Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(6); 2012 > Article
-
Original Article
The Definition of Minimal Extrathyroid Extension in Thyroid Pathology by Analyzing Sizable Intra- and Extrathyroid Blood Vessels - Hyae Min Jeon, Beom Jin Lim, Hang-Seok Chang1, SoonWon Hong
-
Korean Journal of Pathology 2012;46(6):548-553.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.548
Published online: December 26, 2012
Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
1Department of General Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding Author: SoonWon Hong, M.D. Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 135-720, Korea. Tel: +82-2-2019-3543, Fax: +82-2-3463-2103, soonwonh@yuhs.ac
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- To define the exact boundary of the intrathyroid and extrathyroid aspects of a gland when determining the extent of cancer invasion, we plan to clarify the definition of sizable vascular structures, which is one of the helpful histologic clues in determining a minimal extrathyroid extension. We hypothesized that arterial wall thicknesses in extrathyroid soft tissue would be significantly different from the arteries in the thyroid parenchyma.
-
Methods
- Twenty cases of papillary carcinoma were selected. The numbers and wall thicknesses of the arteries and arterioles in intrathyroid and extrathyroid tissue were evaluated. The absence of nerve tissue in the thyroid gland was confirmed using the S-100 protein immunohistochemical stain.
-
Results
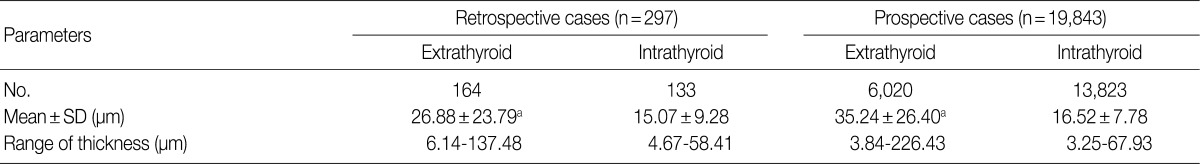
- The comparison of the mean thicknesses of the total arteries between the extrathyroid and intrathyroid tissues in the retrospective study (26.88 µm vs. 15.07 µm, respectively) and the prospective study (35.24 µm vs. 16.52 µm, respectively) revealed significant differences (p=0.000). The greatest thickness of the intrathyroid arteries was 67.93 µm.
-
Conclusions
- According to our results, the study showed that the extrathyroidal arteries were significantly thicker than the intrathyroidal arteries. We suggest that the sizable blood vessels of extrathyroidal arteries should be greater than 67.93 µm in thickness.
- Patient selection
- Patients who were diagnosed with papillary carcinoma or microcarcinoma and underwent surgery at Severance Hospital from January to June 2011 were included in this study. First, a preliminary study was performed in a retrospective manner. Ten cases of total thyroidectomy specimens with papillary carcinoma or microcarcinoma were randomly selected in age- (3rd to 7th decades) and sex-matched pairs. A representative hematoxylin and eosin (H&E) slide for each of the 10 cases was analyzed. Prospectively, the 10 patients were randomly selected. All of these patients had neither a medical history of hypertension or diabetes, nor a history of surgery for vascular diseases. The study design and case selection were identical to the retrospective study, but in the prospective study, the entire unilateral thyroid gland containing the papillary carcinoma or microcarcinoma was serially dissected, sampled, and evaluated for each patient. We defined the location of vessels as either extrathyroid or intrathyroid by drawing imaginary lines in uncertain areas. Afterward, we counted and analyzed the arteries and arterioles.
- Special and immunohistochemical staining
- The thyroid tissue sections that were 3 µm in thickness, fixed in 10% formalin, and embedded in paraffin blocks, were used for histological analysis with routine H&E staining, along with special and immunohistochemical stains of elastic van Gieson (EVG) and S-100 protein. Using H&E stain, we were able to screen sections of thyroid tissue. To identify the types of vessels as either arteries or veins, the number of arteries was counted, and the arterial wall thickness between the intimal lining and external elastic lamina was precisely measured and eventually corrected for errors of sectioning and analysis. We performed a special staining of EVG,15 and an additional immunohistochemical staining of S-100 protein was completed to confirm the absence of nerve tissue in the thyroid parenchyma, as performed in previous studies.2
- Arterial and arteriolar wall thickness
- For comparing the extrathyroid and intrathyroid arterial and arteriolar wall thicknesses, we initially counted and measured the entire detectable artery. However, several large arteries in the thyroid gland could be a part of the branching arteries penetrating the thyroid along the interlobular spaces.2 In addition, diagnostic problems, that is whether the cancer extended to the extrathyroid tissue or was confined to the intrathyroid region, occurred when the cancer locations were not definitive. As a result, definite arteries and arterioles located in the intrathyroid and interlobular areas were excluded. Therefore, we decided not to include arteries or arterioles in the thyroid parenchyma that were greater than 2 mm from the surface.
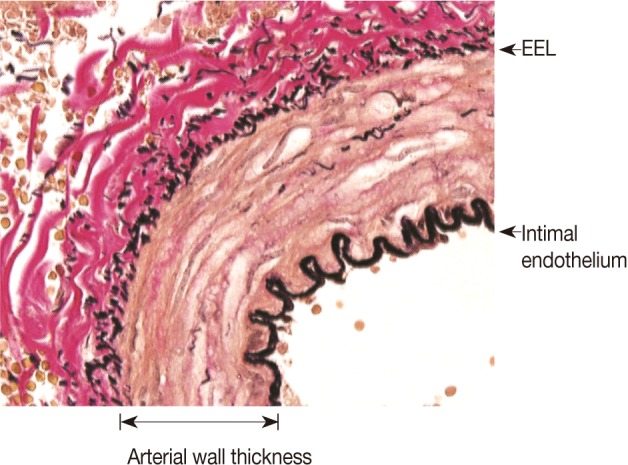
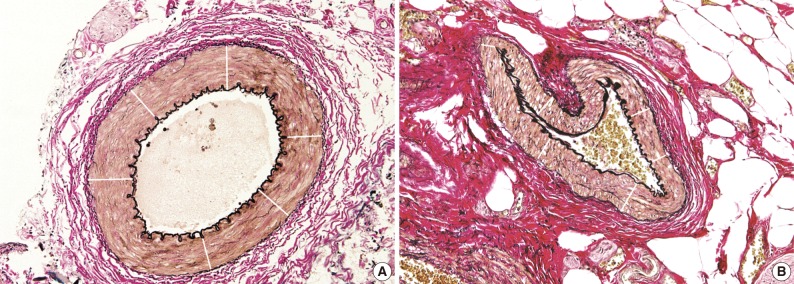
- We measured the arterial and arteriolar wall thicknesses, the distance between the intimal endothelium and external elastic lamina, which refers to the sum of the tunica intima and tunica media15 using the Image J program, a computer-based morphometric analysis programs (Fig. 1).16 To avoid bias, we randomly measured the thicknesses six times, for example, at 12, 2, 4, 6, 8, and 10 o'clock, in each blood vessel17,18 and we then used the mean thicknesses as statistical data, as shown in Fig. 2.
- Statistical analysis
- Data was analyzed using statistical software (IBM SPSS ver. 20.0, IBM Corp., Armonk, NY, USA). A two-tailed independent Student's t-test and Pearson's simple correlation coefficient were used for the continuous variables of the two independent groups in both of the studies. Statistical significance was inferred at p<0.05.
MATERIALS AND METHODS
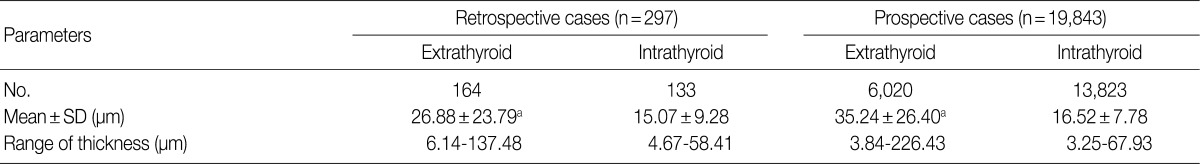
- A total of 297 and 19,843 arteries were counted in ten retrospective and ten prospective analyses, respectively. In the retrospective study, the extrathyroid arteries and arterioles (n=164) outnumbered the intrathyroid ones (n=133). In the prospective study, the extrathyroid arteries and arterioles (n=6,020) were fewer in number than the intrathyroid ones (n=13,823). The mean wall thicknesses of the extrathyroid arteries (26.88 µm and 35.24 µm, respectively, from the retrospective and prospective studies) were thicker (p=0.000) than the intrathyroid arteries and arterioles (15.07 µm and 16.52 µm, respectively, from the retrospective and prospective studies). The respective ranges of the mean thicknesses of the blood vessels in the extrathyroid and intrathyroid tissues were 3.84 to 226.43 µm and 3.25 to 67.93 µm, respectively from the 20 total patients (Table 1).
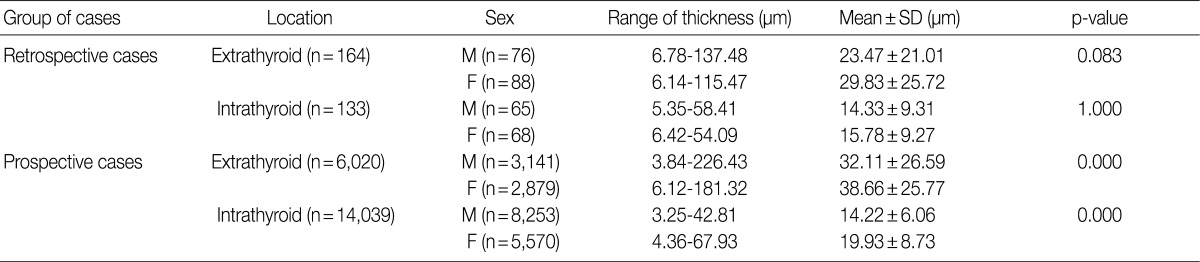
- The wall thickness comparison between the sexes according to the location of the arteries is shown in Table 2. Counting the number of the observed total arteries and arterioles demonstrated that male patients (n=11,535) had more arteries than female patients (n=8,605). This discrepancy was also seen in the number of extrathyroid (M, 3,217; F, 2,967) and intrathyroid (M, 8,318; F, 5,638) arteries for all of the cases. In male patients, the mean extrathyroid arterial walls (23.47 µm and 32.11 µm) were thicker than the intrathyroid arterial walls (14.33 µm and 14.22 µm) in the preliminary (p=0.001) and prospective studies (p= 0.000), respectively. Likewise, the female patients revealed similar results, with the mean extrathyroid arterial walls (29.83 µm and 38.66 µm) being thicker than the intrathyroid arterial walls (15.78 µm and 19.93 µm) in the preliminary (p=0.000) and prospective studies (p=0.000), respectively.
- Table 3 shows that there was no significant difference in the mean extrathyroid (p=0.083) or intrathyroid (p=1.000) arterial wall thicknesses between the male and female patients in the preliminary study. However, in the prospective study, a comparison between the paired mean extrathyroid (p=0.000) and intrathyroid (p=0.000) arterial wall thicknesses demonstrated that the male arteries (32.11 µm and 14.22 µm) were thinner than female arteries (38.66 µm and 19.93 µm), respectively.
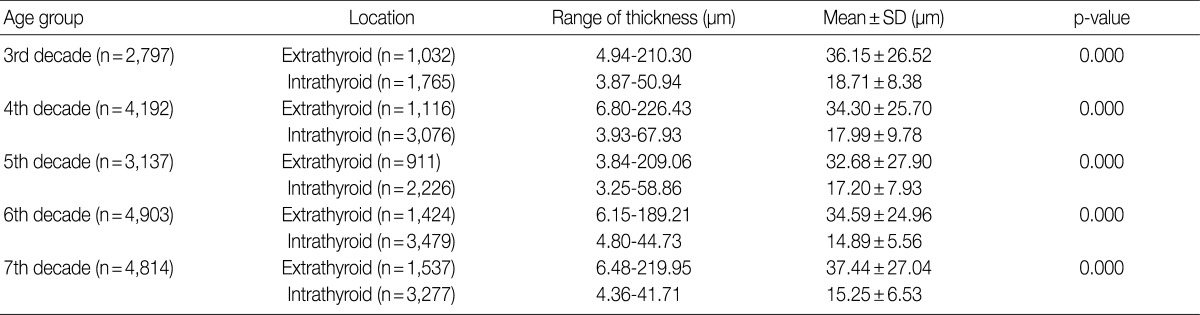
- We identified the correlations between the arterial wall thicknesses and age groups in the prospective cases (Table 4). All five age groups (3rd to 7th decades) revealed that the mean arterial wall thicknesses in the extrathyroid tissue was thicker than the mean arterial wall thicknesses in the intrathyroid tissue (p=0.000). In each age group, the mean wall thicknesses of the arteries in the extrathyroid and intrathyroid tissues exhibited significant differences (p=0.000) in ascending order (from 3rd to 7th decades) as follows: 36.15 vs. 18.71, 34.30 vs. 17.99, 32.68 vs. 17.20, 34.59 vs. 14.89, and 37.44 vs. 15.25. The preliminary study was excluded due to the limited number of cases.
- When comparing among the age groups, a decreasing tendency in the intrathyroid arterial wall thickness with increasing age was seen (p=0.01). On the other hand, the extrathyroid arterial wall thicknesses did not exhibit any significant relationship to age (p=0.09).
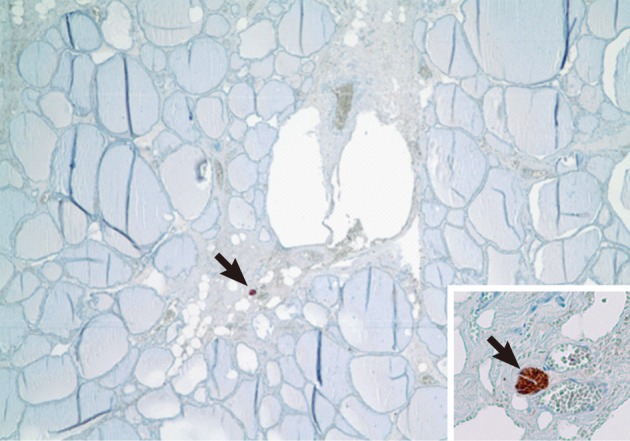
- In addition, nerve tissues were not identified in the thyroid parenchyma itself, but in all of the cases, some nerve tissues in the interlobular septum were identified by using immunohistochemical staining for S-100 protein (Fig. 3).
RESULTS
- Through the practice of surgical pathology, we found many difficulties in determining the extent of the tumors for diagnosing papillary carcinomas or papillary microcarcinomas of the thyroid gland. According to the primary tumor stage (T), the T3 stage classification was determined when the tumor size was greater than 4 cm in size in its greatest dimension, was limited to the thyroid parenchyma, or had any cancer with minimal ETE. Even though the anatomic stage/prognostic groups for papillary carcinomas in the patients younger than 45 years included had only two stages (I, II) whether metastasis was present or not, the T stage could be an important parameter for determining the prognostic groups of patients 45 years and older.7-10 Because of this significance, pathologists should discretely judge whether tumors are limited to the thyroid. Searching previous articles and textbooks, however, we could not find discrete clarifying parameters of minimal ETE. Therefore, we focused on the aspect of "sizable blood vessels" in determining minimal ETE.5
- In this study, we found that the mean thickness of the extrathyroid arteries were greater than that of the intrathyroid arteries in both retrospective and prospective studies. In all of the cases, the extrathyroid arteries were 1.78 and 2.13 times thicker than the intrathyroid arteries in the retrospective and prospective studies, respectively. The thinnest extrathyroid artery was 3.84 µm in thickness, while the thickest intrathyroid artery was 67.93 µm in thickness. In the male group, the extrathyroid arteries were 1.64 and 2.26 times thicker than the intrathyroid arteries in the retrospective and prospective studies, respectively. In the female group, the extrathyroid arteries were 1.89 and 1.94 times thicker than the intrathyroid arteries in the retrospective and prospective studies, respectively. In other words, the arteries in the extrathyroid tissue were about two times thicker than arteries in thyroid parenchyma.
- The total numbers of arteries were counted in each study. The retrospective study revealed that extrathyroid arteries (n=164) were greater in number than the intrathyroid arteries (n=133), but the prospective study revealed the opposite results (6,020 vs. 13,823) for extrathyroid and intrathyroid arteries, respectively. One potential reason for this was that each representative slide in the ten prospective cases included papillary carcinomas or papillary microcarcinomas. As a result, the area of thyroid parenchyma that could be examined decreased. Comparing the number of arteries between the sexes, we found that the male group had more arteries than the female group.
- We also found that the extrathyroid and intrathyroid arteries exhibited no differences based on sex in the retrospective study. However, the prospective study revealed that the arteries in the female were 1.20 and 1.40 times thicker than those in males in the extrathyroid and intrathyroid tissues, respectively.
- A correlation was not identified in the age differences in the retrospective study due to the limited number of cases. In the prospective study, the total number of arteries revealed that the 6th decade group had the most arteries (n=4,903), while the 3rd decade group exhibited the fewest number of arteries (n= 2,797). The relationships among age groups in the 3rd, 4th, 5th, 6th, and 7th decades were analyzed in the prospective study as follows: 1) all five age groups revealed that the arteries in the extrathyroid tissue were thicker than in the intrathyroid tissue, 2) in each decade, the results of the differences of the arterial wall thicknesses between the location of the arteries increased in ascending order of age with the extrathyroid arteries being 1.93, 1.90, 1.90, 2.32, and 2.43 times thicker than the intrathyroid arteries, respectively. For all of the cases, a two-fold difference in thickness of the extrathyroid and intrathyroid arterial walls was seen.
- In addition, we confirmed that nerve tissue did not exist in the thyroid parenchyma, in agreement with previous studies.3
- Previous studies have only mentioned the "sizable blood vessels" as one of the factors determining the boundary between intrathyroid and extrathyroid tissues, whereas our study presented the "sizable blood vessels" numerically. However, the number of patients was insufficient to establish numeric criteria for sizable blood vessels. We only included arteries and arterioles as vessels in order to remove measurement error. We also calculated the mean thicknesses of the arteries and arterioles by randomly measuring each artery and arteriole a total of six times, as performed in previous studies.17,18 Further larger studies are required in order to establish new criteria for sizable blood vessels and should include more precise measurement methods. With the publication of investigations about the prognostic differences according to minimal ETE, the demand for realistic criteria of sizable blood vessels will increase.
- In conclusion, the retrospective study exhibited differences between extrathyroid and intrathyroid arterial and arteriolar wall thicknesses regardless of sex. The prospective study revealed that extrathyroid arteries were thicker than intrathyroid arteries in all 20 cases regardless of sex or age group.
- Of the total 20 patients, the ranges of the mean thicknesses of the blood vessels in the extrathyroid and intrathyroid tissues were 3.84 to 226.43 µm and 3.25 to 67.93 µm, respectively. Therefore, we suggest that the sizable blood vessel of extrathyroid arteries can be expected to be greater than 67.93 µm in thickness.
DISCUSSION
- 1. Ito Y, Tomoda C, Uruno T, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survival. World J Surg 2006; 30: 780-786. ArticlePubMedPDF
- 2. Carcangiu ML. In: Mills SE, ed. Thyroid. Histology for pathologists. 2007; 3rd ed. Philadelphia: Lippincott Williams & Wilkins, 1129-1148.
- 3. Mete O, Rotstein L, Asa SL. Controversies in thyroid pathology: thyroid capsule invasion and extrathyroidal extension. Ann Surg Oncol 2010; 17: 386-391. ArticlePubMedPDF
- 4. Komorowski RA, Hanson GA. Occult thyroid pathology in the young adult: an autopsy study of 138 patients without clinical thyroid disease. Hum Pathol 1988; 19: 689-696. ArticlePubMed
- 5. Ghossein R. Update to the College of American Pathologists reporting on thyroid carcinomas. Head Neck Pathol 2009; 3: 86-93. ArticlePubMedPMCPDF
- 6. Gnepp DR, Ogorzalek JM, Heffess CS. Fat-containing lesions of the thyroid gland. Am J Surg Pathol 1989; 13: 605-612. ArticlePubMed
- 7. DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumours: pathology and genetics of tumours of endocrine organs. 2004; Lyon: IARC Press.
- 8. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 2010; 7th ed. New York: Springer.
- 9. Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006; 16: 109-142. ArticlePubMed
- 10. Shaha AR. TNM classification of thyroid carcinoma. World J Surg 2007; 31: 879-887. ArticlePubMedPDF
- 11. Hu A, Clark J, Payne RJ, Eski S, Walfish PG, Freeman JL. Extrathyroidal extension in well-differentiated thyroid cancer: macroscopic vs microscopic as a predictor of outcome. Arch Otolaryngol Head Neck Surg 2007; 133: 644-649. ArticlePubMed
- 12. Jukkola A, Bloigu R, Ebeling T, Salmela P, Blanco G. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocr Relat Cancer 2004; 11: 571-579. ArticlePubMed
- 13. Gluckman JL. In: Johnson JT, Gluckman JL, eds. Clinical manifestations of thyroid cancer. Carcinoma of the thyroid. 1999; Oxford: Isis Medical Media, 33-37.
- 14. Ito Y, Tomoda C, Uruno T, et al. Minimal extrathyroid extension does not affect the relapse-free survival of patients with papillary thyroid carcinoma measuring 4 cm or less over the age of 45 years. Surg Today 2006; 36: 12-18. ArticlePubMedPDF
- 15. Aravind B, Saunders B, Navin T, et al. Inhibitory effect of TIMP influences the morphology of varicose veins. Eur J Vasc Endovasc Surg 2010; 40: 754-765. ArticlePubMed
- 16. Van Vré EA, van Beusekom HM, Vrints CJ, Bosmans JM, Bult H, Van der Giessen WJ. Stereology: a simplified and more time-efficient method than planimetry for the quantitative analysis of vascular structures in different models of intimal thickening. Cardiovasc Pathol 2007; 16: 43-50. ArticlePubMed
- 17. Basta-Jovanovic G, Venkataseshan VS, Gil J, Kim DU, Dikman SH, Churg J. Morphometric analysis of glomerular basement membranes (GBM) in thin basement membrane disease (TBMD). Clin Nephrol 1990; 33: 110-114. PubMed
- 18. Rayat CS, Joshi K, Sakhuja V, Datta U. Glomerular basement membrane thickness in normal adults and its application to the diagnosis of thin basement membrane disease: an Indian study. Indian J Pathol Microbiol 2005; 48: 453-458. PubMed
REFERENCES
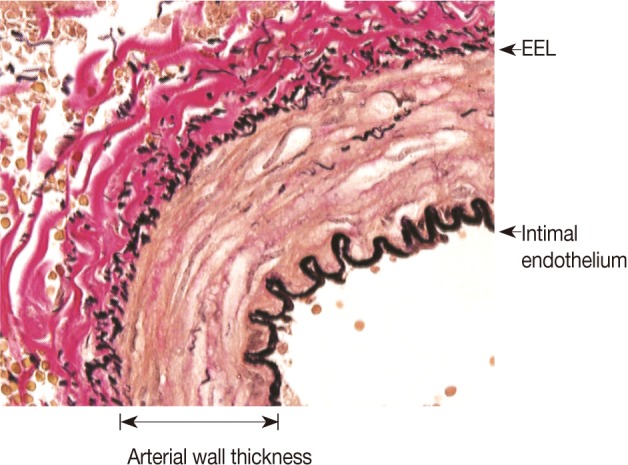
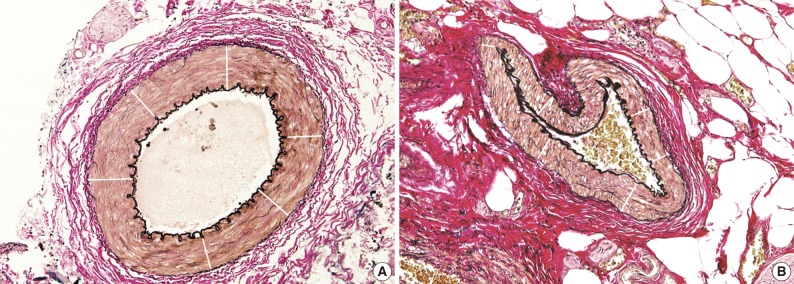
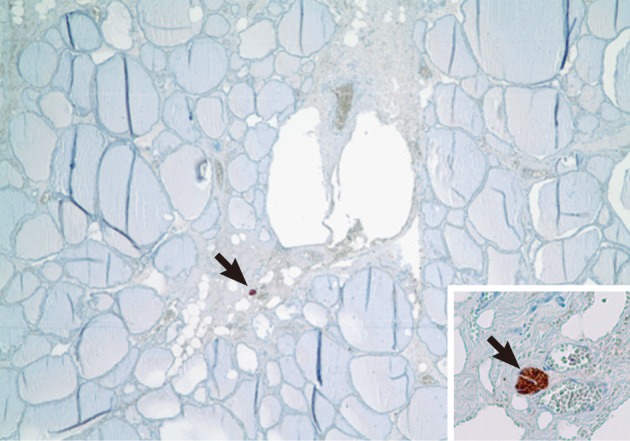
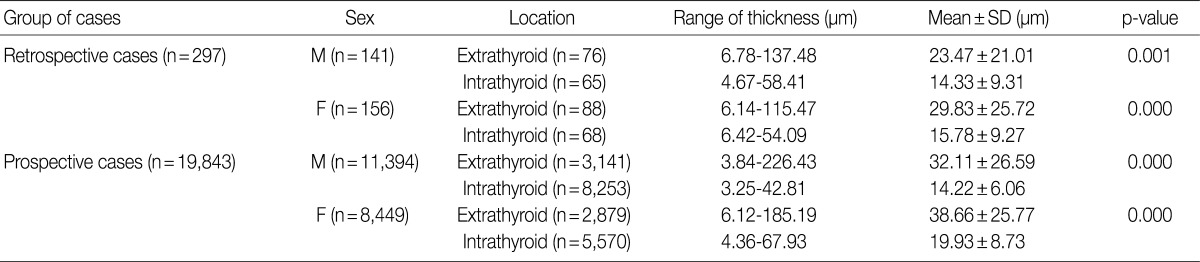
Figure & Data
References
Citations

- Invasion in thyroid cancer: Controversies and best practices
Michiya Nishino, Jack Jacob
Seminars in Diagnostic Pathology.2020; 37(5): 219. CrossRef - MiR-221/222 promote migration and invasion, and inhibit autophagy and apoptosis by modulating ATG10 in aggressive papillary thyroid carcinoma
Hao Shen, Zaikai Lin, Haiyan Shi, Lingling Wu, Baojin Ma, Hong Li, Baobing Yin, Jun Tang, Hongjin Yu, Xiaoxing Yin
3 Biotech.2020;[Epub] CrossRef - Minimal extrathyroidal extension affects the prognosis of differentiated thyroid cancer: Is there a need for change in the AJCC classification system?
Zeming Liu, Yihui Huang, Sichao Chen, Di Hu, Min Wang, Ling Zhou, Wei Zhou, Danyang Chen, Haifeng Feng, Wei Wei, Chao Zhang, Wen Zeng, Liang Guo, Scott M. Langevin
PLOS ONE.2019; 14(6): e0218171. CrossRef - miR-199a-3p downregulation in thyroid tissues is associated with invasion and metastasis of papillary thyroid carcinoma
Chengbiao Liu, Meiling Xing, Liping Wang, Kejun Zhang
British Journal of Biomedical Science.2017; 74(2): 90. CrossRef - Clinicopathological Significance of Minimal Extrathyroid Extension in Solitary Papillary Thyroid Carcinomas
Chang Gok Woo, Chang Ohk Sung, Yun Mi Choi, Won Gu Kim, Tae Yong Kim, Young Kee Shong, Won Bae Kim, Suck Joon Hong, Dong Eun Song
Annals of Surgical Oncology.2015; 22(S3): 728. CrossRef - Intraoperative Frozen Section for the Evaluation of Extrathyroidal Extension in Papillary Thyroid Cancer
Om Prakash Prajapati, A. K. Verma, M. Sabaretnam
World Journal of Surgery.2015; 39(7): 1855. CrossRef - Tumor Sprouting in Papillary Thyroid Carcinoma Is Correlated with Lymph Node Metastasis and Recurrence
Eunjung Lee, Wonkyung Jung, Jeong-Soo Woo, Jae Bok Lee, Bong Kyung Shin, Han Kyeom Kim, Aeree Kim, Baek-hui Kim
Korean Journal of Pathology.2014; 48(2): 117. CrossRef



Fig. 1
Fig. 2
Fig. 3



SD, standard deviation. ap=0.000, between extrathyroid and intrathyroid.
SD, standard deviation; M, male; F, female.
SD, standard deviation; M, male; F, female.
SD, standard deviation.

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article