Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(1); 2012 > Article
-
Original Article
Detection of Survivin and COX-2 in Thyroid Carcinoma: Anaplastic Carcinoma Shows Overexpression of Nuclear Survivin and Low COX-2 Expression - Young A Kim, Meesoo Chang, Young Joo Park1, Ji Eun Kim
-
Korean Journal of Pathology 2012;46(1):55-60.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.1.55
Published online: February 23, 2012
Department of Pathology, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- Corresponding Author: Ji Eun Kim, M.D. Department of Pathology, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, 425 Sindaebang 2-dong, Dongjak-gu, Seoul 156-707, Korea. Tel: +82-2-870-2642, Fax: +82-2-831-0261, npol181@snu.ac.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Overexpression of survivin, a member of the inhibitors of apoptosis protein, has been reported in various carcinomas, and its interaction with cyclooxygenase 2 (COX-2) results in accelerated tumor progression. The purpose of this study is to investigate the immunohistochemical expression of survivin and COX-2 in benign and malignant thyroid tissues and to define its association with pathologic and clinical features.
-
Methods
- We examined expression of survivin and COX-2 by immunohistochemistry in 334 benign and malignant thyroid tissues and evaluated their clinical significance.
-
Results
- Expression of survivin showed an increase along the spectrum of thyroid carcinoma progression; rarely positive in adenomatous goiter, moderately positive in papillary carcinoma, and strongly positive in anaplastic carcinoma (AC). Papillary microcarcinoma revealed the highest COX-2 positivity and AC demonstrated the lowest positivity among thyroid cancers. Node negative carcinomas showed higher COX-2 expression than node positive tumors. Survivin expression did not correlate with COX-2.
-
Conclusions
- Our findings suggest that survivin overexpression may be related to the pathogenesis of AC and can be a predictor of disease progression. COX-2 may be involved in the early phase of thyroid carcinoma.
- Case selection and specimens
- We estimated 338 thyroidectomy specimens from the files of the Department of Pathology, Seoul National University Hospital and Boramae Hospital, from 1993 to 2003. These tissue samples were from patients with adenomatous goiter (AG, n=57), follicular adenoma (FA, n=58), follicular carcinoma (FC, n=57), papillary carcinoma (PC, n=149, including 14 papillary microcarcinoma), or AC (n=17). Clinical data such as age, gender, tumor-node-metastasis stage, histological features, and clinical stage according to American Joint Committee on Cancer (AJCC) were reviewed and their association with protein expression levels was analyzed statistically in 168 patients who had thyroid cancer with available clinical data. This study was approved by the Institutional Review Board of Seoul National University Hospital.
- Immunohistochemistry on tissue array blocks
- Two cores of 2.0 mm tissue were obtained from the most representative part of individual cases and a new array block was constituted as previously described.12 Immunohistochemistry for survivin (1:400, rabbit polyclonal, R&D Systems, Minneapolis, MN, USA) and COX-2 (1:400, cx129, mouse monoclonal, Cayman, Ann Arbor, MI, USA) was performed in paraffin embedded tissue microarray sections. Briefly, all tissue sections underwent heat-induced epitope retrieval in pH 6.0 citrate buffer (Dako, Carpinteria, CA, USA) followed by 3% H2O2 solution for 10 minutes. Detection was performed using DAKO EnVision+ system (Dako), according to the manufacturer's instructions. Diaminobenzidine (Dako) was used as a chromogen, and the slides were counterstained with Mayer's hematoxylin (Dako) for 3 minutes.
- Evaluation of immunostain
- Nuclear expression of survivin and cytoplasmic expression of COX-2 were scored by evaluating the grade of stain intensity as well as the percentage of positive cells according to the previous study, with minor modification.13 Intensity of staining was graded along the following scale: 0 (no staining), 1+ (weak staining), 2+ (strong staining). For survivin, only distinct nuclear expression was counted as positive. The distribution of staining was estimated as 1 (less than 50% of cells staining) and 2 (50% or greater of cells staining). The final score was calculated by multiplying the intensity grade by the distribution grade.
- Statistical analysis
- All statistical analyses were conducted using the SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). Data were compared using the non-parametric Kruskal-Wallis test and the Mann-Whitney U test to assess differences between individual data sets. Correlation coefficients were generated using the Spearman's nonparametric correlation. Values of p<0.05 were considered statistically significant.
MATERIALS AND METHODS
- Clinicopathologic findings
- We analyzed clinical data of thyroid carcinoma cases, excluding AG and FA cases. Among 223 thyroid cancer cases, 168 cases (57 FC, 94 PC, and 17 AC) were available for the clinical data. There were 138 women and 30 men (age range, 19 to 88 years; median age, 45 years) in this pool and their median follow-up period was 18.5 months (range, 6.8 to 107 months). Fifty-one patients presented with stage I, 35 with stage II, 52 with stage III and 30 with stage IV disease. At the time of this study, 10 of 168 (5.1%) carcinoma patients died of disease including 9 AC and 1 FC. Summaries of the immunohistochemical findings in various thyroid lesions are presented in Tables 1 and 2 and the representative features are presented in Figs. 1-3.
- AC showed frequent expression of survivin and rare COX-2 expression
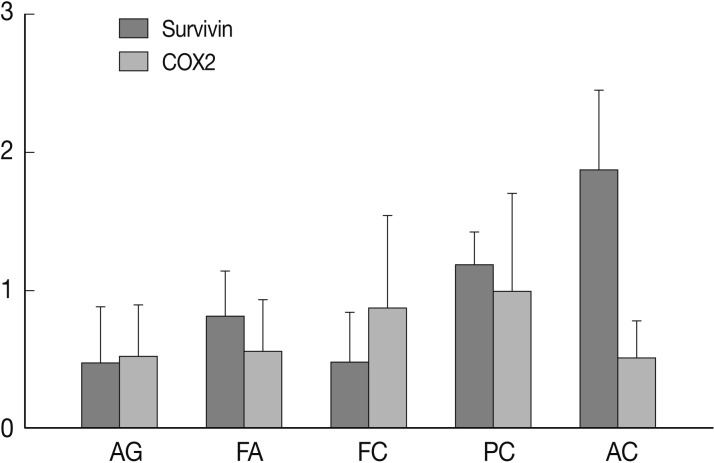
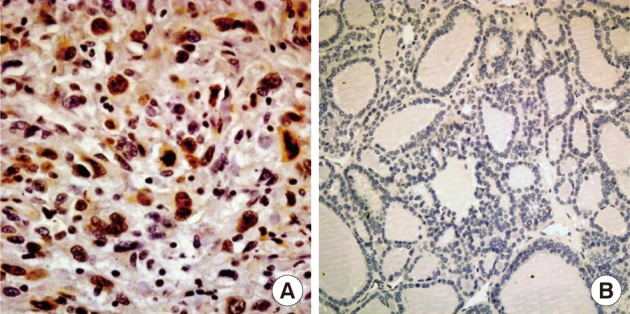
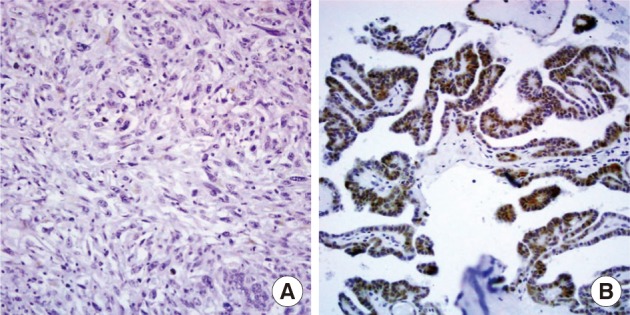
- Nuclear survivin expression was found in 10 of 57 (18%) AG, 21 of 58 (36%) FA, 13 of 57 (23%) FC, 73 of 149 (49%) PC at low levels, and 14 of 17 (82%) AC at high levels. Expression of survivin showed an increase along the spectrum of thyroid carcinoma progression, and AC showed the highest expression (p=0.047) (Fig. 1). Cytoplasmic expression of COX-2 was found in 10 of 57 (18%) AG, 10 of 58 (17%) FA, 18 of 58 (31%) FC, 61 of 149 (40%) PC, and 2 of 17 (12%) AC. COX-2 expression was generally low without significant differences among the histological types except AC which showed the lowest COX-2 expression (p=0.014) (Table 1).
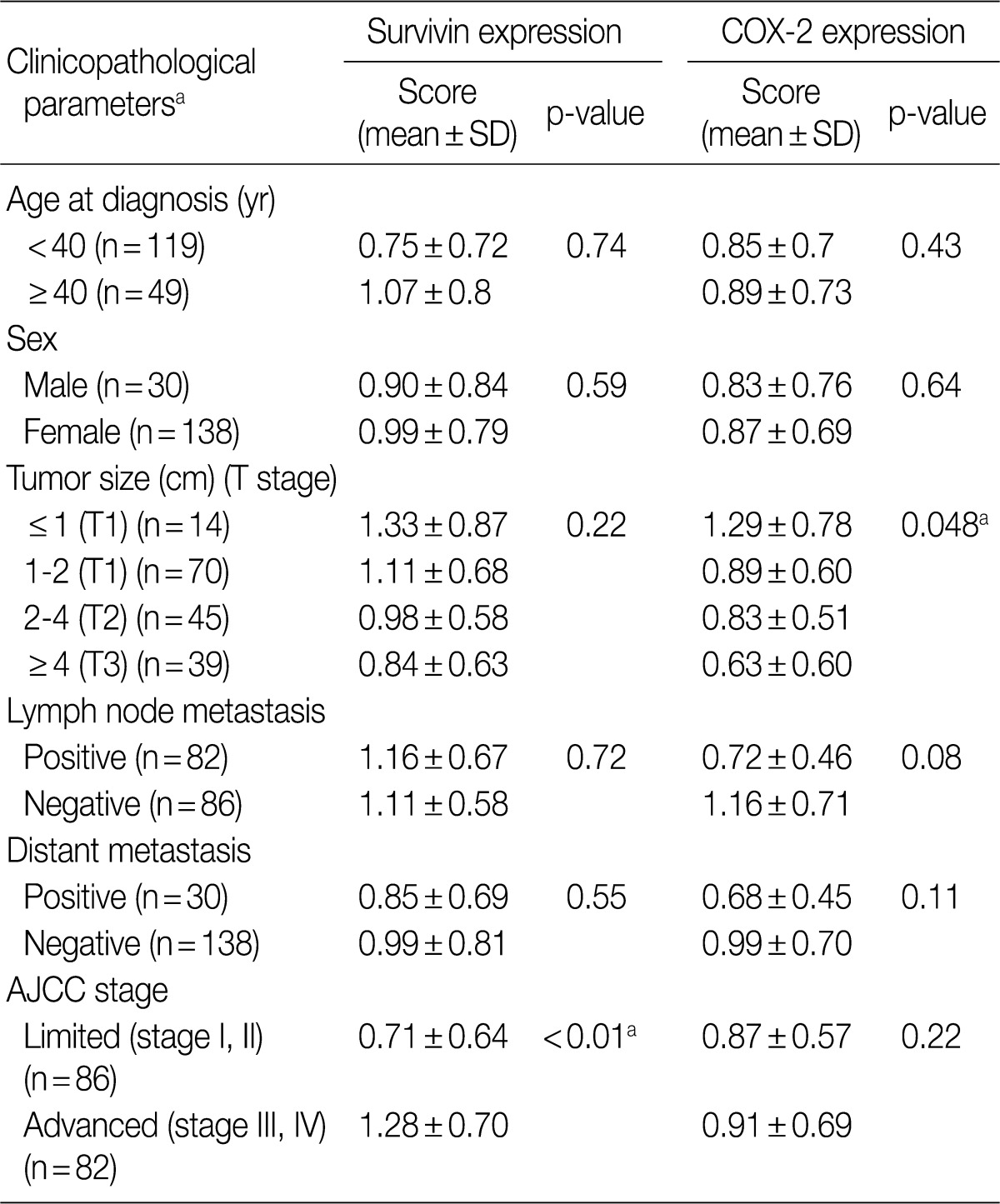
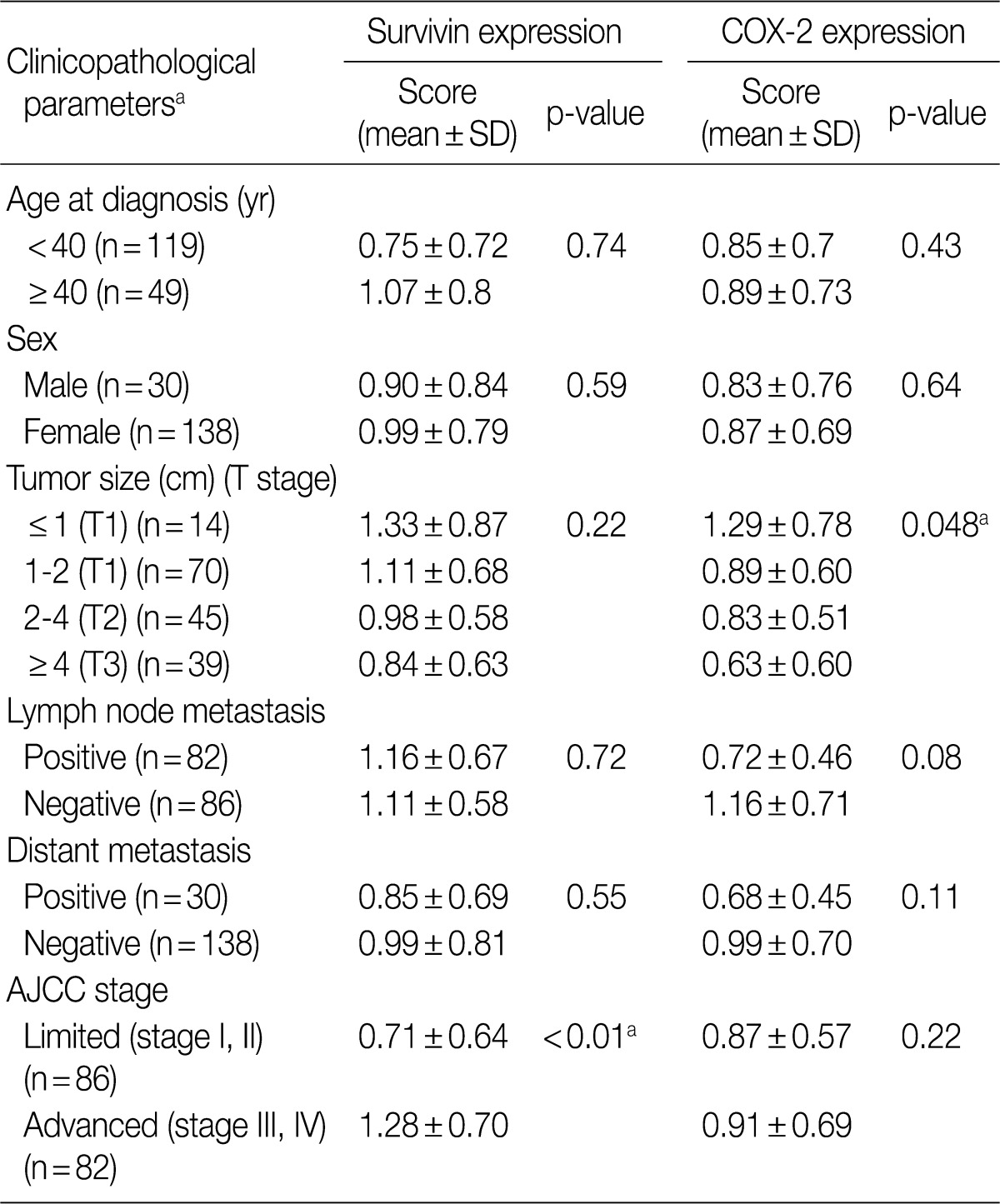
- Survivin expression correlated with advanced stage and COX-2 was frequently expressed in lymph node negative tumors and papillary microcarcinoma
- The relationship between clinicopathological parameters and expression of survivin and COX-2 in thyroid carcinomas (PC, FC, and AC) is summarized in Table 2. Survivin expression was significantly higher in clinically advanced cases (AJCC stage III and IV) than in limited cases (stage I, II) (p<0.01) and correlated well with the AJCC stage (p=0.02). No significant correlation was found between age, gender, distant metastasis, and the expression levels of survivin and COX-2. COX-2 immunoreactivity showed an inverse relationship with tumor size with papillary microcarcinomas (tumor size ≤1 cm) showing the highest expression among thyroid cancers (p=0.048). Lymph node positive tumors revealed lower COX-2 positivity than node negative tumors but the statistical power was borderline (p=0.08). Expression of survivin did not correlate with that of COX-2 among benign and malignant thyroidal lesions (p=0.35).
RESULTS
- More than 90% of all thyroid cancers including PC and FC are derived from malignant transformation of follicular cells. Although most thyroid cancer patients present favorable prognosis with proper management, a small number of patients develop AC, a more aggressive form of cancer that is unresponsive to radioactive iodine and chemotherapy. Although less than 1-3% of all thyroid cancers are anaplastic tumors, it contributes up to 14-50% of the annual mortality associated with thyroid cancer, and AC is mostly arise from the dedifferentiation of PCs.14 However, the exact pathogenesis of AC remains uncertain.
- Dysregulation of apoptosis is fundamentally involved in the development of many tumors including AC.14 AC cell lines express several antiapoptotic proteins, including survivin,15 and AC cells are extremely resistant to apoptosis after exposure to ionizing radiation therapy.16 Survivin, a known inhibitor of mitochondrial apoptosis, is associated with the aggressive characteristics of various cancers.3,5,12 In this study, we discovered a stepwise increase of survivin expression from hyperplastic tissue to PC to AC, and a correlation of survivin expression with clinical stage. Our results suggest that expression of survivin may be involved in disease progression of thyroid carcinoma and transformation of PC to AC. However, survivin may not be involved in FA to FC transition because FA showed a slightly higher expression of survivin than FC, although the level of expression in both lesions was very low compared to AC.
- There are several conflicting reports regarding the significance of survivin expression in various neoplasms. Some of these discrepancies are based on different interpretations of the cellular localization of survivin.10,17-19 We observed vague background staining patterns in the cytoplasm of some cases, especially in degenerated cysts and Hurthle cell lesions. Only distinct nuclear expression was counted as positive because survivin was shown to be bound to microtubules,20 which justifies its nuclear localization.
- There has been an increasing number of reports about COX-2 and its dysregulation of apoptosis.7,21 Sun et al.21 demonstrated that COX-2 can confer resistance to mitochondrial apoptosis and up-regulation of BCL-2. COX-2 expression has been found in thyroid carcinoma but its clinical relevance is controversial.22-24 COX-2 mRNA and protein levels are elevated in thyroid cancer tissues when compared with benign thyroid tissues,25 but contradictory results have also been reported.22 In this study, we observed that COX-2 was also present in benign tissues, and there was no significant difference in COX-2 expression between benign and malignant thyroid lesions. In fact, AC showed the lowest COX-2 expression. However, among the T1 stage tumors, only papillary microcarcinomas (tumors sized less than or equal to 1 cm) showed higher COX-2 immunoreactivity. Lymph node negative cancers and cancers without distant metastasis expressed less COX-2 positivity than their counterparts, although it failed to reach a statistically significant power. These findings suggest that COX-2 expression may contribute to development of early phase thyroid carcinoma, and the role of COX-2 diminishes as the cancer progresses. Our findings are in agreement with previous reports demonstrating that COX-2 expression is reduced in PC cases with large size and advanced stage,23 and papillary microcarcinomas upregulate COX-2 in comparison to ordinary PC.22 Regrettably, we cannot find evidences that COX-2 is a reliable marker for the differentiation of PC from other thyroid lesions as some researchers have suggested.25 Furthermore, it does not appear to be a diagnostically useful marker in thyroid cancer. However, low expression of COX-2 may reflect the relatively indolent nature of thyroid carcinomas compared with other carcinomas.
- A positive correlation between the expression of survivin and COX-2 has been demonstrated in some cancers including breast, endometrial, and ovarian carcinomas.9,11,26 Both proteins are involved in mitochondrial antiapoptotic mechanisms and angiogenesis, and are upregulated by external stresses like hypoxia.27,28 In this present study, we could not find a positive correlation between the expressions of these two proteins. COX-2 immunoreactivity was very low in most thyroid carcinomas, compared to the reported breast, endometrial and ovarian cancers.9,11,26 Such differences in the prevalence of expression may be explained by the intrinsic biological differences between the above aggressive female cancers and thyroid cancer, which is characterized by a more indolent nature.
- New developments in cancer therapy are focusing on apoptosis-based therapy. Clinical trials of the antagonists of survivin and COX-2 have already been performed in a wide range of cancers as chemoprevention or as adjuvant therapy of established disease.29,30 As seen from our results, survivin signaling can be considered as a therapeutic target in AC whereas the role of COX-2 in thyroid carcinoma is controversial.
- In summary, our study demonstrates that nuclear expression of survivin is frequent and characteristic of AC, which may implicate survivin as a significant role player in the pathogenesis of AC. Furthermore, a gradual increase of survivin expression from benign tissue to AC, and correlation of survivin expression with clinical stage suggest that survivin is also involved in the disease progression of thyroid carcinoma. COX-2 expression may be related to the pathogenesis of thyroid carcinoma during the early stages. This may be demonstrated by papillary microcarcinomas and node negative cancers showing significantly higher COX-2 expression than later stage cancers. Survivin and COX-2 may contribute independently in different phases of thyroid carcinogenesis.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Segev DL, Umbricht C, Zeiger MA. Molecular pathogenesis of thyroid cancer. Surg Oncol 2003; 12: 69-90. ArticlePubMed
- 2. Ambrosini G, Adida C, Sirugo G, Altieri DC. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem 1998; 273: 11177-11182. ArticlePubMed
- 3. Tracey L, Pérez-Rosado A, Artiga MJ, et al. Expression of the NF-kappaB targets BCL2 and BIRC5/Survivin characterizes small B-cell and aggressive B-cell lymphomas, respectively. J Pathol 2005; 206: 123-134. PubMed
- 4. Adida C, Haioun C, Gaulard P, et al. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood 2000; 96: 1921-1925. PubMed
- 5. Kawasaki H, Toyoda M, Shinohara H, et al. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer 2001; 91: 2026-2032. ArticlePubMed
- 6. Herschman HR, Fletcher BS, Kujubu DA. TIS10, a mitogen-inducible glucocorticoid-inhibited gene that encodes a second prostaglandin synthase/cyclooxygenase enzyme. J Lipid Mediat 1993; 6: 89-99. PubMed
- 7. Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem 2001; 276: 18563-18569. ArticlePubMed
- 8. Krysan K, Dalwadi H, Sharma S, Põld M, Dubinett S. Cyclooxygenase 2-dependent expression of survivin is critical for apoptosis resistance in non-small cell lung cancer. Cancer Res 2004; 64: 6359-6362. ArticlePubMedPDF
- 9. Erkanli S, Bolat F, Kayaselcuk F, Demirhan B, Kuscu E. COX-2 and survivin are overexpressed and positively correlated in endometrial carcinoma. Gynecol Oncol 2007; 104: 320-325. ArticlePubMed
- 10. Costa P, Catarino AL, Silva F, Sobrinho LG, Bugalho MJ. Expression of prolactin receptor and prolactin in normal and malignant thyroid: a tissue microarray study. Endocr Pathol 2006; 17: 377-386. ArticlePubMedPDF
- 11. Barnes N, Haywood P, Flint P, Knox WF, Bundred NJ. Survivin expression in in situ and invasive breast cancer relates to COX-2 expression and DCIS recurrence. Br J Cancer 2006; 94: 253-258. ArticlePubMedPMCPDF
- 12. Ito Y, Yoshida H, Uruno T, et al. Survivin expression is significantly linked to the dedifferentiation of thyroid carcinoma. Oncol Rep 2003; 10: 1337-1340. ArticlePubMed
- 13. Kleinschmidt-DeMasters BK, Heinz D, McCarthy PJ, et al. Survivin in glioblastomas: protein and messenger RNA expression and comparison with telomerase levels. Arch Pathol Lab Med 2003; 127: 826-833. PubMed
- 14. Nagaiah G, Hossain A, Mooney CJ, Parmentier J, Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol 2011; 2011: 542358.ArticlePubMedPMCPDF
- 15. Tirrò E, Consoli ML, Massimino M, et al. Altered expression of c-IAP1, survivin, and Smac contributes to chemotherapy resistance in thyroid cancer cells. Cancer Res 2006; 66: 4263-4272. ArticlePubMedPDF
- 16. Namba H, Saenko V, Yamashita S. Nuclear factor-kB in thyroid carcinogenesis and progression: a novel therapeutic target for advanced thyroid cancer. Arq Bras Endocrinol Metabol 2007; 51: 843-851. ArticlePubMed
- 17. Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997; 3: 917-921. ArticlePubMedPDF
- 18. Antonaci A, Consorti F, Mardente S, Natalizi S, Giovannone G, Della Rocca C. Survivin and cyclin D1 are jointly expressed in thyroid papillary carcinoma and microcarcinoma. Oncol Rep 2008; 20: 63-67. ArticlePubMed
- 19. Martinez A, Bellosillo B, Bosch F, et al. Nuclear survivin expression in mantle cell lymphoma is associated with cell proliferation and survival. Am J Pathol 2004; 164: 501-510. ArticlePubMedPMC
- 20. Li F, Ambrosini G, Chu EY, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998; 396: 580-584. ArticlePubMedPDF
- 21. Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res 2002; 62: 6323-6328. PubMed
- 22. García-González M, Abdulkader I, Boquete AV, Neo XM, Forteza J, Cameselle-Teijeiro J. Cyclooxygenase-2 in normal, hyperplastic and neoplastic follicular cells of the human thyroid gland. Virchows Arch 2005; 447: 12-17. ArticlePubMedPDF
- 23. Ito Y, Yoshida H, Nakano K, et al. Cyclooxygenase-2 expression in thyroid neoplasms. Histopathology 2003; 42: 492-497. ArticlePubMedPDF
- 24. Lee KJ, Jung YS, Kim WH, Yoon TI, Joo HJ, Soh EY. Cyclooxygenase-2 expression in human thyroid disease. J Endocrinol Invest 2008; 31: 111-118. ArticlePubMedPDF
- 25. Lo CY, Lam KY, Leung PP, Luk JM. High prevalence of cyclooxygenase 2 expression in papillary thyroid carcinoma. Eur J Endocrinol 2005; 152: 545-550. ArticlePubMed
- 26. Athanassiadou P, Grapsa D, Athanassiades P, et al. The prognostic significance of COX-2 and survivin expression in ovarian cancer. Pathol Res Pract 2008; 204: 241-249. ArticlePubMed
- 27. Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest 2004; 114: 1117-1127. ArticlePubMedPMC
- 28. Dong Z, Venkatachalam MA, Wang J, et al. Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia: Hif-1-independent mechanisms. J Biol Chem 2001; 276: 18702-18709. PubMed
- 29. Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer 2003; 3: 46-54. ArticlePubMedPDF
- 30. Mrozek E, Kloos RT, Ringel MD, et al. Phase II study of celecoxib in metastatic differentiated thyroid carcinoma. J Clin Endocrinol Metab 2006; 91: 2201-2204. ArticlePubMed
REFERENCES
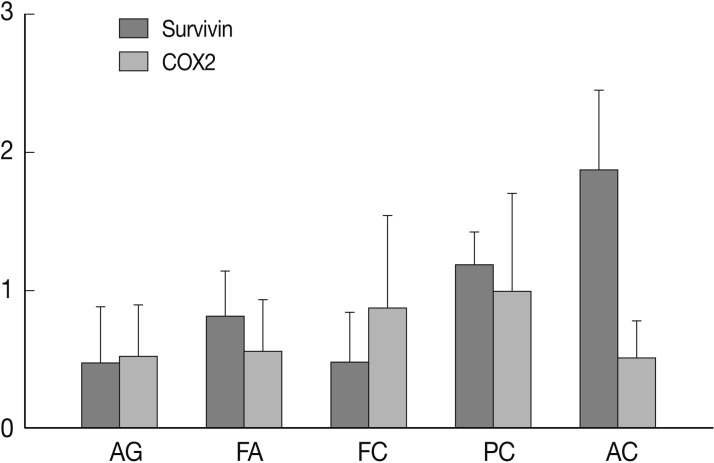
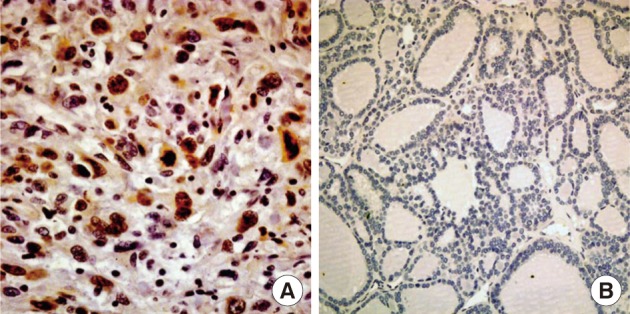
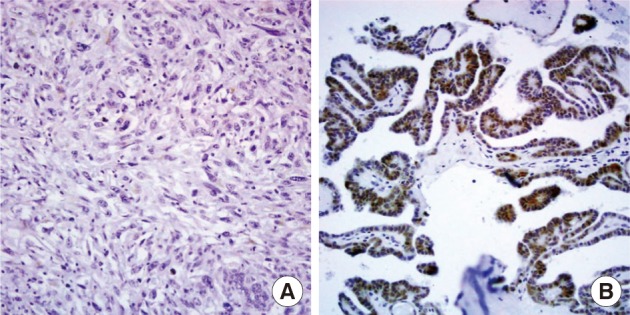
Figure & Data
References
Citations

- Survivin as a diagnostic and therapeutic marker for thyroid cancer
Mohammad-Reza Mahmoudian-Sani, Arash Alghasi, Ali Saeedi-Boroujeni, Akram Jalali, Mohammad Jamshidi, Ali Khodadadi
Pathology - Research and Practice.2019; 215(4): 619. CrossRef - TFAP2B overexpression contributes to tumor growth and progression of thyroid cancer through the COX-2 signaling pathway
Xiaoyan Fu, Huayong Zhang, Zhipeng Chen, Zhongyuan Yang, Dingbo Shi, Tianrun Liu, Weichao Chen, Fan Yao, Xuan Su, Wuguo Deng, Miao Chen, Ankui Yang
Cell Death & Disease.2019;[Epub] CrossRef - Expression of nm23-H1 and COX-2 in thyroid papillary carcinoma and microcarcinoma
Marija Milkovic Perisa, Bozena Sarcevic, Koraljka Gall Troselj, Kresimir Grsic, Sanda Sitic, Sven Seiwerth
Oncology Letters.2017; 13(5): 3547. CrossRef - The Diagnostic Usefulness of HMGA2, Survivin, CEACAM6, and SFN/14-3-3 δ in Follicular Thyroid Carcinoma
Min Hye Jang, Kyeong Cheon Jung, Hye Sook Min
Journal of Pathology and Translational Medicine.2015; 49(2): 112. CrossRef - Evaluation of survivin expression and its prognostic value in papillary thyroid carcinoma
Sonja Selemetjev, Tijana Isic Dencic, Ilona Marecko, Jelena Jankovic, Ivan Paunovic, Svetlana Savin, Dubravka Cvejic
Pathology - Research and Practice.2014; 210(1): 30. CrossRef



Fig. 1
Fig. 2
Fig. 3

SD, standard deviation; AG, adenomatous goiter; FA, follicular adenoma; FC, follicular carcinoma; PC, papillary carcinoma; AC, anaplastic carcinoma.
SD, standard deviation; AJCC, American Joint Committee on Cancer. aStatistically significant values.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article