Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(5); 2012 > Article
-
Original Article
Expression of CHOP in Squamous Tumor of the Uterine Cervix - Hyun Hee Chu1, Jun Sang Bae1, Kyoung Min Kim1, Ho Sung Park1, Dong Hyu Cho2, Kyu Yun Jang1,3, Woo Sung Moon1,3, Myoung Jae Kang1, Dong Geun Lee1, Myoung Ja Chung1,3,4
-
Korean Journal of Pathology 2012;46(5):463-469.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.5.463
Published online: October 25, 2012
1Department of Pathology, Chonbuk National University Medical School, Jeonju, Korea.
2Department of Obstetrics and Gynecology, Chonbuk National University Medical School, Jeonju, Korea.
3Research Institute for Endocrine Sciences, Chonbuk National University, Jeonju, Korea.
4Research Institute for Medical Sciences, Chonbuk National University, Jeonju, Korea.
- Corresponding Author: Myoung Ja Chung, M.D. Department of Pathology, Chonbuk National University Medical School, 567 Baekje-daero, Deokjin-gu, Jeonju 561-756, Korea. Tel: +82-63-270-3072, Fax: +82-63-270-3135, mjchung@jbnu.ac.kr
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- High-risk human papillomavirus (HR-HPV) infection and abnormal p53 expression are closely involved in carcinogenesis of squamous cell carcinoma (SqCC) of uterine cervix. Recent studies have suggested that virus-induced endoplasmic reticulum (ER) stress modulates various cell survival and cell death signaling pathways. The C/EBP homologous protein (CHOP) is associated with ER stress-mediated apoptosis and is also involved in carcinogenesis of several human cancers. We hypothesized that CHOP is involved in the carcinogenesis of uterine cervical cancer in association with HR-HPV and/or p53.
-
Methods
- Immunohistochemistry was used to analyze CHOP and p53 protein expression of tissue sections from 191 patients with invasive cancer or preinvasive lesions of the uterine cervix (61 cases of SqCC, 66 cases of cervical intraepithelial neoplasia [CIN] III, and 64 cases of CIN I).
-
Results
- CHOP was expressed in 59.4% of CIN I, 48.5% of CIN III, and 70.5% of SqCC cases. It was also significantly more frequent in invasive SqCC than in preinvasive lesions (p=0.042). Moreover, CHOP expression significantly correlated with HR-HPV infection and p53 expression (p=0.009 and p=0.038, respectively).
-
Conclusions
- Our results suggest that CHOP is involved in the carcinogenesis of the uterine cervix SqCC via association with HR-HPV and p53.
- Patients and specimens
- We selected 191 female patients with CIN or invasive SqCC for the study. Tissues were obtained from 61 invasive SqCC, 66 grade 3 CIN (CIN 3), and 64 CIN 1 patients who underwent surgery at the Department of Obstetrics and Gynecology, Chonbuk National University Hospital between June 2005 and June 2011. For the present study, we selected patients with available tissue blocks and who had PAP smear and HR-HPV tests before tissue collection. All cytological specimens were taken within 60 days prior to the tissue collection date. To investigate the association between HPV infection and CHOP expression, we incorporated half of the HPV negative cases into each group. Among the 130 CIN cases, 64 were HR-HPV negative and 66 cases were HR-HPV positive. However, a small number of HPV-negative cases (15 cases, 25% of invasive cancer) were included in the invasive SqCC group because of the high prevalence of HPV infection in cervical cancer. Clinicopathological information was obtained through a computerized tumor registry database. Thirty cases of normal cervical tissues with no HPV infection were used as normal control tissue. Histological typing for CIN was performed according to the three-tiered classification of the World Health Organization. The mean ages of the patient groups at the time of diagnosis were 34.53 years for CIN 1, 39.68 years for CIN 3, and 53.43 years for SqCC (range for the entire sample, 26 to 85 years). This study was approved by the Human Ethics Committee of Chonbuk National University Medical School. Informed consent was provided according to the Declaration of Helsinki.
- Determination of HPV infection
- Endo/exocervical specimens were obtained by a cytobrush. The presence of HPV infection was determined using the Hybrid Capture system (Digene Inc., Gaithersburg, MD, USA). The Hybrid Capture test is a nucleic acid hybridization assay in which specimens containing the target DNA hybridize with a specific HPV RNA probe mixture, including probes for the following HR-HPV types-16/18/31/35/45/51/52/56. The resultant RNA : DNA hybrids are captured onto the surface of a microplate well coated with antibodies specific for RNA:DNA hybrids. Immobilized hybrids are then reacted with alkaline phosphatase conjugated antibodies specific for the RNA:DNA hybrids, and detected with a chemiluminescent substrate. Emitted light is measured by a luminometer as relative light units (RLU). Samples were classified as positive for HR-HPV if the RLU value was >1.0.17
- Immunohistochemistry
- Immunohistochemical studies were performed on 5-µm sections of formalin-fixed, paraffin-embedded tissue specimens. All specimens were examined with the Dako Envision system, which uses dextran polymers conjugated with horseradish peroxidase (Dako, Carpinteria, CA, USA). Antigen retrieval was achieved by incubating tissue sections in boiling 10 µmol/L citrate buffer (pH 6.0) for 10 minutes in a microwave oven prior to incubation with primary antibodies against CHOP. Tissue sections for p53 detection were incubated for three 5-minute intervals in a scientific cooker for antigen retrieval (10 µmol/L citrate buffer, pH 6.0). After blocking endogenous peroxidase, the sections were incubated with Protein Block Serum-Free (Dako) at room temperature for 10 minutes to block non-specific staining. Thereafter, sections were incubated for 2 hours at room temperature with mouse anti-p53 monoclonal antibody (1:100, Dako) and with mouse anti-CHOP monoclonal antibody (1:50, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) overnight at 4℃. Peroxidase activity was detected using the enzyme substrate 3-amino-9-ethyl carbazole. Negative control sections were treated in the same way except that they were incubated with Tris-buffered saline without primary antibody.
- All immunostained sections were evaluated by two pathologists (M.J. Chung and H.H. Chu) who were blinded to the patients' clinicopathological information. CHOP assays were interpreted for immunoreactivity according to the intensity of staining and percentage of positive cells. The intensity of cytoplasmic staining was classified into four categories: no immunostaining (0), weak immunostaining (1), moderate immunostaining (2), and intense immunostaining (3). The percentage of positive cells was scored as 0 (≤30%), 1 (>30% and ≤50%), and 2 (>50%). The sum index was obtained by totaling the scores of intensity and the percentages. If the final score was ≥3, the tumor was considered positive; if otherwise, the tumor was considered negative. Nuclear staining in >10% of the neoplastic cells was considered positive for p53 expression.18,19
- Statistical analysis
- SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The association between the staining index and other clinicopathological variables was analyzed using Pearson's chi-square test. Differences were considered significant when p-values were <0.05.
MATERIALS AND METHODS
- CHOP and p53 expression in association with clinicopathological parameters
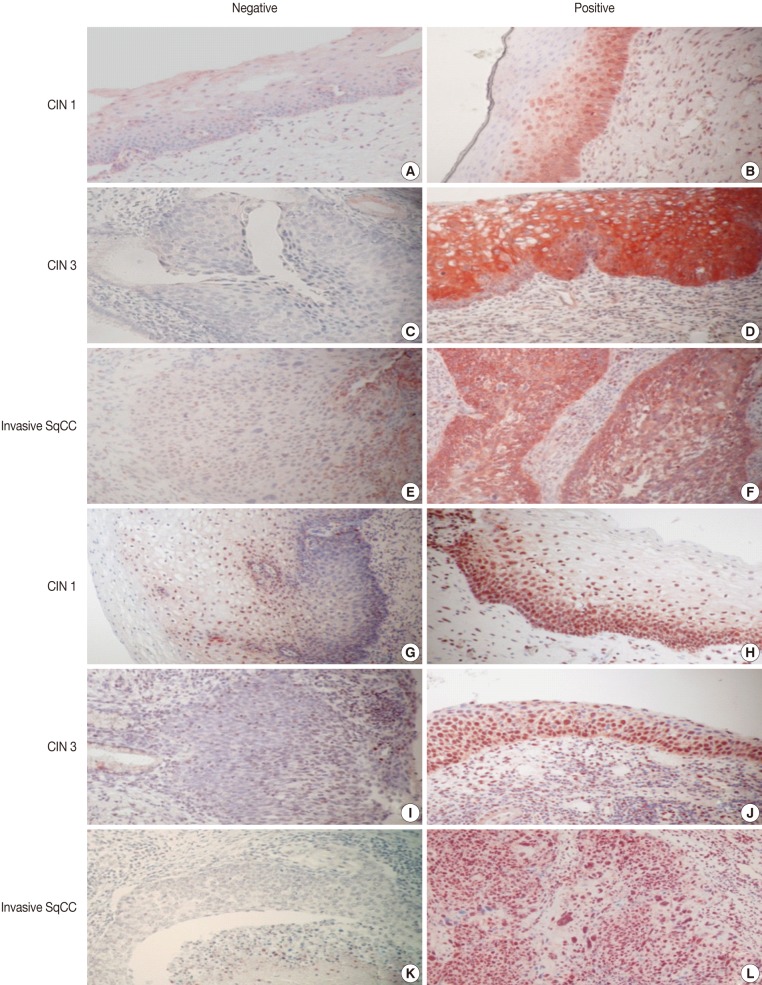
- Imunohistochemical stainings of CHOP and p53 in CIN and invasive SqCC are shown in Fig. 1. CHOP and p53 immunoreactivities were observed primarily in the cytoplasm and nuclei, respectively. The basal layer of normal cervical mucosa showed very weak immunoreactivity to CHOP and p53. Therefore, all cases of normal cervical tissues were negative for CHOP and p53. The associations between protein expression and clinicopathologic parameters are summarized in Table 1. Lesions of invasive SqCC showed significantly more frequent CHOP expression compared to those of CIN (70.5% vs 53.8%, p=0.04). CHOP expression was significantly higher in CIN (53.8%) than in control tissues (0%). However, there was no gradual increase in CHOP expression along the continuum of CIN. CHOP expression rates were higher in CIN 1 compared with those in CIN 3 (59.4% vs 48.5%, p=0.224) (data not shown). Meanwhile, p53 expression gradually increased with tumor progression and was significantly higher in invasive carcinomas (91.8%) than in CIN (70.8%) (p=0.001). Lesions of CIN 3 showed significantly more frequent p53 expression compared to those of CIN 1 (84.8% vs 57.8%, p<0.001). CHOP expression was correlated with p53 expression (p=0.038) (Table 2).
- CHOP expression in association with HPV infection
- Overall, the study of 191 cases of squamous lesions revealed that CHOP expression correlated significantly with HR-HPV infection (p=0.009) (Table 1). When we conducted correlation analysis after sorting the tumors by histopathological grade, CHOP expression correlated significantly with HPV infection for CIN 1 and invasive cancer (p=0.024 and p=0.007, respectively) (Table 3). On the other hand, we found no correlation between HPV infection and CHOP expression in CIN 3 (p=0.460).
RESULTS
- Conditions that induce ER stress, such as hypoxia, oxidative stress, nutrient deprivation, and changes in pH (acidosis) are frequently encountered in tumor cells.20 Several studies have reported evidence of UPR activation in various tumors. Therefore, it is important to establish the pathway of UPR activation in tumors or the role of UPR activation in tumorigenesis. Although HPV infection is recognized as the main pathogenetic factor of cervical cancer, it is not sufficient to induce tumor development. In the present study, we examined CHOP expression in uterine cervical tumor and its correlation with HR-HPV and/or p53. We demonstrated that lesions of invasive SqCC showed significantly more frequent CHOP expression compared to those of CIN, and CHOP expression significantly correlated with HR-HPV infection and p53 expression.
- There are a few studies which have considered the clinical relevance of CHOP expression in human cancers. The present study showed that relative to the normal cervix, CHOP expression increased in CIN and invasive cancer, and was significantly more frequent in invasive cancer than in CIN. These results are similar to the findings of our earlier study21 and to those found by others.22 Accordingly, Rask et al.22 found that CHOP overexpression is correlated with cancer invasiveness and unfavorable clinicopathological factors in colorectal cancer. We also observed that CHOP expression was significantly less frequent in premalignant glandular lesion (atypical adenomatous hyperplasia) than in invasive adenocarcinoma of lung.21 In contrast, increased CHOP expression has been associated with lower tumor stage in colon cancer, and with a higher survival rate in melanoma.5 De Marco et al.16 reported that ERp57, an ER stress marker, was significantly elevated in squamous cell carcinoma tissues compared with both dysplastic and control tissues of uterine cervix. Based on these results, we suggest that CHOP expression might be involved in tumorigenesis of uterine cervical squamous tumors. Further studies are needed to elucidate the role of CHOP in the development or progression of cancer.
- Accumulating evidence suggests that viruses, including HPV, manipulate the programs emanating from the ER in a complex manner, which in turn, may facilitate viral replication or pathogenesis.14,15 HPV16 E5 represses the cellular ER stress response in genital keratinocytes.15 HPV 16 E5 is a HPV 16 transforming protein, which localizes predominantly in the ER, and can enhance cell immortalization by E6E7. Accordingly, repression of ER stress response may assist the persistence of viral infection. De Marco et al.16 reported that the expression of ER stress marker was significantly increased in HPV 16 positive cancer tissues compared with both dysplastic and control tissue. In the present study, we found that HR-HPV infection correlated significantly with CHOP expression in invasive SqCC. We assume that persistent HPV infection may induce ER stress (and eventually CHOP overexpression), and CHOP overexpression along with apoptosis may be involved in the tumorigenesis of cervical cancer. At any rate, further studies are needed to clarify the role of CHOP overexpression in carcinogenesis of cervical cancer and to identify the mechanism underlying the relationship between HPV infection and CHOP expression.
- A well-known abnormal gene expression pattern in cervical cancer is p53 degradation. Unlike most solid tumors, p53 mutation is infrequently found in cervical cancers. In cervical cancers, viral early protein E6 of HR-HPV induces p53 degradation via direct binding to the ubiquitin ligase E6AP, allowing cells to bypass apoptosis and survive with DNA damage; thus, contributing to tumorigenesis.23-25 Both CHOP and p53 are associated with apoptosis and there are reports indicating that CHOP is a novel p53 target gene.9,10 The present study showed that CHOP expression significantly correlated with p53 expression indicating the link between CHOP and p53 in cervical cancer. In contrast, a previous study showed that environmental stress causes DNA damage, resulting in the expression of p53 and GADD genes and GADD153 (CHOP) expression was independent of p53.26 Indeed, the exact mechanism underlying the relationship between CHOP and p53 expression should be clarified.
- In conclusion, our results suggest that CHOP is involved in tumorigenesis of squamous cell tumors of the uterine cervix via association with HR-HPV and p53. Our data also indicate that CHOP expression may also be associated with tumor progression. Additional studies are required to determine the exact molecular mechanisms explaining the roles of CHOP in the development and progression of cervical cancer, and the relationship between CHOP expression and HPV infection and/or p53 expression.
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519-529. ArticlePubMedPDF
- 2. Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ 2006; 13: 374-384. ArticlePubMedPDF
- 3. Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005; 74: 739-789. ArticlePubMed
- 4. Luethy JD, Holbrook NJ. Activation of the gadd153 promoter by genotoxic agents: a rapid and specific response to DNA damage. Cancer Res 1992; 52: 5-10. PubMed
- 5. Korabiowska M, Cordon-Cardo C, Betke H, et al. GADD153 is an independent prognostic factor in melanoma: immunohistochemical and molecular genetic analysis. Histol Histopathol 2002; 17: 805-811. PubMed
- 6. Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature 1993; 363: 640-644. ArticlePubMedPDF
- 7. Forus A, Flørenes VA, Maelandsmo GM, Fodstad O, Myklebost O. The protooncogene CHOP/GADD153, involved in growth arrest and DNA damage response, is amplified in a subset of human sarcomas. Cancer Genet Cytogenet 1994; 78: 165-171. ArticlePubMed
- 8. Buitrago-Pérez A, Garaulet G, Vázquez-Carballo A, Paramio JM, García-Escudero R. Molecular signature of HPV-induced carcinogenesis: pRb, p53 and gene expression profiling. Curr Genomics 2009; 10: 26-34. ArticlePubMedPMC
- 9. Liu T, Laurell C, Selivanova G, Lundeberg J, Nilsson P, Wiman KG. Hypoxia induces p53-dependent transactivation and Fas/CD95-dependent apoptosis. Cell Death Differ 2007; 14: 411-421. ArticlePubMedPDF
- 10. Seth A, Giunta S, Franceschil C, Kola I, Venanzoni MC. Regulation of the human stress response gene GADD153 expression: role of ETS1 and FLI-1 gene products. Cell Death Differ 1999; 6: 902-907. ArticlePubMedPDF
- 11. Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol 2005; 16: 481-488. ArticlePubMed
- 12. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology 2004; 324: 17-27. ArticlePubMed
- 13. Hamid NA, Brown C, Gaston K. The regulation of cell proliferation by the papillomavirus early proteins. Cell Mol Life Sci 2009; 66: 1700-1717. ArticlePubMedPMCPDF
- 14. He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ 2006; 13: 393-403. ArticlePubMedPDF
- 15. Sudarshan SR, Schlegel R, Liu X. The HPV-16 E5 protein represses expression of stress pathway genes XBP-1 and COX-2 in genital keratinocytes. Biochem Biophys Res Commun 2010; 399: 617-622. ArticlePubMedPMC
- 16. De Marco F, Bucaj E, Foppoli C, et al. Oxidative stress in HPV-driven viral carcinogenesis: redox proteomics analysis of HPV-16 dysplastic and neoplastic tissues. PLoS One 2012; 7: e34366.ArticlePubMedPMC
- 17. Guha D, Chatterjee R. Human papillomavirus detection and genotyping by hybrid capture II in the cervical smears of women at high-risk for HIV infection. Int J Hum Genet 2006; 6: 185-190. Article
- 18. Park CS, Joo IS, Song SY, Kim DS, Bae DS, Lee JH. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol Oncol 1999; 74: 53-60. ArticlePubMed
- 19. Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999; 59: 5830-5835. PubMed
- 20. Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer 2004; 4: 966-977. ArticlePubMedPDF
- 21. Kim KM, Yu TK, Chu HH, et al. Expression of ER stress and autophagy-related molecules in human non-small cell lung cancer and premalignant lesions. Int J Cancer 2012; 131: E362-E370. ArticlePubMed
- 22. Rask K, Thörn M, Pontén F, et al. Increased expression of the transcription factors CCAAT-enhancer binding protein-beta (C/EBBeta) and C/EBzeta (CHOP) correlate with invasiveness of human colorectal cancer. Int J Cancer 2000; 86: 337-343. ArticlePubMed
- 23. Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75: 495-505. ArticlePubMed
- 24. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990; 63: 1129-1136. ArticlePubMed
- 25. Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248: 76-79. ArticlePubMed
- 26. O'Reilly MA, Staversky RJ, Watkins RH, Maniscalco WM, Keng PC. p53-independent induction of GADD45 and GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2000; 278: L552-L559. ArticlePubMed
REFERENCES

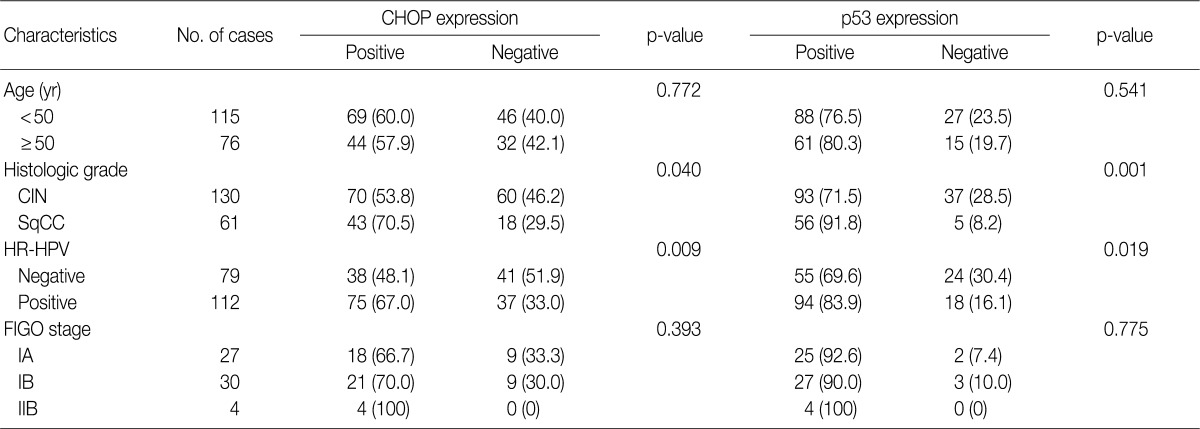
Figure & Data
References
Citations

- Interplay between the cellular stress pathway, stemness markers, and Helicobacter pylori infection in gastric cancer
Mehran Gholamin, Atena Mansouri, Mohammad Reza Abbaszadegan, Mohammad Ali Karimi, Hossein Barzegar, Fatemeh Fardi Golyan, Hanie Mahaki, Hamid Tanzadehpanah, Reihaneh Alsadat Mahmoudian
Gene Reports.2025; 40: 102263. CrossRef - Role of C-reactive protein in cervical intraepithelial neoplasia/cancer
Adriana Pedreañez, Yenddy Carrero, Renata Vargas, Juan P.Hernández Fonseca, Jesús Mosquera
Pathology - Research and Practice.2025; 276: 156274. CrossRef - Expression of GRP78 and its copartners in HEK293 and pancreatic cancer cell lines (BxPC-3/PANC-1) exposed to MRI and CT contrast agents
Ali Ahmed Azzawri, Ibrahim Halil Yildirim, Zeynep Yegin, Abdurrahim Dusak
Nucleosides, Nucleotides & Nucleic Acids.2024; 43(5): 391. CrossRef - Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology
Elif Guzel, Sefa Arlier, Ozlem Guzeloglu-Kayisli, Mehmet Tabak, Tugba Ekiz, Nihan Semerci, Kellie Larsen, Frederick Schatz, Charles Lockwood, Umit Kayisli
International Journal of Molecular Sciences.2017; 18(4): 792. CrossRef - Endoplasmic reticulum stress pathway PERK‐eIF2α confers radioresistance in oropharyngeal carcinoma by activating NF‐κB
Qiao Qiao, Chaonan Sun, Chuyang Han, Ning Han, Miao Zhang, Guang Li
Cancer Science.2017; 108(7): 1421. CrossRef - Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells
Boyun Kim, Hee Seung Kim, Eun-Ji Jung, Jung Yun Lee, Benjamin K. Tsang, Jeong Mook Lim, Yong Sang Song
Molecular Carcinogenesis.2016; 55(5): 918. CrossRef - Down-regulation of C/EBP homologous protein (CHOP) expression in gastric cardia adenocarcinoma: Their relationship with clinicopathological parameters and prognostic significance
Xiao-Juan Zhu, She-Gan Gao, San-Qiang Li, Zhen-Guo Shi, Zhi-Kun Ma, Shan-Shan Zhu, Xiao-Shan Feng
Clinics and Research in Hepatology and Gastroenterology.2015; 39(3): 391. CrossRef - MG289 in <i>Mycoplasma genitalium</i> Enhances Microbial Invasion and Bacterial Persistence in Benign Human Prostate Cells
Wasia Rizwani, Leticia Reyes, Jeongsoon Kim, Steve Goodison, Charles J. Rosser
Open Journal of Urology.2013; 03(06): 232. CrossRef

Fig. 1



Values are presented as number (%). CHOP, C/EBP homologous protein; CIN, cervical intraepithelial neoplasms; SqCC, squamous cell carcinoma; HR-HPV, high-risk human papillomavirus; FIGO, International Federation of Gynecology and Obstetrics.
Values are presented as number (%). CHOP, C/EBP homologous protein.
Values are presented as number (%). CHOP, C/EBP homologous protein; HPV, human papillomavirus; CIN, cervical intraepithelial neoplasms; HR-HPV, high-risk human papillomavirus; SqCC, squamous cell carcinoma.

 E-submission
E-submission


 PubReader
PubReader Cite this Article
Cite this Article