Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(2); 2012 > Article
-
Case Report
Primary Monophasic Synovial Sarcoma Arising in the Mesentery: Case Report of an Extremely Rare Mesenteric Sarcoma Confirmed by Molecular Detection of aSYT-SSX2 Fusion Transcript - Han Suk Ryu, Ilyeong Heo1, Jae Soo Koh1, Sung-Ho Jin2, Hye Jin Kang3, Soo Youn Cho1
-
Korean Journal of Pathology 2012;46(2):187-191.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.2.187
Published online: April 25, 2012
1Department of Pathology, Chung-Ang University Medical Center, Chung-Ang University College of Medicine, Seoul, Korea.
2Department of Pathology, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences (KIRAMS), Seoul, Korea.
3Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences (KIRAMS), Seoul, Korea.
4Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences (KIRAMS), Seoul, Korea.
- Corresponding Author: Soo Youn Cho, M.D. Department of Pathology, Korea Cancer Center Hospital, 75 Nowon-gil, Nowon-gu, Seoul 139-706, Korea. Tel: +82-2-970-2545, Fax: +82-2-970-2430, sycho@kcch.re.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Synovial sarcoma arises in the para-articular tissues, and it can also occur in various unexpected sites. We report a rare case of primary monophasic synovial sarcoma (MSS) arising in the mesentery. A 59-year-old man presented with a palpable abdominal mass. On microscopic examination, the entire tumor comprised a dense proliferation of the spindle cells without epithelial components. The tumor cells were positive for transducin-like enhancer of split 1, bcl-2, epithelial membrane antigen and CD99 but negative for CD34, CD117, alpha-smooth muscle actin, cytokeratin, and calretinin on immunohistochemistry. The reverse transcriptase-polymerase chain reaction revealed a single 151-bp fragment representing the SYT-SSX2 fusion transcript. Because mesenteric MSS is extremely rare and many cases display histologic findings that overlap with those of more frequently involved tumors such as hemangiopericytoma and gastrointestinal stromal tumor, there is a chance of making an incorrect diagnosis that can result in an inappropriate treatment.
- A 59-year-old man was admitted to the hospital because of a 3-month-history of huge abdominal palpable mass in the right lower quadrant. However the patient presented with no other symptoms, such as abdominal pain or indigestion. Physical examination showed a smooth, bulging mass in the right lower abdomen, but revealed no other physical abnormalities. A magnetic resonance imaging of the abdomen revealed a well-defined heterogeneously enhanced giant mass involving the lower abdomen adjacent to the jejunum (Fig. 1). The suspected preoperative diagnosis was a sort of mesenteric or omental tumor, such as gastrointestinal stromal tumor (GIST), hemangiopericytoma or leiomyosarcoma.
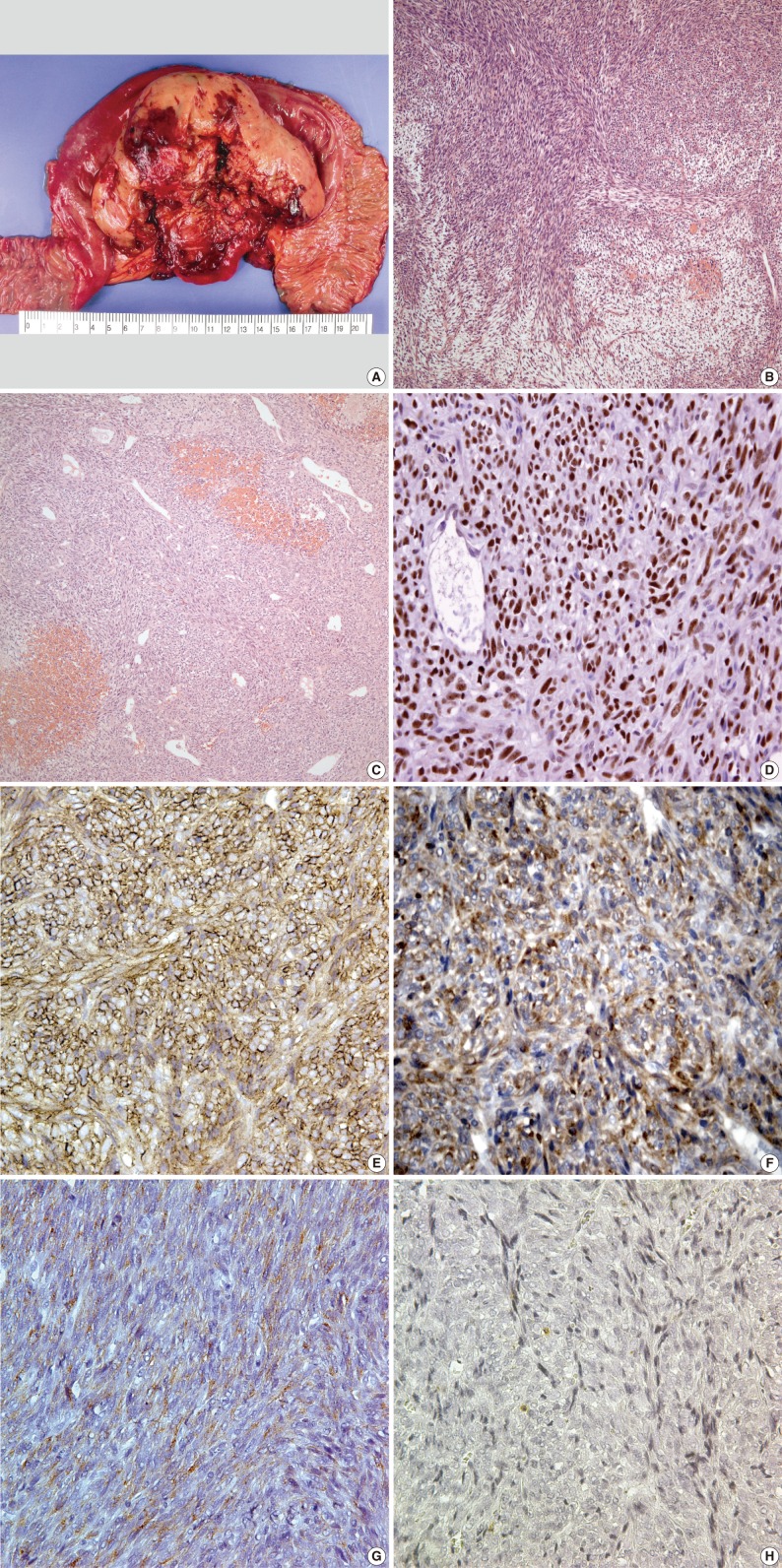
- Wide excision and segmental resection of the jejunum were performed. A large tumor was located in the mesentery, primarily around the jejunum, and it adhered to the outer wall of the jejunum. Postoperative adjuvant chemotherapy was performed using a combination regimen of adriamycin with cis-diamminedichloroplatinum for six cycles. Gross examination revealed a relatively well-circumscribed mass measuring 12×10×5 cm, and it was located throughout the outer wall of the small intestine and mesentery. The cut surface showed a tan, fish-like fleshy appearance with multifocal hemorrhage and friable areas (Fig. 2A). Microscopically, the tumor was well defined by a thin fibrous capsule and it grew into the outer wall of the jejunum from the mesenteric wall. There were dense cellular sheets with alternating hypocellular areas (Fig. 2B). Spindle cells were small, uniform and closely packed with scant cytoplasm. Relatively frequent mitotic figures were observed (more than 10/10 high power fields). Hemangiopericytomatous vascular arrangements were also identified in some areas. Stromal hyalinization was found in a small portion of the neoplasm (Fig. 2C). On immunohistochemistry, the tumor cells were positive for transducin-like enhancer of split 1 (TLE1; 1:20, Santa Cruz Biochemicals, Santa Cruz, CA, USA) (Fig. 2D), CD99 (1:100, Labvision, Neomarkers, Fremont, CA, USA) (Fig. 2E), bcl-2 (1:100, Dako, Glostrup, Denmark) (Fig. 2F), epithelial membrane antigen (EMA; 1:100, Dako) (Fig. 2G) and S-100 protein (1:1,000, Dako), but negative for alpha-smooth muscle actin (1:200, Labvision, Neomarkers), CD34 (1:200, Labvision, Neomarkers), cytokeratin AE1/AE3 (1:400, Biogenex, San Ramon, CA, USA), calretinin (predilution, Labvision, Neomarkers) and CD117 (1:200, Dako) (Fig. 2H). Ki-67 (1:100, Labvision, Neomarkers) was expressed in 5% of tumor cells.
- For identification of SYT-SSX fusion gene transcripts, an additional RT-PCR of t(X;18) was performed. Total RNA was isolated from a 4-µm paraffin-embedded tissue section. The cDNA was synthesized with SuperScript II RNase Reverse Transcriptase (GIBCO BRL Life Technologies, Gaithersburg, MD, USA) in the presence of random primers for an hour at 42℃. The cDNA was amplified using the forward and reverse primers: 5'-CCAGCAGAGGCCTTATGGATA-3'; 5'-GGTGCAGTTGTTTCCCATCG-3' for SYT; 5'-GGTGCAGTTGTTTCCCATCG-3' for SSX1; 5'-GGCACAGCTCTTTCCCATCA-3' for SSX2. The RT-PCR was carried out under the following thermocycling conditions: 35 amplification cycles, each consisting of denaturation at 94℃ for 30 seconds, annealing at 61℃ for 30 seconds and elongation at 72℃ for 30 seconds, with an extension at 72℃ for 1 minute. The amplified products were electrophoresed on a 1.5% agarose gel and then stained with ethidium bromide. The RT-PCR produced a band for the SYT-SSX2 fusion gene transcript (151 bp) (Fig. 3). The final diagnosis was primary mesenteric synovial sarcoma with monophasic fibrous type. Two years later, the patient presented with a recurrence of the multiple masses in the mesocolon. The patient is currently being treated with a combination chemotherapy regimen consisting of etoposide, ifosfamide, and cisplatin.
CASE REPORT
- Given the rarity of spindle cell tumor in the intra-abdominal cavity, it would be difficult to make a diagnosis of it. A total of 50 cases of intra-abdominal synovial sarcoma has been reported up to present.4,5 Of these, there was only one case of primary mesenteric synovial sarcoma with monophasic subtype according to a review of the English literature.3 A diagnostic approach to biphasic synovial sarcoma can also be made even without ancillary tests such as immunohistochemistry or molecular study. But this does not always apply to MSS, that arising in an unusual location. Because the MSS of the mesentery is a very rare, its diagnosis cannot be established before more frequently encountered spindle cell tumors including GIST and other kinds of sarcomas could be completely ruled out. This can be rather worrisome because patients may receive an inappropriate treatment if pathologists or physicians make an incorrect diagnosis of it, particularly in cases of MSS occurring in an uncommon site. As mentioned by Fisher et al.,6 MSS is commonly confused with other spindle cell malignancies such as fibrosarcoma, malignant hemangiopericytoma or malignant peripheral nerve sheath tumor (MPNST) as well as GIST according to microscopic findings due to the overlap of histological features. In the current case, the immunohistochemistry and microscopic findings were most compatible with MSS, rather than the sarcomas mentioned above. No immunoreactivity for cytokeratin AE1/AE3 was identified in the current case, although it is not an uncommon finding in those of MSS. According to Begueret et al.,7 only 58.3% of total cases of MSS had a focal positive reaction to cytokeratin AE1/AE3. These authors also noted that EMA expression was more commonly found in cases of MSS. Immunoreactivity for TLE1, CD99 and bcl-2 in spindle cells is not suggestive of fibrosarcoma but it is consistent with the immunohistochemical findings of synovial sarcoma,8 despite the presence of identical morphologic findings such as herringbone arrangement of spindle cells in some areas. In addition, there are often distinctive morphologic features in cases of MSS and these include thick collagen or a hemangiopericytomatous vascular pattern which are rarely seen in fibrosarcomas.8 Due to the hemangiopericytomatous vascular arrangements and higher incidence than synovial sarcoma of the intra-abdominal cavity,9 it is mandatory to make a differential diagnosis of MSS from hemangiopericytoma. It should be noted, however, the MSS has the "hemangiopericytomatous vascular arrangement" throughout the entire neoplasm. In addition, the MSS generally has a greater cellularity with a higher nuclear to cytoplasmic ratio, more mitotic figures and straighter cell alignments.10 Finally, the expression of CD34 is absent in cases of synovial sarcoma but it is typically seen in most cases of hemangiopericytoma.10 A differential diagnosis of MPNST should be made from MSS based on the differences in chemosensitivity between the two sarcomas.8 A differential diagnosis between the two tumors can be made not only with an ancillary test using a combination of S-100 protein and CD99 but also based on distinct nuclear findings such as wavy contour on microscopic examination. The GIST should also be considered in the diagnosis, although it can be distinguished from the MSS based on some distinctive histologic and immunohistochemical findings.8,11 GISTs are almost always positive for CD34 or CD117, whereas synovial sarcoma is negative for both markers.12
- According to Begueret et al.,7 however, unusual immunohistochemical results might occur in the intrathoracic MSS. These authors reported that CD34, S-100 protein and CD117 were expressed in 4-8% of MSS on the immunohistochemistry.7 In addition, TLE1 was also expressed in a number of tumors when it has been considered as specific markers for synovial sarcoma.13 Therefore, the molecular detection of the SYT-SSX fusion gene transcripts is necessary along with immunohistochemistry. Coindre et al.14 conducted a prospective study to investigate the utility of molecular testing in 204 patients with synovial sarcoma and concluded that it was very useful in detecting the MSS that occurred in uncommon or unexpected sites including the retroperitoneum and lung. We have therefore performed the RT-PCR to detect the SYT-SSX fusion gene transcript and thereby detected it in the current case. The t(X;18)(p11.2;q11.2) translocation is found in about 90% of synovial sarcomas and it is considered a pathogenic factor of this tumor.15 Two different types of breakpoints on chromosome X have been reported to correspond to the chimeric genes SYT-SSX1 and SYT-SSX2.16 Nearly all monophasic tumors bear the SYT-SSX2 fusion, as shown in the current case.8 Because the primary MSS of the mesentery is an extremely rare condition, it can be misdiagnosed as another type of spindle cell tumor. It is mandatory to recognize the occurrence of monophasic synovial sarcoma of the mesentery based on the morphological and genetic characteristics, which may help in making a correct diagnosis.
DISCUSSION
- 1. Morland B, Cox G, Randall C, Ramsay A, Radford M. Synovial sarcoma of the larynx in a child: case report and histological appearances. Med Pediatr Oncol 1994; 23: 64-68. ArticlePubMed
- 2. Paláu L MA, Thu Pham T, Barnard N, Merino MJ. Primary synovial sarcoma of the kidney with rhabdoid features. Int J Surg Pathol 2007; 15: 421-428. ArticlePubMedPDF
- 3. Buiga-Potcoavă R, Crişan D, Olinici CD. Primary intraabdominal synovial sarcoma: a case report. Rom J Gastroenterol 2005; 14: 67-69. PubMed
- 4. Cadman NL, Soule EH, Kelly PJ. Synovial sarcoma: an analysis of 34 tumors. Cancer 1965; 18: 613-627. ArticlePubMed
- 5. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer 1993; 72: 469-477. ArticlePubMed
- 6. Fisher C, Folpe AL, Hashimoto H, Weiss SW. Intra-abdominal synovial sarcoma: a clinicopathological study. Histopathology 2004; 45: 245-253. ArticlePubMed
- 7. Begueret H, Galateau-Salle F, Guillou L, et al. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol 2005; 29: 339-346. PubMed
- 8. Christopher DM, Fletcher K, Unni KK, Mertens F. World Health Organization classification of tumours: pathology and genetics of tumours of soft tissue and bone. 2002; Lyon: IARC Press, 200-204.
- 9. Enzinger FM, Smith BH. Hemangiopericytoma: an analysis of 106 cases. Hum Pathol 1976; 7: 61-82. PubMed
- 10. Weiss SW, Folpe AL. Enzinger and Weiss's soft tissue tumors. 2007; St. Louis: Mosby, 1161-1182.
- 11. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002; 33: 459-465. ArticlePubMed
- 12. Makhlouf HR, Ahrens W, Agarwal B, et al. Synovial sarcoma of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 10 cases. Am J Surg Pathol 2008; 32: 275-281. PubMed
- 13. Kosemehmetoglu K, Vrana JA, Folpe AL. TLE1 expression is not specific for synovial sarcoma: a whole section study of 163 soft tissue and bone neoplasms. Mod Pathol 2009; 22: 872-878. ArticlePubMedPDF
- 14. Coindre JM, Pelmus M, Hostein I, Lussan C, Bui BN, Guillou L. Should molecular testing be required for diagnosing synovial sarcoma? A prospective study of 204 cases. Cancer 2003; 98: 2700-2707. ArticlePubMed
- 15. Dal Cin P, Rao U, Jani-Sait S, Karakousis C, Sandberg AA. Chromosomes in the diagnosis of soft tissue tumors. I. Synovial sarcoma. Mod Pathol 1992; 5: 357-362. PubMed
- 16. de Leeuw B, Balemans M, Olde Weghuis D, Geurts van Kessel A. Identification of two alternative fusion genes, SYT-SSX1 and SYT-SSX2, in t(X;18)(p11.2;q11.2)-positive synovial sarcomas. Hum Mol Genet 1995; 4: 1097-1099. ArticlePubMed
REFERENCES
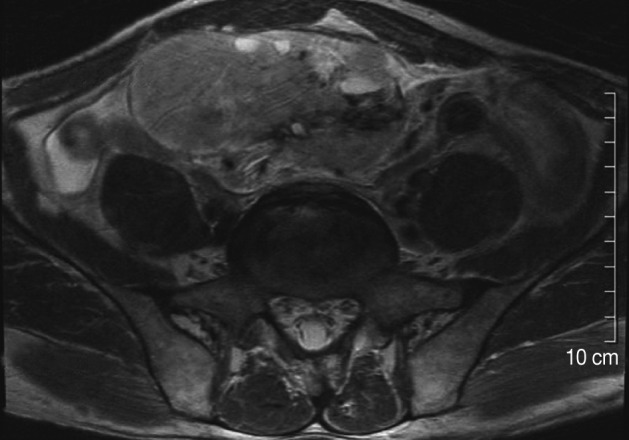
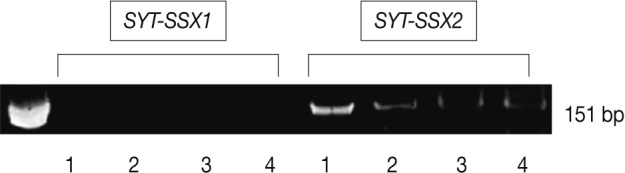
Figure & Data
References
Citations

- A case of primary mesenteric synovial sarcoma: a challenging presentation
Nihed Abdessayed, Malek Barka, Samiha Mabrouk, Zeineb Nfikha, Zeineb Maatoug, Yosra Fejji, Mohamed Salah Jarrar, Sabri Youssef, Moncef Mokni
Surgical Case Reports.2023;[Epub] CrossRef - Giant solitary fibrous tumor of the pelvis
Gerardo Palmieri, Carmine Grassi, Luigi Conti, Filippo Banchini, Maria Diletta Daccò, Gaetano M. Cattaneo, Patrizio Capelli
International Journal of Surgery Case Reports.2020; 77(D): S52. CrossRef - Tumeur neuroectodermique gastro-intestinale (GNET) : à propos d’un cas de tumeur du grêle avec métastases hépatiques
Thibault Kervarrec, Claire Lecointre, Rémy Kerdraon, Guido Bens, Arnaud Piquard, Patrick Michenet
Annales de Pathologie.2015; 35(6): 506. CrossRef




 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article