Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(6); 2012 > Article
-
Original Article
Expression of SIRT1 and DBC1 in Gastric Adenocarcinoma - Youngran Kang, Woon Yong Jung1, Hyunjoo Lee2, Eunjung Lee, Aeree Kim, Baek-hui Kim
-
Korean Journal of Pathology 2012;46(6):523-531.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.523
Published online: December 26, 2012
Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
2Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding Author: Baek-hui Kim, M.D. Department of Pathology, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 152-703, Korea. Tel: +82-2-2626-3255, Fax: +82-2-2626-1486, maelstrom@naver.com
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Sirtuin 1 (SIRT1) and deleted in breast cancer 1 (DBC1) are known as tumor suppressor or promoter genes. This may be due to their diverse functions and interaction with other proteins. Gastric adenocarcinoma is one of the most common malignancies, but little is known about its carcinogenesis. Therefore, we investigated the association of immunohistochemical expression of SIRT1, DBC1, p53, and β-catenin and their variable clinicopathological characteristics.
-
Methods
- We obtained samples from 452 patients who underwent gastrectomy. Tissue microarray blocks were constructed and immonohistochemical staining was performed.
-
Results
- Expression of DBC1 and SIRT1 was associated with lower histologic grade, intestinal type of Lauren classification, and lower pT (p<0.001) and pN stage (DBC1, p=0.002; SIRT1, p<0.001). Association between absence of lymphatic invasion, and SIRT1 (p=0.001) and DBC1 (p=0.004) was observed. Cytoplasmic β-catenin expression was associated with lower histologic grade, pT, pN, tumor-node-metastasis (TNM) stage, DBC1 (p<0.001), and SIRT1 (p=0.001). Expression of SIRT1 and DBC1 was not associated with p53 (p=0.063 and p=0.060). DBC1 was an independent good prognostic factor in multivariate analysis (p=0.012).
-
Conclusions
- SIRC1 and DBC1 can be considered to be good prognostic factors in gastric adenocarcinoma.
- Patients and samples
- Archived formalin-fixed paraffin-embedded (FFPE) samples of gastric adenocarcinomas were obtained from 452 patients who underwent radical gastrectomy at Korea University Guro Hospital from January 2002 to December 2005. No patient had a prior history of neo-adjuvant chemotherapy.
- Clinicopathologic data, including age, sex, and distant metastasis were obtained from medical records. Survival data was obtained from records of the Ministry of Public Administration and Security as well as from medical records. All slide glasses and specimen photos were reviewed and the histopathologic type, histologic grade, Lauren classification, depth of invasion, regional lymph node metastasis, and lymphatic invasion were evaluated. The reviewed cases were reclassified according to the Seventh American Joint Committee on Cancer/International Union Against Cancer Classification (AJCC/UICC) tumor-node-metastasis (TNM) cancer classification system.20 The World Health Organization classification system, which describes three main histological grades, including well, moderately, and poorly differentiated, was applied for histological typing and grading of adenocarcinoma.21 The mean follow-up period was 50.9 months (median, 53.3 months; range, 3 to 83 months). The median age was 60.5 years (range, 23 to 84 years) and the male to female ratio was 2.1. Forty percent (n=183) of patients were diagnosed with early gastric carcinoma and 59.5 percent (n=269) with advanced gastric carcinoma. This study was approved by the Institutional Review Board of Korea University Guro Hospital (KUGGR-2010-033).
- Tissue microarray (TMA) and immunohistochemical staining
- Hematoxylin and eosin-stained slide glasses of selected patients were reviewed. Representative portions from the leading edge of tumor infiltration were marked and used for tissue microarray construction. Tumor tissues of 2.0 mm core diameter were taken from donor FFPE blocks and arranged in empty recipient paraffin blocks to generate a TMA. One core per tumor was arrayed. The TMA blocks were cut into 4 µm slices for immunohistochemical staining. A standard streptavidin-biotin peroxidase complex method was used. After deparaffinization and rehydration, slides were heated in a microwave oven for 15 minutes in 10 mM citrate buffer (pH 6.0) and treated with 3% hydrogen peroxide for 20 minutes. We used the Bond-maX autostainer (Leica, Wetzlar, Germany). Antibodies used in this study were DBC1 (1:200, polyclonal, Abcam, Cambridge, MA, USA), SIRT1 (1:200, clone H-300, Santa Cruz Biotechnology, Santa Cruz, CA, USA), β-catenin (1:500, clone β-catenin-1, Dako, Carpinteria, CA, USA), and p53 (1:500, clone DO-7, Novocastra, Newcastle upon Tyne, England).
- Analysis of immunohistochemical staining by optical microscope
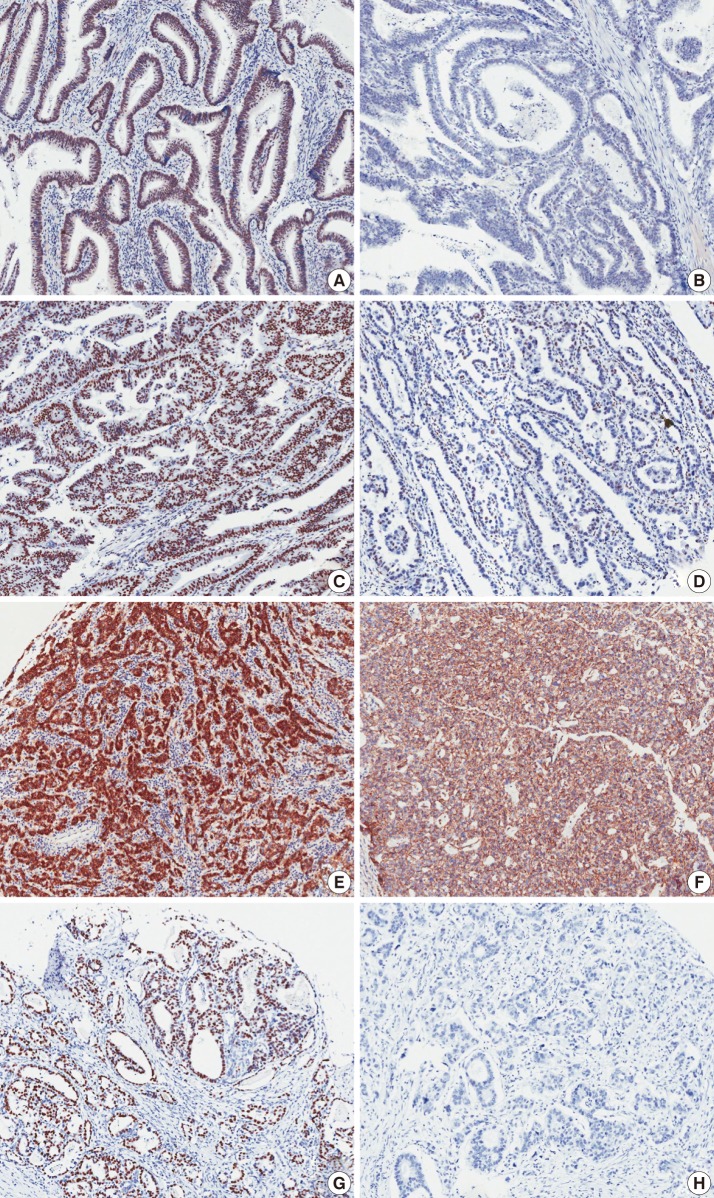
- Immunohistochemical analysis by optical microscope was performed by two pathologist (Y.R.Kang and B.H.Kim). Immunohistochemical staining of p53 showed a nuclear staining pattern (Fig. 1). The nuclear staining intensity (0, no staining; 1, weak; 2, moderate; 3, strong) and percent areas of positive tumor cell nuclei were graded. When more than 50% of tumor cells showed a moderate or strong staining intensity, we considered it to be positive staining. To examine the correlation between the expression of p53 and other proteins, a cut-off value of 50% was applied to the p53.
- To analyze the β-catenin expression in tumor cells, the positivity of nuclear, cytoplasmic, and membranous staining was also scored. For nuclear and cytoplasmic staining, cases with tumor cells expressing more than 30% β-catenin were considered to be positive, and for membranous staining, cases with a pattern of loss of membranous β-catenin staining of more than 30% of tumor cells were regarded as negative (Fig. 1).
- Analysis of immunohistochemical staining by image analyzer
- Expression of DBC1 and SIRT1 were found mainly in the nuclei. To decrease the effects of observer biases, DBC1 and SIRT1 TMA slides were analyzed by an image analyzer program (IA). A Scanscope CS (Aperio, Vista, CA, USA) digital scanner was used for scanning of TMA slides. The scanned images were analyzed using an Aperio image analysis toolbox. A combination of the Genie histology pattern recognition tool (Aperio) and nuclear quantification cellular analysis tool (Aperio) was used to select tumor cells. The algorithm was adjusted to match the requirements of our particular marker and staining set. These adjustments include segmentation type, nuclear curvature threshold, size, roundness, compactness, and elongation. The output data contained the nuclear staining intensity (1+, 2+, 3+, and negative), positive tumor cell percentage, and cell count. The cut-off value of 30% was also applied to DBC1 and SIRT1 staining. When the sum of 2+ and 3+ cells exceeded 30% of the total tumor cells, the case was considered to be positive.
- Statistical analysis
- All statistical analyses were performed using the SPSS ver. 12 (SPSS Inc., Chicago, IL, USA). Kaplan-Meier plots and the log-rank test were used to estimate survival rates. The overall survival period was applied to this study. Overall survival was calculated as the time from diagnosis to the date of death or the last follow-up date. The median survival was 53.3 months. Correlations between the immunohistochemical expression of proteins and the clinicopathological characteristics were analyzed by Pearson's χ2 tests, but histologic grade and pathologic stage were analyzed by a Spearman's rank tests.
- Multivariate analysis was performed using the Cox proportional hazard model. In all statistical analyses, a p<0.05 was considered statistically significant.
MATERIALS AND METHODS
- Expression of DBC1 and SIRT1, and correlation with clinicopathologic characteristics
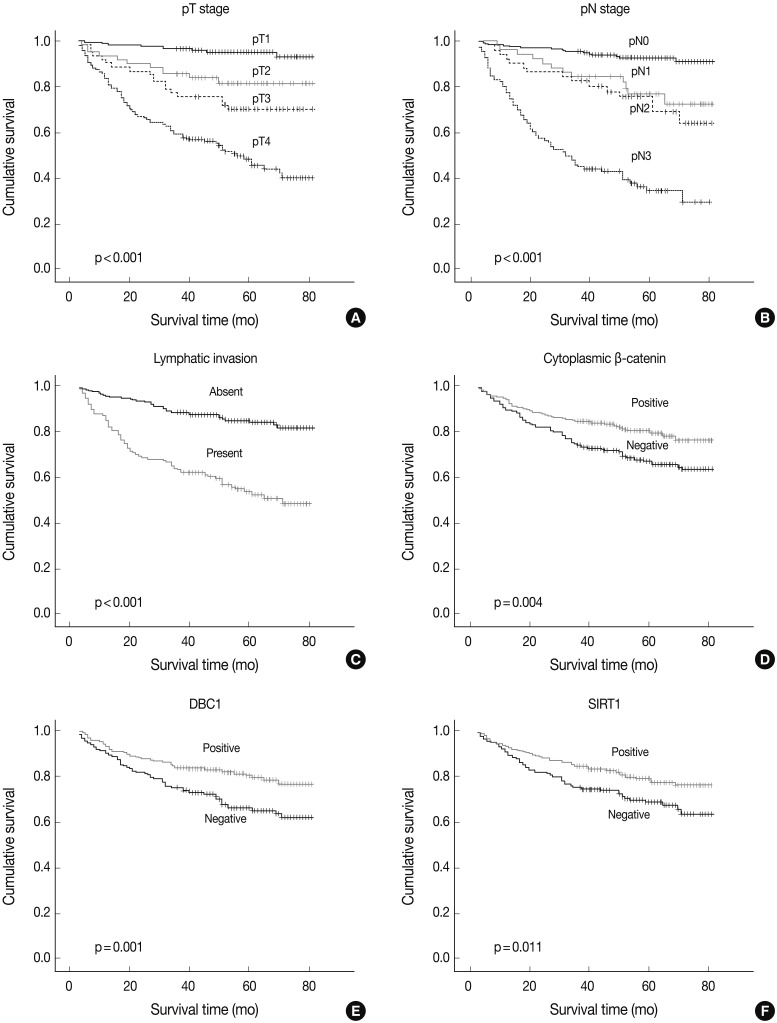
- DBC1 and SIRT1 expression in gastric adenocarcinoma were observed in 271 (60%) and 255 (56%) patients. Expression of DBC1 and SIRT1 were significantly associated with lower histologic grade, intestinal type of Lauren classification, lower pathologic T stage (DBC1 and SIRT1, p<0.001), and lower pathologic N stage (DBC1, p=0.002; SIRT1, p<0.001). The lower TNM stage showed a significant association with SIRT1 (p<0.001), but not with DBC1 (p=0.112). A statistically significant association between the absence of lymphatic invasion and both SIRT1 (p=0.001) and DBC1 (p=0.004) was observed. Statistical associations between younger age (less than 60) and SIRT1 (p=0.023) and DBC1 (p=0.001) were also observed. However, neither sex was associated with SIRT1 (p=0.611) or DBC1 (p=0.080) expression (Table 1). SIRT1 (p=0.011) and DBC1 (p=0.001) showed significant association with better patient survival (Fig. 2). On regression analysis, expression of DBC1 and SIRT1 showed a significant linear association (p<0.001).
- Expression of cytoplasmic β-catenin and p53
- A cytoplasmic, membranous, and nuclear pattern of β-catenin expression was observed in 262 (58%), 367 (81%), and 28 (6%) cases, respectively. The cytoplasmic expression of β-catenin was significantly associated with lower histologic grade (p<0.001), lower pT stage (p<0.001), lower pN stage (p<0.001), lower TNM stage (p<0.001), SIRT1 (p<0.001), and DBC1 (p<0.001) (Table 1). The membranous pattern of β-catenin was also associated with lower histologic grade (p<0.001), lower pT stage (p<0.001), lower pN stage (p=0.003), lower TNM stage (p=0.001), SIRT1 (p=0.002), and DBC1 (p=0.013). Cytoplasmic β-catenin expression also showed a significant association with better patient survival (p=0.004) (Fig. 2). Nuclear β-catenin expression had no significant statistical association with any clinicopathologic factors.
- Expression of p53 showed a statistical association with lower histologic grade (p<0.001). No association between p53 expression and SIRT1 (p=0.063) or DBC1 (p=0.060) expression was observed (Table 1). No pattern of β-catenin showed any correlation with p53 expression (p=0.508, p=0.768, p=0.196, or membranous, nuclear, or cytoplasmic expression of β-catenin, respectively). Expression of p53 had no association with patient survival (p=0.545).
- Correlation of DBC1, SIRT1, cytoplasmic β-catenin, and p53 expression
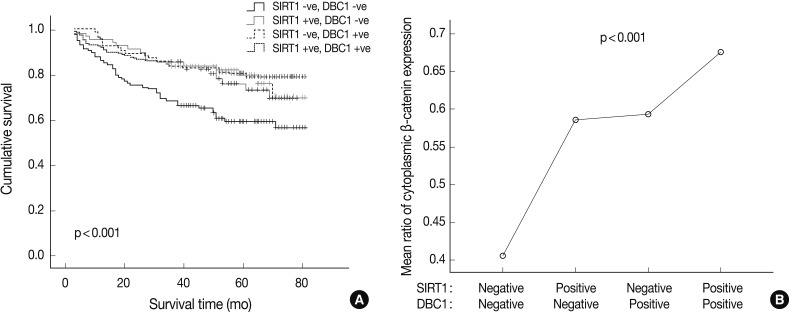
- Simultaneous expression of both DBC1 and SIRT1 was significantly correlated with high expression of cytoplasmic β-catenin (p<0.001). However, the cases of concurrent negative expression of both DBC1 and SIRT1 showed no association with cytoplasmic β-catenin expression. Also, when DBC1 and SIRT1 were expressed as concurrent negative, it was significantly associated with poor patient survival (p<0.001) (Fig. 3). In contrast, there was no association between simultaneous expression of both DBC1 and SIRT1 and expression of p53 (p=0.089).
- Multivariate analysis
- We performed multivariate analysis with the clinicopathologic variables. DBC1 showed statistical significance in multivariate analysis (p=0.012) (Table 2). This means that expression of DBC1 was an independent good prognostic factor in gastric adenocarcinoma.
RESULTS
- SIRT1 is a NAD+-dependent protein deacetylase that deacetylates diverse proteins as mentioned above.22-24 The first identified target of SIRT1 is the p53 tumor suppressor gene. SIRT1 deacetylates p53 and attenuates its ability for cell-cycle arrest and apoptosis, so the role of SIRT1 is considered to be tumor promotion in carcinogenesis.7,22,23,25 Another protein, DBC1, which is a SIRT1-associated protein, negatively regulates SIRT1 activity on p53 and inhibits tumor promoting activity of SIRT1.2,15,26 These two proteins, SIRT1 and DBC1, are thought to play a critical role in carcinogenesis. However, in gastric adenocarcinomas, only a few studies have been conducted with human tissue samples and their results are contrary to the previously known relationship between SIRT1 and DBC1.10,11 Therefore, in this study, we examined the expression of SIRT1 and DBC1 in human gastric adenocarcinomas and analyzed the association with SIRT1, DBC1, p53, β-catenin, and clinicopathologic variables.
- We examined SIRT1 and DBC1 expression with an image analyzer. SIRT1 and DBC1 positive expression showed a direct correlation with each other, a result that is in concordance with previous study data.11 One study reported that expression of DBC1 and SIRT1 was significantly associated with shorter overall survival and greater risk of death, while expression of DBC1 is an independent prognostic factor for disease recurrence and poor survival outcome. However, in this analysis, conflicting results were produced. DBC1 and SIRT1 expression were correlated with better survival and good prognostic factors including lower histologic grade, intestinal type of Lauren classification, lower pathologic T (DBC1 and SIRT1, p<0.001) and N stage (DBC1, p=0.002; SIRT1, p<0.001), and absence of lymphatic invasion (SIRT1, p=0.001; DBC1, p=0.004). Notably, simultaneous expression of both DBC1 and SIRT1 was significantly correlated with high expression of cytoplasmic β-catenin (p<0.001) and better patient survival (p<0.001). We analyzed SIRT1 and DBC1 expression by an image analyzer that was optimized for the counting of nuclear protein expression to decrease intraobserver or interobserver bias in positive cell counting. The resultant data showed mostly similar survival data and a similar relationship between the expression of these two proteins and clinicopathologic variables. We concluded that there was no critical error in interpreting the immunoexpression of SIRT1 and DBC1 in TMA slides. Of course, other biases including selection bias, preservation of tissues, conditions of tissue staining, and so on may lead to an interpretation error, but this is an ineluctable consequence of using both an image analyzer and optical microscope.
- We also analyzed p53 expression of gastric adenocarcinomas in association with SIRT1, DBC1, and β-catenin expression. Only lower histologic grade was associated with p53 expression (p<0.001).
- Some recent study results indicate that SIRT1 could serve as a tumor suppressor by interaction with other proteins such as β-catenin, BRCA1, histone protein, and NF-κB.3,24,27-29 Therefore, we also examined the expression of β-catenin in gastric adenocarcinomas and analyzed its association with SIRT1 and DBC1 expression. Cytoplasmic, membranous, and nuclear expression of β-catenin were separately scored and analyzed. Cytoplasmic and membranous β-catenin expression showed significant associations with SIRT1 and DBC1 expression, lower histologic grade, lower tumor stage, and prolonged patient survival. Correlations between nuclear SIRT1 expression and β-catenin expression in the cytoplasm and cell membrane in concordance with a previous study that investigated colon cancer tumorigenesis.3 On the other hand, nuclear expression of β-catenin in this study was not correlated with SIRT1 expression, which may be due to the difficulty in counting positive nuclei in the presence of cytoplasmic staining. Therefore, we evaluated the relationship between the expression of cytoplasmic β-catenin and the others. Significant associations between SIRT1 and β-catenin in the cytoplasm and cell membrane suggest that SIRT1 expression suppresses the localization of β-catenin to the nucleus, and significantly attenuates its ability to activate transcription. This hypothesis could explain how SIRT1 and cytoplasmic β-catenin expression is correlated in gastric adenocarcinoma patients and how SIRT1 and β-catenin positive patients show better survival results. Similar to this hypothesis in this study, simultaneous expression of both DBC1 and SIRT1 showed significant associations with a high expression of cytoplasmic β-catenin (p<0.001) and better patient survival (p<0.001).
- Multivariate analysis was performed with SIRT1 and DBC1. In multivariate analysis, lymph node metastasis (p<0.001), depth of invasion (p<0.001), and lymphatic invasion (p=0.041) were consistent independent prognostic factors. DBC1 expression was also an independent good prognostic factor (p=0.012). However, in a previous study, expression of DBC1 was an independent prognostic factor for disease recurrence and poor survival outcome.11 This discordant result may be due to observation error in interpretation of immunohistochemical staining slides. SIRT1 did not show prognostic significance in multivariate analysis.
- Recent studies indicate that SIRT1 can serve as both a tumor promoter and a tumor suppressor.3 Some malignant tumors such as breast cancer, colon cancer, and prostate cancer are known to have increased nuclear expression of SIRT1.4,6,7 However, in some tumors including glioblastoma, bladder cancer, and ovarian cancers, reduced expression of SIRT1 was also found.30 These contradictory findings may be attributable to the diverse functions of SIRT1, which can interact with both tumor suppressors and tumor promoters. In this study, we analyzed SIRT1, β-catenin, and p53 together. It appears that expression of SIRT1 is closely associated with the cytoplasmic expression of β-catenin in gastric adenocarcinoma. This finding could explain why SIRT1 positive groups showed better survival. The relationship between SIRT1 and p53 in gastric adenocarcinoma is unclear in these study results. We could not find a relationship between SIRT1 and p53 (p=0.063). Therefore, we believe this weak correlation may be due to a partial relationship between SIRT1 and p53. In another study, expression of p53 showed no significant correlation between SIRT1 expression cases and negative cases.10 In spite of the interaction between SIRT1 and p53, immunohistochemical correlation may or may not exist. More intense research dealing with SIRT1 and SIRT1-related protein is necessary to understand the diverse roles of SIRT1 in gastric carcinogenesis.
- In some studies, the various roles of DBC1 have been examined. First of all, DBC1 was found to augment signals of apoptosis via relocalization of the DBC1 C-terminal fragment into the mitochondria.26 DBC1 is also able to regulate the transcriptional activity of some nuclear hormone receptors including retinoic acid receptor α and AR.26 DBC1 is known to inhibit SIRT1 through a direct protein to protein interaction and thus promotes p53 mediated apoptosis.2 However, mutation of DBC1 does not direct binding to SIRT1, so it should not affect enzymatic activity of SIRT1 and the acetylation state of SIRT1 substrates including p53.2 In this regard, a negative correlation is expected between SIRT1 and DBC1 expressions. However, in this study, expression of DBC1 was significantly correlated with SIRT1 expression (p<0.001).
- In agreement with our data, some study results revealed upregulation of DBC1 in various types of malignancies. Moreover, recent studies have reported a positive correlation of SIRT1 and DBC1 expression in human cancer tissues.8,11 These results may be due to the recently identified diverse functions of DBC1 other than inhibition of SIRT1.16,26 Though DBC1 expression of gastric adenocarcinoma is associated with SIRT1 expression, the role of DBC1 in gastric carcinogenesis may be other than inhibition of SIRT1.
- In summary, expression of DBC1, SIRT1, and cytoplasmic and membranous β-catenin in gastric adenocarcinoma showed significant correlations with each other. Furthermore, expressions of these three proteins were associated with lower histologic grade, lower tumor stage, and better patient survival. However, there was no evident association between SIRT1 and p53 protein. The role of SIRT1 and DBC1 in gastric carcinogenesis should be considered carefully in correlation with other proteins that interact with SIRT1 and DBC1.
DISCUSSION
- 1. Guarente L, Picard F. Calorie restriction: the SIR2 connection. Cell 2005; 120: 473-482. ArticlePubMed
- 2. Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature 2008; 451: 583-586. ArticlePubMedPDF
- 3. Firestein R, Blander G, Michan S, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One 2008; 3: e2020.ArticlePubMedPMC
- 4. Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell 2005; 123: 437-448. ArticlePubMed
- 5. Hida Y, Kubo Y, Murao K, Arase S. Strong expression of a longevity-related protein, SIRT1, in Bowen's disease. Arch Dermatol Res 2007; 299: 103-106. ArticlePubMedPDF
- 6. Kuzmichev A, Margueron R, Vaquero A, et al. Composition and histone substrates of polycomb repressive group complexes change during cellular differentiation. Proc Natl Acad Sci U S A 2005; 102: 1859-1864. ArticlePubMedPMC
- 7. Huffman DM, Grizzle WE, Bamman MM, et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res 2007; 67: 6612-6618. ArticlePubMedPDF
- 8. Lee H, Kim KR, Noh SJ, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis for breast carcinoma. Hum Pathol 2011; 42: 204-213. ArticlePubMed
- 9. Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene 2007; 26: 5489-5504. ArticlePubMedPDF
- 10. Feng AN, Zhang LH, Fan XS, et al. Expression of SIRT1 in gastric cardiac cancer and its clinicopathologic significance. Int J Surg Pathol 2011; 19: 743-750. ArticlePubMedPDF
- 11. Cha EJ, Noh SJ, Kwon KS, et al. Expression of DBC1 and SIRT1 is associated with poor prognosis of gastric carcinoma. Clin Cancer Res 2009; 15: 4453-4459. ArticlePubMedPDF
- 12. Czyzewska J, Guzińska-Ustymowicz K, Ustymowicz M, Pryczynicz A, Kemona A. The expression of E-cadherin-catenin complex in patients with advanced gastric cancer: role in formation of metastasis. Folia Histochem Cytobiol 2010; 48: 37-45. ArticlePubMed
- 13. Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005; 10: 86-103. ArticlePubMedPMC
- 14. Hamaguchi M, Meth JL, von Klitzing C, et al. DBC2, a candidate for a tumor suppressor gene involved in breast cancer. Proc Natl Acad Sci U S A 2002; 99: 13647-13652. ArticlePubMedPMC
- 15. Zhao W, Kruse JP, Tang Y, Jung SY, Qin J, Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature 2008; 451: 587-590. ArticlePubMedPMCPDF
- 16. Fu J, Jiang J, Li J, et al. Deleted in breast cancer 1, a novel androgen receptor (AR) coactivator that promotes AR DNA-binding activity. J Biol Chem 2009; 284: 6832-6840. ArticlePubMedPMC
- 17. Trauernicht AM, Kim SJ, Kim NH, Boyer TG. Modulation of estrogen receptor alpha protein level and survival function by DBC-1. Mol Endocrinol 2007; 21: 1526-1536. PubMed
- 18. Park SH, Lee SI. Recent advances in chemotherapy of gastric cancer. Korean J Med 2012; 82: 417-426. Article
- 19. Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology 2011; 78: 302-310. ArticlePubMedPDF
- 20. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging handbook: from the AJCC cancer staging manual. 2010; 7th ed. New York: Springer.
- 21. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 2010; 4th ed. Lyon: IARC Press.
- 22. Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001; 107: 149-159. ArticlePubMed
- 23. Luo J, Nikolaev AY, Imai S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001; 107: 137-148. ArticlePubMed
- 24. Kyrylenko S, Kyrylenko O, Suuronen T, Salminen A. Differential regulation of the Sir2 histone deacetylase gene family by inhibitors of class I and II histone deacetylases. Cell Mol Life Sci 2003; 60: 1990-1997. ArticlePubMedPMCPDF
- 25. Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res 2005; 65: 10457-10463. ArticlePubMedPDF
- 26. Kim JE, Lou Z, Chen J. Interactions between DBC1 and SIRT 1 are deregulated in breast cancer cells. Cell Cycle 2009; 8: 3784-3785. ArticlePubMed
- 27. Mullan PB, Quinn JE, Harkin DP. The role of BRCA1 in transcriptional regulation and cell cycle control. Oncogene 2006; 25: 5854-5863. ArticlePubMedPDF
- 28. Wang RH, Zheng Y, Kim HS, et al. Interplay among BRCA1, SIRT1, and survivin during BRCA1-associated tumorigenesis. Mol Cell 2008; 32: 11-20. ArticlePubMedPMC
- 29. Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 2004; 23: 2369-2380. ArticlePubMedPMC
- 30. Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 2000; 289: 2126-2128. ArticlePubMed
REFERENCES
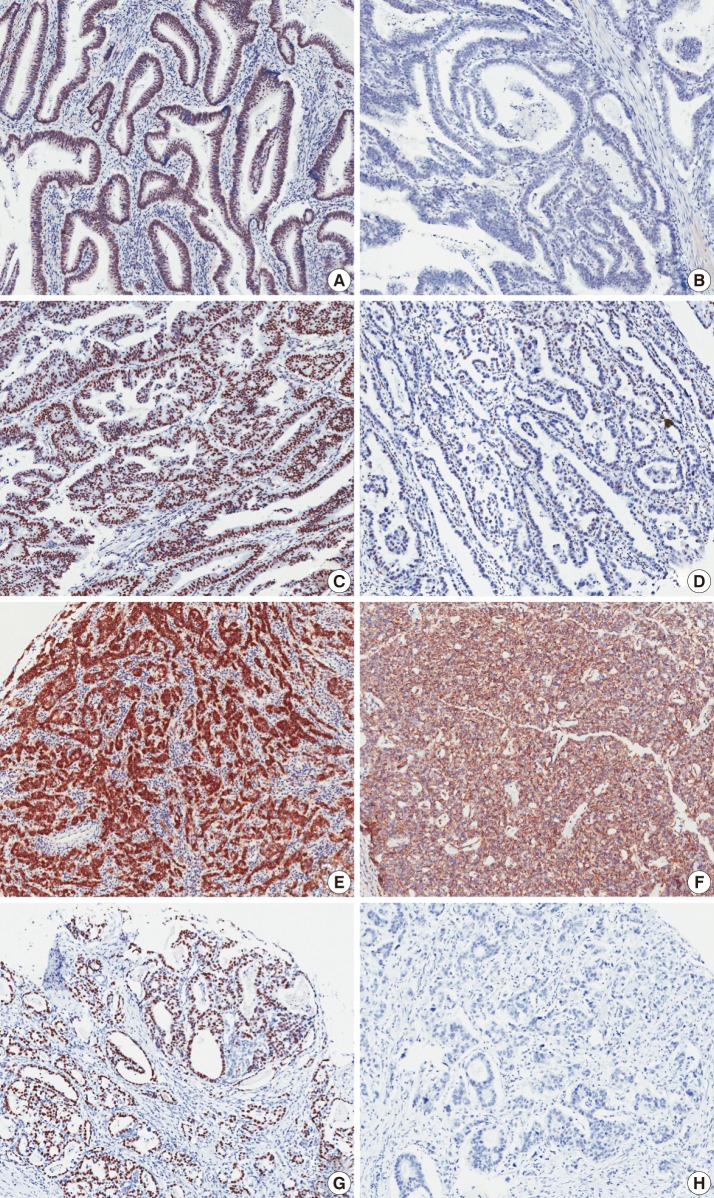
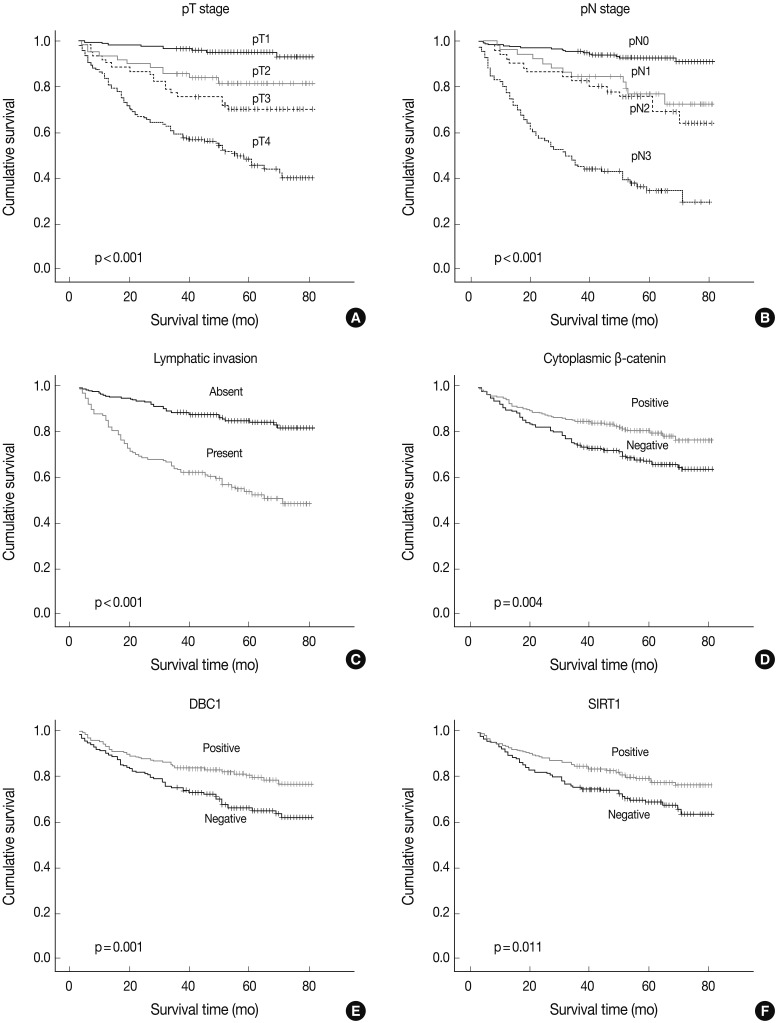
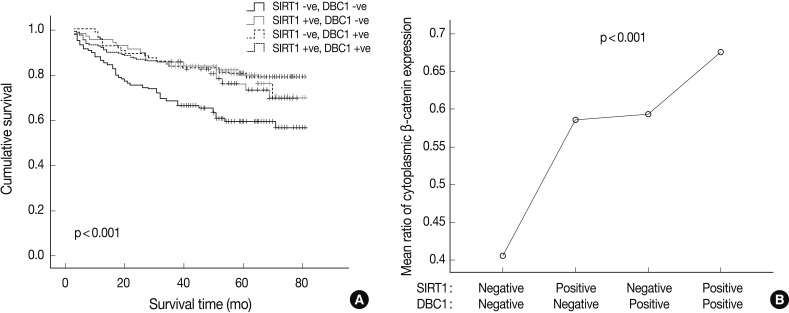
Figure & Data
References
Citations

- Regulatory role of DBC1 in inflammation and autoimmune diseases
Jinzhi Wu, Fan Yang, Guanhua Xu, Xinlei Ma, Jin Lin, Weiqian Chen
Rheumatology & Autoimmunity.2025; 5(1): 15. CrossRef - Prognostic and clinicopathological value of dbc1 expression in human cancers: a systematic review and meta-analysis
Haojia Wang, Xinhong Cheng, Bruce Xianzhuo Zhang, Yong Wang, Shuo Gao, Fanghui Ding, Xiaojing Song, Dandan Li, Haixu Ni, Yang Luo, Xun Li
Frontiers in Oncology.2025;[Epub] CrossRef - Mechanistic insights into the dual role of CCAR2/DBC1 in cancer
Hwa Jin Kim, Sue Jin Moon, Jeong Hoon Kim
Experimental & Molecular Medicine.2023; 55(8): 1691. CrossRef - Sirtuins (SIRTs) As a Novel Target in Gastric Cancer
Agata Poniewierska-Baran, Paulina Warias, Katarzyna Zgutka
International Journal of Molecular Sciences.2022; 23(23): 15119. CrossRef - Histone Deacetylase Functions in Gastric Cancer: Therapeutic Target?
Amandine Badie, Christian Gaiddon, Georg Mellitzer
Cancers.2022; 14(21): 5472. CrossRef - Advances on the role of the deleted in breast cancer (DBC1) in cancer and autoimmune diseases
Qiannan Fang, Joseph A Bellanti, Song Guo Zheng
Journal of Leukocyte Biology.2021; 109(2): 449. CrossRef - miR-1301-3p Promotes Cell Proliferation and Facilitates Cell Cycle Progression via Targeting SIRT1 in Gastric Cancer
Dakui Luo, Hao Fan, Xiang Ma, Chao Yang, Yu He, Yugang Ge, Mingkun Jiang, Zekuan Xu, Li Yang
Frontiers in Oncology.2021;[Epub] CrossRef - CCAR1 and CCAR2 as gene chameleons with antagonistic duality: Preclinical, human translational, and mechanistic basis
Gavin S. Johnson, Praveen Rajendran, Roderick H. Dashwood
Cancer Science.2020; 111(10): 3416. CrossRef - Role of Histone Acetylation in Gastric Cancer: Implications of Dietetic Compounds and Clinical Perspectives
Danielle Q Calcagno, Fernanda Wisnieski, Elizangela R da Silva Mota, Stefanie B Maia de Sousa, Jéssica M Costa da Silva, Mariana F Leal, Carolina O Gigek, Leonardo C Santos, Lucas T Rasmussen, Paulo P Assumpção, Rommel R Burbano, Marília AC Smith
Epigenomics.2019; 11(3): 349. CrossRef - Survival and Clinicopathological Significance of SIRT1 Expression in Cancers: A Meta-Analysis
Min Sun, Mengyu Du, Wenhua Zhang, Sisi Xiong, Xingrui Gong, Peijie Lei, Jin Zha, Hongrui Zhu, Heng Li, Dong Huang, Xinsheng Gu
Frontiers in Endocrinology.2019;[Epub] CrossRef - Cell cycle and apoptosis regulator 2 at the interface between DNA damage response and cell physiology
Martina Magni, Giacomo Buscemi, Laura Zannini
Mutation Research/Reviews in Mutation Research.2018; 776: 1. CrossRef - NANOGP8 expression regulates gastric cancer cell progression by transactivating DBC1 in gastric cancer MKN‑45 cells
Li Li, Ru Feng, Sujuan Fei, Jiang Cao, Qinqin Zhu, Guozhong Ji, Jianwei Zhou
Oncology Letters.2018;[Epub] CrossRef - Overexpression of DBC1, correlated with poor prognosis, is a potential therapeutic target for hepatocellular carcinoma
Changcan Li, Jianhua Liao, Shaohan Wu, Junwei Fan, Zhihai Peng, Zhaowen Wang
Biochemical and Biophysical Research Communications.2017; 494(3-4): 511. CrossRef - Association of sirtuins with clinicopathological parameters and overall survival in gastric cancer
Xiaobing Shen, Pengfei Li, Yuchao Xu, Xiaowei Chen, Haixiang Sun, Ying Zhao, Mengqi Liu, Wenwen Zhang
Oncotarget.2017; 8(43): 74359. CrossRef - Co-ordinated overexpression of SIRT1 and STAT3 is associated with poor survival outcome in gastric cancer patients
Shu Zhang, Shuling Huang, Chao Deng, Yu Cao, Jun Yang, Guangxia Chen, Bin Zhang, Chaoqin Duan, Jiong Shi, Bo Kong, Helmut Friess, Nanyi Zhao, Chen Huang, Xiaoli Huang, Lei Wang, Xiaoping Zou
Oncotarget.2017; 8(12): 18848. CrossRef - The prognostic role of Sirt1 expression in solid malignancies: a meta-analysis
Changwen Wang, Wen Yang, Fang Dong, Yawen Guo, Jie Tan, Shengnan Ruan, Tao Huang
Oncotarget.2017; 8(39): 66343. CrossRef - SIRT1 induces tumor invasion by targeting epithelial mesenchymal transition-related pathway and is a prognostic marker in triple negative breast cancer
Min-Sun Jin, Chang Lim Hyun, In Ae Park, Ji Young Kim, Yul Ri Chung, Seock-Ah Im, Kyung-Hun Lee, Hyeong-Gon Moon, Han Suk Ryu
Tumor Biology.2016; 37(4): 4743. CrossRef - Prognostic and clinical value of Sirt1 expression in gastric cancer: A systematic meta-analysis
Bin Jiang, Jin-huang Chen, Wen-zheng Yuan, Jin-tong Ji, Zheng-yi Liu, Liang Wu, Qiang Tang, Xiao-gang Shu
Journal of Huazhong University of Science and Technology [Medical Sciences].2016; 36(2): 278. CrossRef - Significance of silent mating type information regulation 2 homolog 1 and claudin 4 expression in gastric carcinoma and precursor lesions
Nashwa M. Emara, Ranih Z. Amer, Khaled M. Elsadek Attia, Heba M. Rashad, Adel Z. Elseady, Abd El-Latif M. Elbalshy
Egyptian Journal of Pathology.2016; 36(2): 158. CrossRef - Distinctive role of SIRT1 expression on tumor invasion and metastasis in breast cancer by molecular subtype
Yul Ri Chung, Hyojin Kim, Soo Young Park, In Ae Park, Ja June Jang, Ji-Young Choe, Yoon Yang Jung, Seock-Ah Im, Hyeong-Gon Moon, Kyung-Hun Lee, Koung Jin Suh, Tae-Yong Kim, Dong-Young Noh, Wonshik Han, Han Suk Ryu
Human Pathology.2015; 46(7): 1027. CrossRef - SIRT1 is a regulator of autophagy: Implications in gastric cancer progression and treatment
Guanglin Qiu, Xuqi Li, Xiangming Che, Chao Wei, Shicai He, Jing Lu, Zongliang Jia, Ke Pang, Lin Fan
FEBS Letters.2015; 589(16): 2034. CrossRef - DBC1 Functions as a Tumor Suppressor by Regulating p53 Stability
Bo Qin, Katherine Minter-Dykhouse, Jia Yu, Jun Zhang, Tongzheng Liu, Haoxing Zhang, SeungBaek Lee, JungJin Kim, Liewei Wang, Zhenkun Lou
Cell Reports.2015; 10(8): 1324. CrossRef - DBC1 promotes anoikis resistance of gastric cancer cells by regulating NF-κB activity
YONGWEI HUAN, DEPING WU, DAYONG ZHOU, BO SUN, GUOXIN LI
Oncology Reports.2015; 34(2): 843. CrossRef - Resveratrol relieves ischemia‑induced oxidative stress in the hippocampus by activating SIRT1
Zhuangzhi Meng, Jianguo Li, Honglin Zhao, Haiying Liu, Guowei Zhang, Lingzhan Wang, He Hu, Di Li, Mingjing Liu, Fulong Bi, Xiaoping Wang, Geng Tian, Qiang Liu, Batu Buren
Experimental and Therapeutic Medicine.2015;[Epub] CrossRef - CCAR2 deficiency augments genotoxic stress-induced apoptosis in the presence of melatonin in non-small cell lung cancer cells
Wootae Kim, Joo-Won Jeong, Ja-Eun Kim
Tumor Biology.2014; 35(11): 10919. CrossRef - Radioprotective and Antioxidant Effect of Resveratrol in Hippocampus by Activating Sirt1
Jianguo Li, Li Feng, Yonghua Xing, Yan Wang, Liqing Du, Chang Xu, Jia Cao, Qin Wang, Saijun Fan, Qiang Liu, Feiyue Fan
International Journal of Molecular Sciences.2014; 15(4): 5928. CrossRef - SIRT1 expression is associated with a poor prognosis, whereas DBC1 is associated with favorable outcomes in gastric cancer
Akira Noguchi, Keiji Kikuchi, Huachuan Zheng, Hiroyuki Takahashi, Yohei Miyagi, Ichiro Aoki, Yasuo Takano
Cancer Medicine.2014; 3(6): 1553. CrossRef - Sirtuins and Cancer: New Insights and Cell Signaling
Marcos Vinícius Macedo de Oliveira, João Marcus Oliveira Andrade, Alanna Fernandes Paraíso, Sérgio Henrique Sousa Santos
Cancer Investigation.2013; 31(10): 645. CrossRef - Deleted in breast cancer-1 (DBC-1) in the interface between metabolism, aging and cancer
Eduardo Nunes Chini, Claudia C. S. Chini, Veronica Nin, Carlos Escande
Bioscience Reports.2013;[Epub] CrossRef - SIRT1 Expression Is Associated with Good Prognosis in Colorectal Cancer
Wonkyung Jung, Kwang Dae Hong, Woon Yong Jung, Eunjung Lee, Bong Kyung Shin, Han Kyeom Kim, Aeree Kim, Baek-hui Kim
Korean Journal of Pathology.2013; 47(4): 332. CrossRef - Clinicopathological significance of SIRT1 and p300/CBP expression in gastroesophageal junction (GEJ) cancer and the correlation with E-cadherin and MLH1
Li-Hua Zhang, Qin Huang, Xiang-Shan Fan, Hong-Yan Wu, Jun Yang, An-Ning Feng
Pathology - Research and Practice.2013; 209(10): 611. CrossRef



Fig. 1
Fig. 2
Fig. 3


SIRT1, sirtuin 1; DBC1, deleted in breast cancer 1; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; TNM, tumor-node-metastasis. aAmerican Joint Committee on Cancer Cancer Staging Manual, 7th edition.
CI, confidence interval; EGC, early gastric cancer; AGC, advanced gastric cancer; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; SIRT1, sirtuin 1; DBC1, deleted in breast cancer 1.

 E-submission
E-submission

 PubReader
PubReader Cite this Article
Cite this Article