3 to 8 years, significantly younger than the typical MS patient population [26,27]. The vast majority of cases (70%–80%) are associated with either a recent viral illness (typically an upper respiratory tract infection) or, less commonly, recent vaccination [28]. Imaging findings show diffuse, large, poorly demarcated lesions within the white matter, sparing the periventricular areas [12]. This is in contrast to MS lesions which are well-demarcated and nearly always involve the periventricular white matter. MS should be considered if 2 or more of the following are present on imaging: 2 or more periventricular lesions, presence of ‘black holes,’ and absence of bilateral involvement [26]. ADEM lesions can be histologically distinct from MS in that all lesions appear of similar age with diffuse infiltration of lymphocytes and macrophages with ill-defined borders [12]. Unlike MS, there will be varying degree of axonal damage, with increased levels of microtubule-associated proteins such as tau detected in the CSF [28]. ADEM is generally self-limited with a full recovery within 4–6 weeks and no lasting neurologic sequelae in 50%–80% of cases [26,27]. A recent development is the recognition of anti–myelin oligodendrocyte glycoprotein antibodies associated with the development of recurrent forms of ADEM, which challenges the prior understanding of ADEM but is still considered clinically distinct from MS [26].
- Progressive multifocal leukoencephalopathy
- Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease caused by reactivation of JC polyomavirus (JCV) infection in the CNS [29]. Patients who develop PML are typically immunosuppressed, traditionally in patients with poorly controlled human immunodeficiency virus infection but increasingly seen in other immunosuppression contexts, such as therapy-related (e.g., monoclonal antibody therapy, anti-rejection therapy for transplant), or other etiologies [30,31]. Patients with MS are not immunosuppressed at initial presentation, though treatment with natalizumab, an effective immune modulating drug used in patients with RRMS, is strongly associated with the development of PML in certain at-risk individuals [32,33]. On imaging, PML will appear as T2 hyperintense lesions within the subcortical and deep white matter, though unlike MS they do not respect the border between the gray and white matter [29]. JCV viral studies can be performed on CSF, which can confirm the diagnosis. Other CSF parameters are typically within normal limits [29]. Histologically, there will be multiple foci of demyelination with infiltration of macrophages, though unlike MS there will be minimal lymphocytes. In addition, classic viral inclusions will be present in the oligodendrocytes with background bizarre-appearing astrocytes, which are not seen in MS [29]. These infected cells can be highlighted by an SV40 immunohistochemical stain, which will be negative in MS.
- Steroid treated primary CNS lymphoma
- Steroid administration can lead to transient radiologic improvement and histopathologic disappearance of malignant cells in patients with primary CNS lymphoma, sometimes referred to as the “vanishing tumor phenomenon”. This can lead to non-diagnostic biopsy with subsequent recurrence of the malignancy several weeks later [34], and, similar to tumefactive MS, is a potential diagnostic pitfall for surgical neuropathologists. Occasionally, there is a residual inflammatory reaction in the brain with associated demyelination (“sentinel lesion”), raising the possibility of MS and other primary inflammatory demyelinating conditions in the differential diagnosis [35]. Several features can help to distinguish MS from a steroid-treated primary central nervous system lymphoma (PCNSL). Steroid-treated lymphoma causes incomplete and irregular demyelination, in contrast to the confluent and sharply demarcated lesions in MS. The presence of apoptotic bodies and extensive T cell predominant inflammatory infiltrates also favors steroid-treated PCNSL over MS [36].
DIFFERENTIAL DIAGNOSIS
- The treatment of MS depends on both the phase of the disease and the clinical subtype. The mainstay of treatment for an acute exacerbation (or for a fulminant demyelinating disorder) is intravenous (IV) or oral corticosteroids [12,37,38]. If the patient exhibits a poor response to corticosteroid therapy, more aggressive options such as therapeutic plasma exchange or intravenous immunoglobulin may be considered, though their efficacy is limited [37,38].
- In recent years, there has been development of several disease-modifying therapies (DMTs) for the treatment of RRMS. Previously, medications such as interferons and glatiramer acetate were considered first-line therapy. However, the current standard treatment is IV monoclonal antibody therapy with either ocrelizumab or natalizumab [39]. As noted above, due to the increased risk of PML associated with natalizumab therapy, all patients are tested for latent JC virus prior to the initiation of treatment. Patients who test positive for JC virus are typically started on ocrelizumab, and patients who test negative can be safely started on natalizumab. Other monoclonal antibodies such as alemtuzumab have also shown efficacy in the treatment of RRMS; however, it is typically not recommended as first-line therapy due to significant toxicities [37,39]. Oral medications such as dimethyl fumarate or the oral sphingosine-1-phosphate receptor modulators such as fingolimod and siponimod are additional options [39].
- The treatment for SPMS is essentially the same as that of RRMS, though siponimod and ocrelizumab have shown the most promising, albeit modest, results in slowing progression of disease [37,39]. In cases of PPMS, ocrelizumab is the only U.S. Food and Drug Administration–approved medication that has been shown to slow the progression of disease [39,40].
- With the advent of these new therapies for the treatment of MS, the prognosis for patients with established disease, particularly RRMS, has improved significantly [37]. However, there are no consistently reliable prognostic factors to predict disease course and severity. Clinical characteristics that have been associated with a worse prognosis are bowel or bladder symptoms at disease onset, incomplete recovery from the first attack, a short interval between the first and second attacks, and an early accumulation of disability [41]. Anovulation through pregnancy, breastfeeding, or being peri- or post-menopausal is protective against the development of MS. Breastfeeding in particular has been shown to reduce the risk of postpartum relapse in patients with established MS [42]. While patients with MS are routinely monitored with serial imaging studies, the extent of T2 lesions seen on MRI does not necessarily correlate with disease severity [43,44].
TREATMENT AND PROGNOSTIC FACTORS
- MS is the most common immune-mediated demyelinating disorder. While the etiology is still poorly understood, the constellation of characteristic clinical, laboratory, and imaging findings can help in arriving at the diagnosis. We have discussed the varied clinical presentations of MS with corresponding imaging findings, the histologic appearance of MS, and the importance of differentiating MS from other demyelinating diseases and mimickers. The long-term survival of patients with the disease has greatly improved in recent years with the advent of several new DMTs. However, MS is still a chronic disease with a high rate of disability, unclear etiology, and currently incurable. Additional work is necessary to further improve the outcomes of patients with this disease.
CONCLUSION
Ethics Statement
Not applicable.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization: JTA. Supervision: JTA. Writing—original draft: all authors. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest.
Funding Statement
No funding to declare.



- 1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018; 378: 169-80. ArticlePubMedPMC
- 2. Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022; 375: 296-301. ArticlePubMed
- 3. Alfredsson L, Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb Perspect Med 2019; 9: a028944.ArticlePubMedPMC
- 4. Hedstrom AK, Alfredsson L, Lundkvist Ryner M, Fogdell-Hahn A, Hillert J, Olsson T. Smokers run increased risk of developing anti-natalizumab antibodies. Mult Scler 2014; 20: 1081-5. ArticlePubMedPDF
- 5. Hedstrom AK, Baarnhielm M, Olsson T, Alfredsson L. Tobacco smoking, but not Swedish snuff use, increases the risk of multiple sclerosis. Neurology 2009; 73: 696-701. ArticlePubMed
- 6. Ramagopalan SV, Sadovnick AD. Epidemiology of multiple sclerosis. Neurol Clin 2011; 29: 207-17. ArticlePubMed
- 7. Leray E, Moreau T, Fromont A, Edan G. Epidemiology of multiple sclerosis. Rev Neurol (Paris) 2016; 172: 3-13. ArticlePubMed
- 8. G.B.D. Multiple Sclerosis Collaborators. Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18: 269-85. ArticlePubMedPMC
- 9. Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers 2018; 4: 43.ArticlePubMedPDF
- 10. Klineova S, Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8: a028928.ArticlePubMedPMC
- 11. Capet N, Levraut M, Delourme A, et al. Marburg multiple sclerosis variant: complete remission with very early administration of mitoxantrone: a case report. Neurol Ther 2022; 11: 507-13. ArticlePubMedPDF
- 12. Rahmlow MR, Kantarci O. Fulminant demyelinating diseases. Neurohospitalist 2013; 3: 81-91. ArticlePubMedPMCPDF
- 13. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162-73. ArticlePubMed
- 14. Arrambide G, Tintore M, Espejo C, et al. The value of oligoclonal bands in the multiple sclerosis diagnostic criteria. Brain 2018; 141: 1075-84. ArticlePubMed
- 15. Filippi M, Preziosa P, Banwell BL, et al. Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain 2019; 142: 1858-75. ArticlePubMedPMCPDF
- 16. Sahraian MA, Radue EW, Haller S, Kappos L. Black holes in multiple sclerosis: definition, evolution, and clinical correlations. Acta Neurol Scand 2010; 122: 1-8. ArticlePubMed
- 17. Hemond CC, Bakshi R. Magnetic resonance imaging in multiple sclerosis. Cold Spring Harb Perspect Med 2018; 8: a028969.ArticlePubMedPMC
- 18. Matthews PM, Roncaroli F, Waldman A, et al. A practical review of the neuropathology and neuroimaging of multiple sclerosis. Pract Neurol 2016; 16: 279-87. ArticlePubMed
- 19. Kuhlmann T, Ludwin S, Prat A, Antel J, Bruck W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 2017; 133: 13-24. ArticlePubMedPDF
- 20. Lassmann H. Multiple sclerosis pathology: evolution of pathogenetic concepts. Brain Pathol 2005; 15: 217-22. ArticlePubMed
- 21. Nunes JC, Radbruch H, Walz R, et al. The most fulminant course of the Marburg variant of multiple sclerosis-autopsy findings. Mult Scler 2015; 21: 485-7. ArticlePubMedPDF
- 22. Stadelmann C, Bruck W. Lessons from the neuropathology of atypical forms of multiple sclerosis. Neurol Sci 2004; 25 Suppl 4: S319-22. ArticlePubMedPDF
- 23. Frohman EM, Racke MK, Raine CS. Multiple sclerosis: the plaque and its pathogenesis. N Engl J Med 2006; 354: 942-55. ArticlePubMed
- 24. Jarius S, Wildemann B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol 2013; 23: 661-83. ArticlePubMedPMC
- 25. Jurynczyk M, Craner M, Palace J. Overlapping CNS inflammatory diseases: differentiating features of NMO and MS. J Neurol Neurosurg Psychiatry 2015; 86: 20-5. ArticlePubMed
- 26. Paolilo RB, Deiva K, Neuteboom R, Rostasy K, Lim M. Acute disseminated encephalomyelitis: current perspectives. Children (Basel) 2020; 7: 210.ArticlePubMedPMC
- 27. Wang CX. Assessment and management of acute disseminated encephalomyelitis (ADEM) in the pediatric patient. Paediatr Drugs 2021; 23: 213-21. ArticlePubMedPMCPDF
- 28. Esposito S, Di Pietro GM, Madini B, Mastrolia MV, Rigante D. A spectrum of inflammation and demyelination in acute disseminated encephalomyelitis (ADEM) of children. Autoimmun Rev 2015; 14: 923-9. ArticlePubMedPMC
- 29. Schweitzer F, Laurent S, Cortese I, et al. Progressive multifocal leukoencephalopathy: pathogenesis, diagnostic tools, and potential biomarkers of response to therapy. Neurology 2023; 101: 700-13. ArticlePubMedPMC
- 30. Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 2010; 9: 425-37. ArticlePubMedPMC
- 31. Ahrendsen JT, Sehgal K, Sarangi S, et al. Progressive multifocal leukoencephalopathy after chimeric antigen receptor T-cell therapy for recurrent non-Hodgkin lymphoma. J Hematol 2021; 10: 212-6. ArticlePubMedPMC
- 32. Bohra C, Sokol L, Dalia S. Progressive multifocal leukoencephalopathy and monoclonal antibodies: a review. Cancer Control 2017; 24: 1073274817729901.ArticlePubMedPMC
- 33. Toboso I, Tejeda-Velarde A, Alvarez-Lafuente R, et al. New algorithms improving PML risk stratification in MS patients treated with natalizumab. Front Neurol 2020; 11: 579438.ArticlePubMedPMC
- 34. Okita Y, Narita Y, Miyakita Y, et al. Long-term follow-up of vanishing tumors in the brain: how should a lesion mimicking primary CNS lymphoma be managed? Clin Neurol Neurosurg 2012; 114: 1217-21. ArticlePubMed
- 35. Kvarta MD, Sharma D, Castellani RJ, et al. Demyelination as a harbinger of lymphoma: a case report and review of primary central nervous system lymphoma preceded by multifocal sentinel demyelination. BMC Neurol 2016; 16: 72.ArticlePubMedPMC
- 36. Barrantes-Freer A, Engel AS, Rodriguez-Villagra OA, et al. Diagnostic red flags: steroid-treated malignant CNS lymphoma mimicking autoimmune inflammatory demyelination. Brain Pathol 2018; 28: 225-33. ArticlePubMedPDF
- 37. Travers BS, Tsang BK, Barton JL. Multiple sclerosis: diagnosis, disease-modifying therapy and prognosis. Aust J Gen Pract 2022; 51: 199-206. ArticlePubMed
- 38. Arrambide G, Iacobaeus E, Amato MP, et al. Aggressive multiple sclerosis (2): treatment. Mult Scler 2020; 26: 1352458520924595.ArticlePubMed
- 39. Hauser SL, Cree BA. Treatment of Multiple Sclerosis: A Review. Am J Med 2020; 133: 1380-90. ArticlePubMedPMC
- 40. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376: 209-20. ArticlePubMed
- 41. Langer-Gould A, Popat RA, Huang SM, et al. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: a systematic review. Arch Neurol 2006; 63: 1686-91. ArticlePubMed
- 42. Langer-Gould A, Smith JB, Hellwig K, et al. Breastfeeding, ovulatory years, and risk of multiple sclerosis. Neurology 2017; 89: 563-9. ArticlePubMedPMC
- 43. Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. The natural history of multiple sclerosis: a geographically based study 9: observations on the progressive phase of the disease. Brain 2006; 129: 584-94. ArticlePubMed
- 44. Li DK, Held U, Petkau J, et al. MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology 2006; 66: 1384-9. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
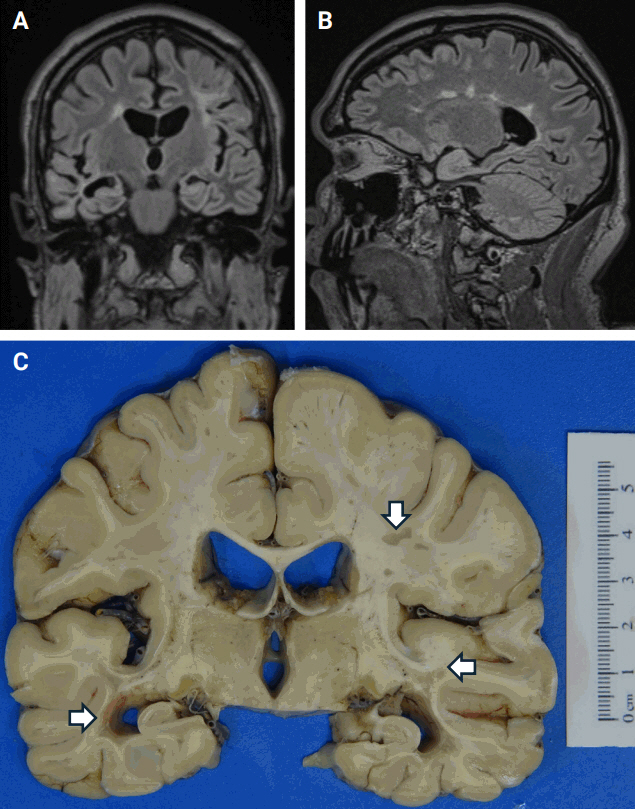


Fig. 1.
Fig. 2.
Fig. 3.
| Pathologic entity | Similarities to multiple sclerosis | Key differences from multiple sclerosis |
|---|---|---|
| Subacute cerebral infarct | Numerous macrophages | Disrupted axonal network |
| Loss of myelin | Involvement of a vascular territory | |
| Presence of other features suggestive of ischemia (acute neuronal necrosis, etc.) | ||
| Neuromyelitis optica | Primary inflammatory demyelinating pathology with T cells, B cells, and macrophages | Presence of anti-AQP4 IgG antibodies |
| Involvement of the optic nerve and spinal cord | Bilateral severe optic neuritis | |
| Spinal lesions span several vertebral segments | ||
| Extensive involvement of gray matter and more destructive than multiple sclerosis lesions with axonal loss | ||
| Presence of acute inflammatory infiltrates | ||
| Acute disseminated encephalomyelitis | Primary inflammatory demyelinating pathology with T cells, B cells, and macrophages | Distinct demographic (younger patients) and clinical symptomatology (monophasic illness, usually self-limited) |
| Relatively preserved axonal network | Small perivenous areas of demyelination | |
| Periventricular white matter often spared | ||
| Progressive multifocal leukoencephalopathy | Demyelinating pathology | Immune compromised patients |
| Relatively preserved axonal network | Enlarged oligodendrocytes with viral cytopathic change (plum-colored) | |
| Presence of bizarre astrocytes | ||
| SV-40 positive cells | ||
| Steroid treated primary CNS lymphoma | Demyelinating pathology with B cells and macrophages | Recent history of corticosteroid treatment |
| Relatively preserved axonal network | Variable presence of residual malignant lymphoma cells | |
| Scattered axonal spheroids | Scattered apoptotic bodies | |
| Patchy and incomplete demyelination | ||
| Greater T cell infiltration |
AQP4, Aquaporin 4; CNS, central nervous system.

 E-submission
E-submission





