Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(3); 2012 > Article
-
Original Article
Urinary Decoy Cell Grading and Its Clinical Implications - Myoung Ju Koh, Beom Jin Lim, Songmi Noh, Yon Hee Kim, Hyeon Joo Jeong
-
Korean Journal of Pathology 2012;46(3):233-236.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.3.233
Published online: June 22, 2012
Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding Author: Hyeon Joo Jeong, M.D. Department of Pathology, Yonsei University College of Medicine, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-752, Korea. Tel: +82-2-2228-1766, Fax: +82-2-362-0860, jeong10@yuhs.ac
• Received: February 14, 2012 • Revised: May 11, 2012 • Accepted: May 11, 2012
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Examination of urine for decoy cells (DCs) is a useful screening test for polyomavirus (PV) activation. We explored the significance of the amount of DCs in persistent shedding, PV nephropathy and acute rejection.
-
Methods
- A case-controlled study was performed in 88 renal allograft patients who had DCs detected at least once in four or more urine samples.
-
Results
- Fifty one patients were classified into the high-grade shedding group (HG) and 37 patients into the low-grade shedding group (LG) according to DC shedding (≥10 or <10 DCs/10 high power field [HPF]). DC shedding of more than three consecutive months was significantly more prevalent in the HG as compared with their LG counterparts (p<0.0001). Urinary DCs were present for more than one year in 29.4% of the HG and 8.1% of the LG. Real-time polymerase chain reaction for PV was higher in both urine (51.4% vs. 11.1%) and plasma (9.1% vs. 0%) of the HG than the LG. The prevalence of PV nephropathy was higher in the HG than the LG (p=0.019). However, there was no significant difference in the prevalence of acute rejection.
-
Conclusions
- Shedding of ≥10 DCs/10 HPF is associated with sustained shedding, polymerase chain reaction positivity and PV nephropathy, but not a predictor of acute rejection.
- During a period ranging from January 2003 to December 2011, a total of 1,956 urine samples were collected for DC testing in 652 renal transplant recipients. Of these, 88 renal allograft patients who had DCs detected at least once in four or more urine samples (n=601), served as the DC shedding group. One hundred forty-two patients who had no DCs in four or more urine samples (n=734) served as the control group. The urinary DC test was routinely done on 1, 3, 6, 9, and 12 months after transplant. If DCs were present, urine cytology was repeated at the next visit. After one year, the duration of urinary DC test became irregular. In our series of patients, there was a variability in its duration. That is, the duration of urinary DC test was less than two consecutive years in 78.4% of total patients. In addition, it was more than two years in 21.6% of patients of the DC shedding group and 5.6% of patients of the control group.
- The presence of DCs was originally reported as negative or no DCs, a few (1-3 DCs/10 high power field [HPF] in routine smear slides or in cytospin slide), several (4-9 DCs/10 HPF in routine smear slides or in cytospin slide) and many DCs (≥10 DCs/10 HPF in routine smear slides or in cytospin slide).6 In the current study, we classified cases with a few and several DCs into the low-grade (LG) shedding group and those with many DCs detected at least once during the study into the high-grade (HG) shedding group (Fig. 1). If DCs were suspected to be present, but not confirmed, an immunocytochemistry for SV-40 large T antigen (1:100, room temperature, 32 minutes, Calbiochem, Cambridge, MA; Ventana, Tucson, AZ, USA) was performed (Fig. 2). Urinary PV DNA replication was examined by quantitative real-time PCR in 35 patients of the HG shedding group and nine patients of the LG shedding group. Plasma PV DNA replication was examined by quantitative real-time PCR in 33 patients of the HG shedding group and seven patients of the LG shedding group. The indications for renal allograft biopsies were serum creatinine elevation, proteinuria of ≥1 g/24 hr or persistent microscopic hematuria. Thirty-two renal allograft biopsies were performed from 88 DC positive patients, which were compared with 42 biopsies from 142 patients of the control group. The biopsy results were also compared with the DC data. PV nephropathy was diagnosed by renal biopsy when tubular epithelial cells with characteristic viral inclusion bodies showed SV-40 immunoreactivity. Statistical analysis was done with the Chi-square method.
MATERIALS AND METHODS
- In the current study, 88 DC-positive patients who were assigned to the DC shedding group were classified into the HG shedding group (n=51) and the LG shedding group (n=37). Urinary DCs first appeared within three months in 29.5%, between three to six months in 23.9%, between six months to one year in 35.2% and more than one year post-transplant in 11.4% of our series of patients. DC shedding frequently waxed and waned. Sustained shedding was defined by the presence of DCs during a consecutive period of more than three months, and it occurred in 66.7% of patients of the HG shedding group and 18.9% of patients of the LG shedding group. Sustained shedding was significantly more prevalent in patients of the HG group as compared with their LG counterparts (p<0.0001). Fifteen patients (29.4%) of the HG shedding group had intermittent secretion of DCs for more than one year and two patients did for more than three years. But only three patients (8.1%) of the LG shedding group had secretion of DCs for more than one year. At the latest follow-up, there was a persistent presence of DCs in 21 patients (41.2%) of the HG shedding group and four patients (10.8%) of the LG shedding group. The number of DCs was decreased in eight patients of the HG shedding group and two patients of the LG group and it was increased in two patients of the HG shedding group and one patient of the LG group (Table 1).
- Quantitative real-time PCR was positive with a range of 132,000-6,150,000,000 copies/mL in urine samples collected from 18 of 35 patients of the HG shedding group and one of nine patients of the LG shedding group. Plasma PCR was positive with a range of 12,200-593,000 copies/mL in three of 33 patients of the HG shedding group, but negative in patients of the LG shedding group. A diagnosis of PV nephropathy was made in seven patients of the HG group within a median period of 17 months (range, 4 to 28 months) post-transplant. Of the six patients with PV nephropathy, for whom the plasma and urine PCR were performed, five and two were positive for urinary and plasma PCR, respectively. The prevalence of PV nephropathy was higher in the HG shedding group than the LG shedding group (p=0.019).
- Immunosuppression was decreased in seven patients who were diagnosed with PV nephropathy. In nine patients of the HG shedding group who were not diagnosed with PV nephropathy, the dose of mycophenolate was lowered or its treatment was discontinued. DCs disappeared in three patients on 3, 4, and 7 months after modulation. The number of DCs was decreased in four patients, but they were persistently present in two patients. In six patients with viruria or viremia, confirmed on the real-time PCR, however, the renal function was stable without immunomodulation.
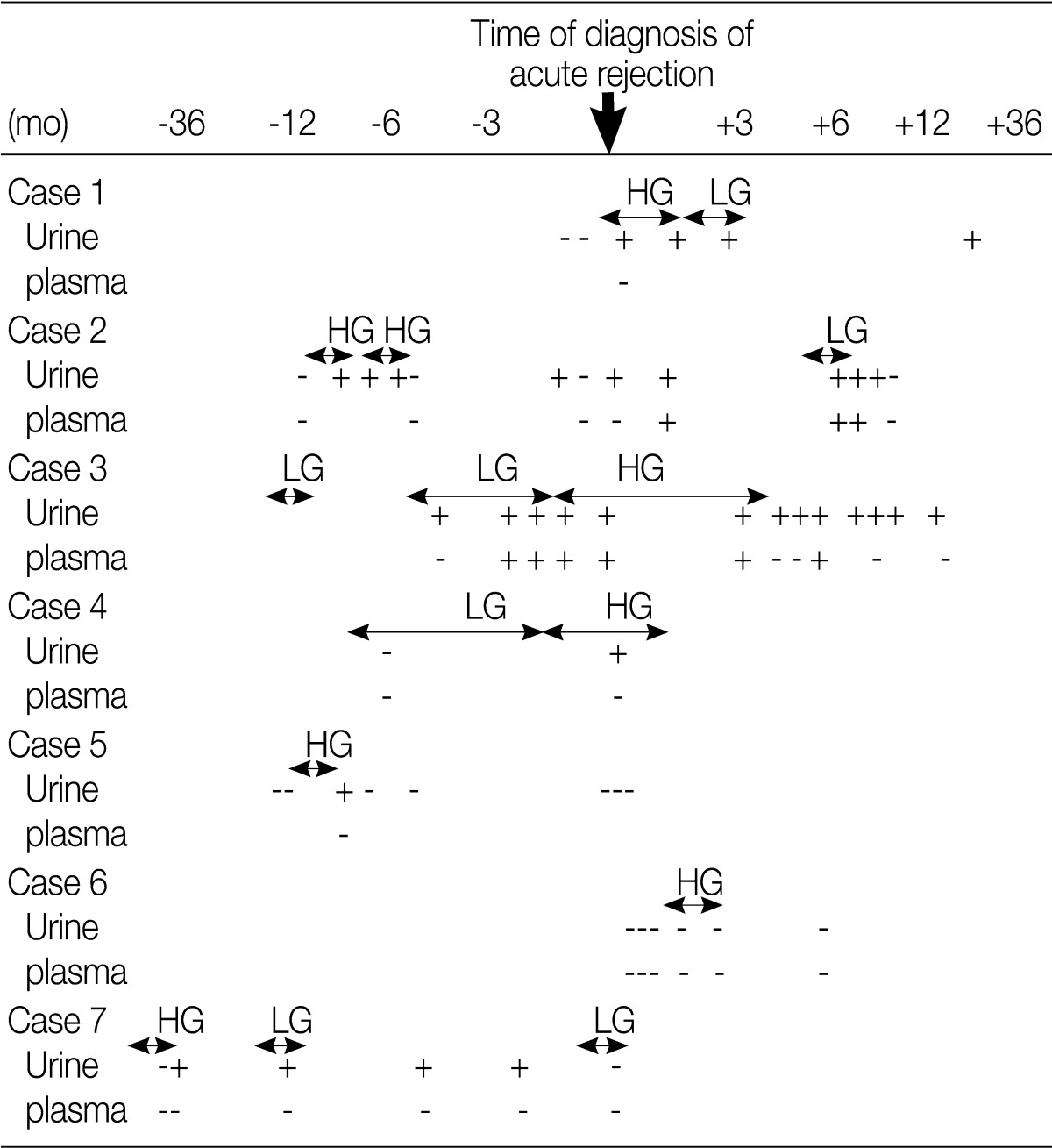
- Acute rejection episodes were proven on biopsy in seven patients of the HG shedding group and in 12 patients of the control group. Five patients of the HG shedding group shed DCs prior to the onset of acute rejection. Plasma PCR for PV was positive in one patient at the onset of acute rejection and it became positive after treatment of acute rejection in one patient (Table 2). In addition, there was no significant difference in the prevalence of acute rejection between the HG shedding group and the control group (p=0.875).
RESULTS
- The current study demonstrated that the degree of urinary DCs was correlated with sustained shedding, urinary PCR and development of PV nephropathy. The duration of urinary DC shedding was longer in the HG shedding group than LG shedding group (shedding of more than one year, 29.4% vs. 8.1%; sustained shedding of more than three consecutive months, 66.7% vs. 18.9%). Urinary PCR was positive in 51.4% of patients of the HG shedding group and 11.1% of patients of the LG shedding group. In addition, plasma PCR was positive in 17.6% of patients of the HG shedding group who were positive for urine PCR. Furthermore, plasma PCR was negative in one patient of the LG shedding group who was positive for urine PCR. All the seven patients with PV nephropathy experienced HG shedding of DCs.
- In our screening program of PV, the urinary DC test was performed at a 1- and then 3-month interval during the first post-transplant year. Most of the DC shedders could be detected during this period as urinary DCs first appeared within a year post-transplant in 88.6% of total patients. But the duration of urinary DC test remains uncertain. Hirsch et al.7 recommended that it be done as a routine screening procedure at a 3-month interval during the first two years of post-transplant. Singh et al.8 recommended that it be more frequently for the first six months and then on a yearly basis. In the current study, urinary DC test was done at a 3-month interval for more than two years in 5% of patients of the LG shedding group and 33.3% of patients of the HG shedding group. But there were two patients who developed PV nephropathy more than two years post-transplant. Furthermore, at the latest follow-up, there was a persistent presence of DCs in more than 40% of patients of the HG shedding group. It would therefore be valid to perform urinary DC test at least on a yearly basis after the first year post-transplant in patients who have experienced more than a single episode of HG shedding.
- Another thing to consider is immunomodulation. It has been reported that the allograft function is preserved following reduced immunosuppression even in presumptive cases of PV nephropathy.9 There was a gradual decrease in immunosuppression in nine patients of the HG shedding group. But there were two patients where DCs were not decreased in number or disappeared. By contrast, DCs were decreased or disappeared without reduction of immunosuppression in a substantial percentage of patients, which was clearly in patients of the LG shedding group. In six patients with viruria or viremia, confirmed on the real-time PCR, the renal function was stable without immunomodulation. The viral load is the most important factor for producing PV nephropathy. It remains obscure, however, who can benefit from the decreased immunosuppression. It is therefore necessary to identify viral genotypes and to determine host's immune reaction to the virus, both of which may play a role in tissue injury.10
- Acute rejection is frequently associated in allograft patients with PV nephropathy.11 Our results showed that the prevalence of acute rejection was relatively higher after DC shedding as compared with prior to it. But there was no difference in the prevalence of acute rejection between the DC shedding group and the control group. This indicates that DC shedding is not a predictor of acute rejection. Acute rejection may be present concurrently with PV nephropathy. Renal biopsy should therefore be considered in patients with graft dysfunction accompanied by HG DC shedding.
- Limitation of the current study originated from a variability in the methods of urine sampling. The study was conducted using either routine smear slides or cytospin slides to estimate the amount of DCs for PV activation. We think this variability might not have a serious impact on the results since there were numerous DCs in most patients of the HG shedding group with no respect to the methods of urine sampling.
- In conclusion, our results indicate that shedding of ≥10 DCs/10 HPF is a clinically significant indicator for sustained shedding and risk for PV nephropathy but it is not a predictor of acute rejection. This implies that a continuous monitoring would be needed for patients with HG shedding even after a transient clearance of DCs.
DISCUSSION
- 1. Drachenberg CB, Beskow CO, Cangro CB, et al. Human polyomavirus in renal allograft biopsies: morphological findings and correlation with urine cytology. Hum Pathol 1999; 30: 970-977. ArticlePubMed
- 2. Nickeleit V, Klimkait T, Binet IF, et al. Testing for polyomavirus type BK DNA in plasma to identify renal-allograft recipients with viral nephropathy. N Engl J Med 2000; 342: 1309-1315. ArticlePubMed
- 3. Gautam A, Patel V, Pelletier L, Orozco J, Francis J, Nuhn M. Routine BK virus surveillance in renal transplantation: a single center's experience. Transplant Proc 2010; 42: 4088-4090. ArticlePubMed
- 4. Drachenberg C, Hirsch HH, Papadimitriou JC, et al. Cost efficiency in the prospective diagnosis and follow-up of polyomavirus allograft nephropathy. Transplant Proc 2004; 36: 3028-3031. ArticlePubMed
- 5. Prince O, Savic S, Dickenmann M, Steiger J, Bubendorf L, Mihatsch MJ. Risk factors for polyomavirus nephropathy. Nephrol Dial Transplant 2009; 24: 1024-1033. ArticlePubMedPMC
- 6. Ahn HJ, Ju MK, Jeong HJ, et al. Immunologic control for polyomavirus infection after kidney transplantation. Nephron Clin Pract 2008; 108: c148-c154. ArticlePubMedPDF
- 7. Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation 2005; 79: 1277-1286. ArticlePubMed
- 8. Singh HK, Bubendorf L, Mihatsch MJ, Drachenberg CB, Nickeleit V. In: Ahsan E, ed. Urine cytology findings of polyomavirus infections. Polyomaviruses and human diseases. 2005; Georgetown, TX: Landes Bioscience/Eurekah, 201.Article
- 9. Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant 2010; 10: 2615-2623. ArticlePubMed
- 10. Fishman JA. BK virus nephropathy: polyomavirus adding insult to injury. N Engl J Med 2002; 347: 527-530. ArticlePubMed
- 11. Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyomavirus nephropathy in 67 renal transplant patients. J Am Soc Nephrol 2002; 13: 2145-2151. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Polyomavirus nephropathy: diagnosis, histologic features, and differentiation from acute rejection
Cynthia C. Nast
Clinical Transplantation and Research.2024; 38(2): 71. CrossRef - Challenges and opportunities in research on BK virus infection after renal transplantation
Yukun Tang, Zipei Wang, Dunfeng Du
International Immunopharmacology.2024; 141: 112793. CrossRef - BK Virus-Associated Nephropathy after Renal Transplantation
Yasuhito Funahashi
Pathogens.2021; 10(2): 150. CrossRef - Diagnostic utility of urine cytology in detection of decoy cells in renal transplant patients: Report of five cases and review of literature
Santosh Tummidi, Kanchan Kothari, Mona Agnihotri, Leena Naik, Amey Rojekar
Diagnostic Cytopathology.2020; 48(3): 222. CrossRef - Association of Pretransplant BK Polyomavirus Antibody Status with BK Polyomavirus Infection After Kidney Transplantation: A Prospective Cohort Pilot Study of 47 Transplant Recipients
Yu Hisadome, Hiroshi Noguchi, Yuki Nakafusa, Kukiko Sakihama, Takanori Mei, Keizo Kaku, Yasuhiro Okabe, Kosuke Masutani, Yuki Ohara, Kazuyuki Ikeda, Yoshinao Oda, Masafumi Nakamura
Transplantation Proceedings.2020; 52(6): 1762. CrossRef - Association Between the Polyomaviruses Titers and Decoy Cell Positivity Rates After Renal Transplantation
Y. Funahashi, M. Kato, T. Fujita, S. Ishida, A. Mori, M. Gotoh
Transplantation Proceedings.2016; 48(3): 921. CrossRef
Urinary Decoy Cell Grading and Its Clinical Implications


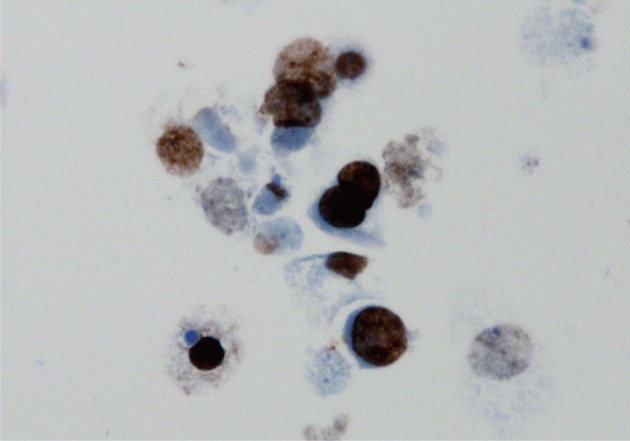
Fig. 1 Voided urine cytology shows many decoy cells displaying nuclear enlargement, high N/C ratio and basophilic 'ground-glass' intranuclear inclusion with marginated chromatin.
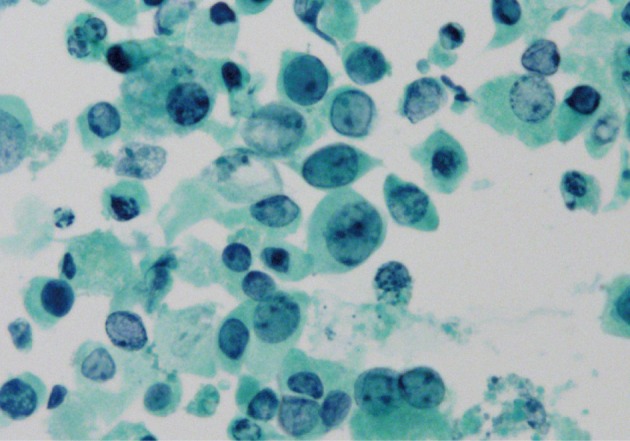
Fig. 2 Decoy cells show a strong nuclear positivity for the SV-40 immunostain.
Fig. 1
Fig. 2
Urinary Decoy Cell Grading and Its Clinical Implications
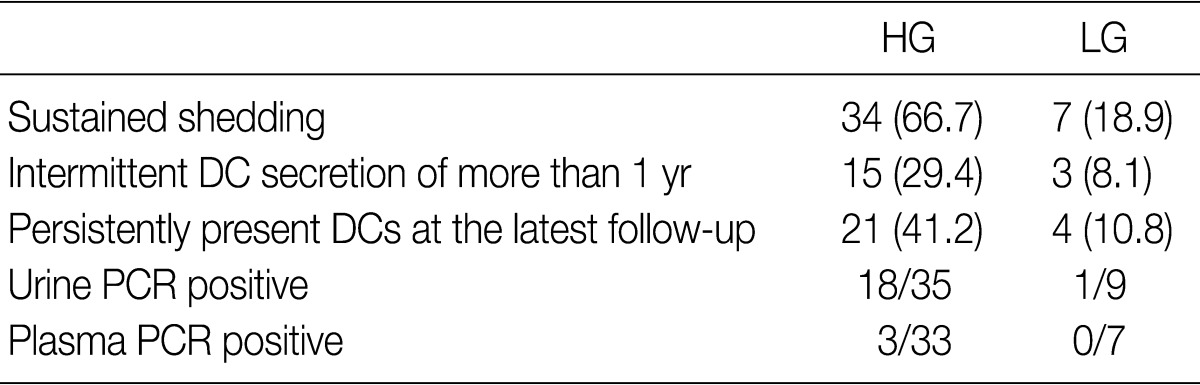
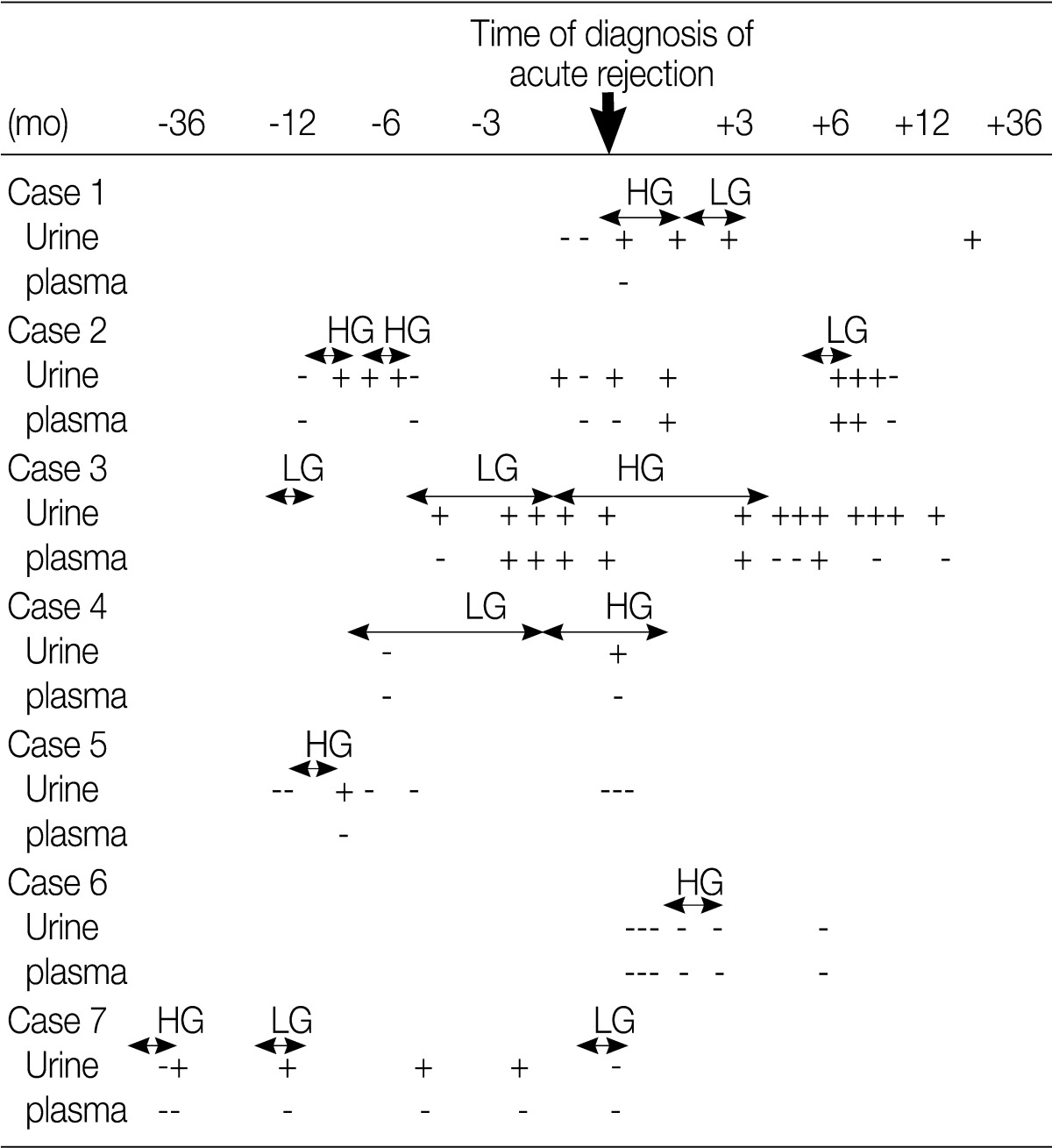
Table 1 Comparison between the HG shedding group and the LG one
Values are presented as number (%). HG, high-grade; LG, low-grade; DC, decoy cells; PCR, polymerase chain reaction.
Table 2 Results of the urinary DC and urinary/plasma PV PCR test in patients with acute rejection
DC, decoy cells; PV PCR, polyomavirus polymerase chain reaction.

 E-submission
E-submission


 PubReader
PubReader Cite this Article
Cite this Article