Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 46(6); 2012 > Article
-
Case Report
Primary Mucinous Cystadenocarcinoma of the Breast: Cytologic Finding and Expression of MUC5 Are Different from Mucinous Carcinoma - Sung Eun Kim,, Ji Hye Park, SoonWon Hong, Ja Seung Koo, Joon Jeong1, Woo-Hee Jung
-
Korean Journal of Pathology 2012;46(6):611-616.
DOI: https://doi.org/10.4132/KoreanJPathol.2012.46.6.611
Published online: December 26, 2012
Department of Pathology, Gangnam Sevrance Hsopital, Yonsei University College of Medicine, Seoul, Korea.
1Department of Surgery, Gangnam Sevrance Hsopital, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding Author: Woo-Hee Jung, M.D. Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, 211 Eonju-ro, Gangnam-gu, Seoul 135-720, Korea. Tel: +82-2-2019-3541, Fax: +82-2-3463-2103, Jungwh96@yuhs.ac
- *Sung Eun Kim's present address: Department of Pathology, Cha Gangnam Medical Center, Cha University, Seoul, Korea.
© 2012 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations

- BMP2 alterations in mucinous cystadenocarcinoma of the breast: insights from whole-exome sequencing
Zhenyu Li, Yi Gong, Guangxin Li, Qingming Jiang, Juanhui Dong, Rui Chen
PeerJ.2025; 13: e19948. CrossRef - Primary mucinous cystadenocarcinoma of the breast commonly harbours TP53 mutations and PI3K/AKT pathway alterations
Cheng Xu, Jing Wu, Ying Ding, Zhihong Zhang, Cong Wang
Virchows Archiv.2025; 487(6): 1235. CrossRef - Mucinous cystadenocarcinoma of the breast harbours TRPS1 expressions and PIK3CA alterations
Wei‐Yu Chen, Yu‐Hsuan Hu, Yu‐Hsin Tsai, Jen‐Fan Hang, Puay Hoon Tan, Chih‐Jung Chen
Histopathology.2024; 84(3): 550. CrossRef - Pure mucinous adenocarcinoma of the breast with the rare lymphoplasmacytic infiltration: A case report with review of literature
Yash Hasmukhbhai Prajapati, Vishal Bhabhor, Kahan Samirkumar Mehta, Mithoon Barot, Husen Boriwala, Mohamed Omar
Clinical Case Reports.2024;[Epub] CrossRef - HER2‐positive mucinous cystadenocarcinoma of the breast coexisting with invasive lobular carcinoma: A case report and review of the literature
Ismail Guzelis, Betul Bolat Kucukzeybek, Mehmet Ali Uyaroglu, Melek Bekler Gokova, Gulten Sezgin, Yuksel Kucukzeybek
Diagnostic Cytopathology.2024;[Epub] CrossRef - Primary mucinous cystadenocarcinoma of the breast: A case report and literature review
Xi Cao, Yongchao Luo, Songjie Shen, Xinyu Ren
Oncology Letters.2024;[Epub] CrossRef - Mammary mucinous cystadenocarcinoma with long-term follow-up: molecular information and literature review
Ting Lei, Yong Qiang Shi, Tong Bing Chen
Diagnostic Pathology.2023;[Epub] CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast Intermixed with Pleomorphic Invasive Lobular Carcinoma: The First Report of This Rare Association
Federica Vegni, Nicoletta D’Alessandris, Angela Santoro, Giuseppe Angelico, Giulia Scaglione, Angela Carlino, Damiano Arciuolo, Michele Valente, Stefania Sfregola, Maria Natale, Alejandro Martin Sanchez, Valeria Masciullo, Gian Franco Zannoni, Antonino Mu
Journal of Personalized Medicine.2023; 13(6): 948. CrossRef - Special Histologic Type and Rare Breast Tumors – Diagnostic Review and Clinico-Pathological Implications
Benjamin Yongcheng Tan, Elaine Hsuen Lim, Puay Hoon Tan
Surgical Pathology Clinics.2022; 15(1): 29. CrossRef - Mucinous cystadenocarcinoma of the breast: a new entity with broad differentials—a case report
Kanwalpreet Kaur, Ashini Shah, Jahnvi Gandhi, Priti Trivedi
Journal of the Egyptian National Cancer Institute.2022;[Epub] CrossRef - Mucinous carcinoma of the breast: distinctive histopathologic and genetic characteristics
Minjung Jung
Kosin Medical Journal.2022; 37(3): 176. CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast: A Rare Case Report With Review of Literature
Ekta Jain, Abhishek Kumar, Raajul Jain, Shivani Sharma
International Journal of Surgical Pathology.2021; 29(7): 740. CrossRef - Mucinous Cystadenocarcinoma of the Breast: Report of 2 Cases Including One With Long-Term Local Recurrence
Anupma Nayak, Ira J. Bleiweiss, Kimberly Dumoff, Tawfiqul A. Bhuiya
International Journal of Surgical Pathology.2018; 26(8): 749. CrossRef - Mucinous breast carcinoma with tall columnar cells
N Tsoukalas, M Kiakou, M Tolia, ID Kostakis, M Galanopoulos, G Nakos, D Tryfonopoulos, G Kyrgias, G Koumakis
The Annals of The Royal College of Surgeons of England.2018; 100(5): e132. CrossRef - Radiologic Findings of Primary Mucinous Cystadenocarcinoma of the Breast: A Report of Two Cases and a Literature Review
Minjung Seong, Eun Young Ko, Boo-Kyung Han, Soo Youn Cho, Eun Yoon Cho, Se Kyung Lee, Jeong Eon Lee
Journal of Breast Cancer.2016; 19(3): 330. CrossRef - Primary Mucinous Cystadenocarcinoma of the Breast with Endocervical-Like Mucinous Epithelium
Dong-Liang Lin, Ji-Lin Hu, Shi-Hong Shao, Dong-Mei Sun, Ji-Gang Wang
Breast Care.2013; 8(6): 445. CrossRef



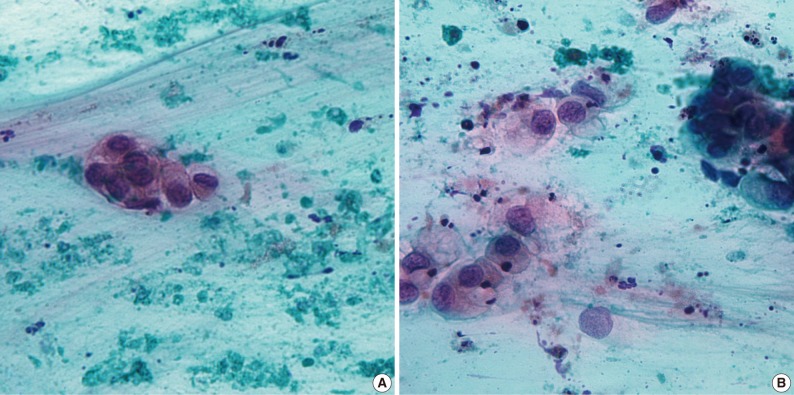
Fig. 1
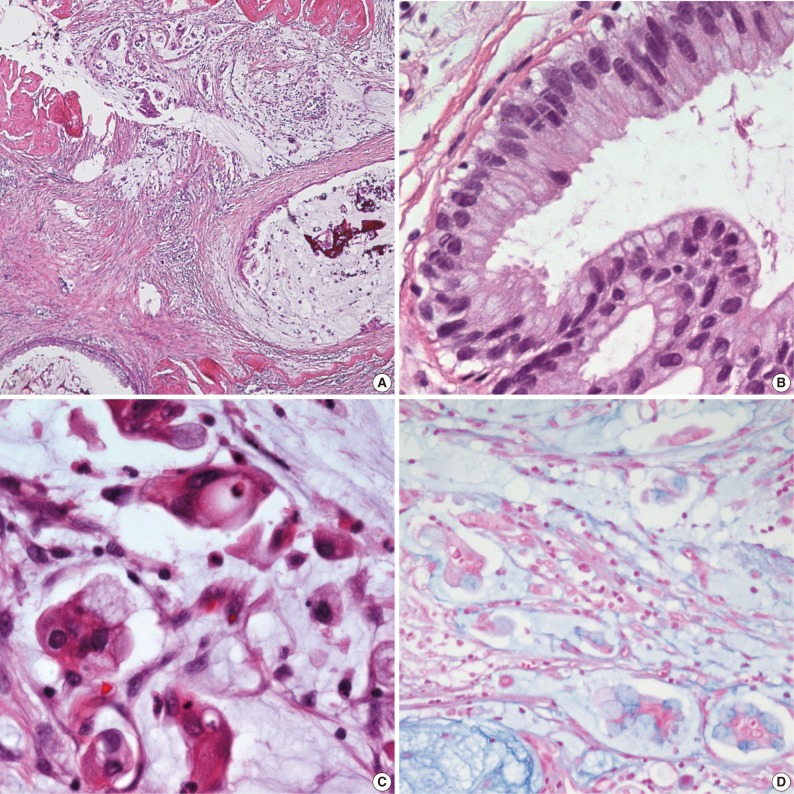
Fig. 2
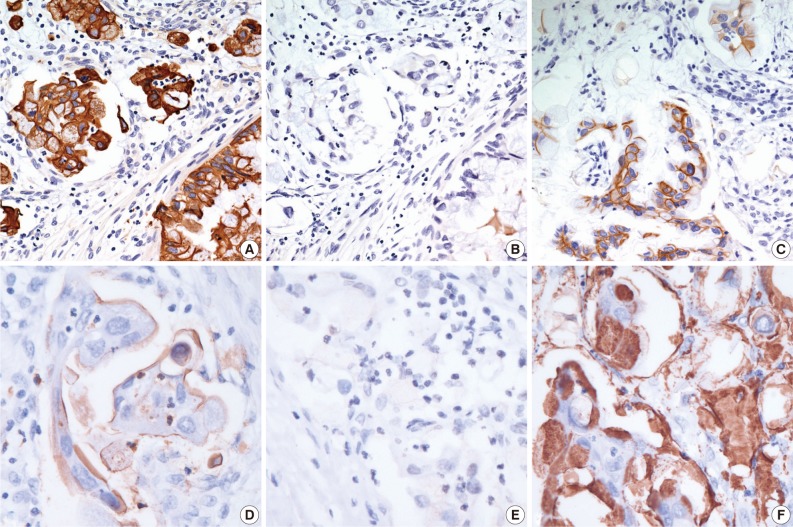
Fig. 3

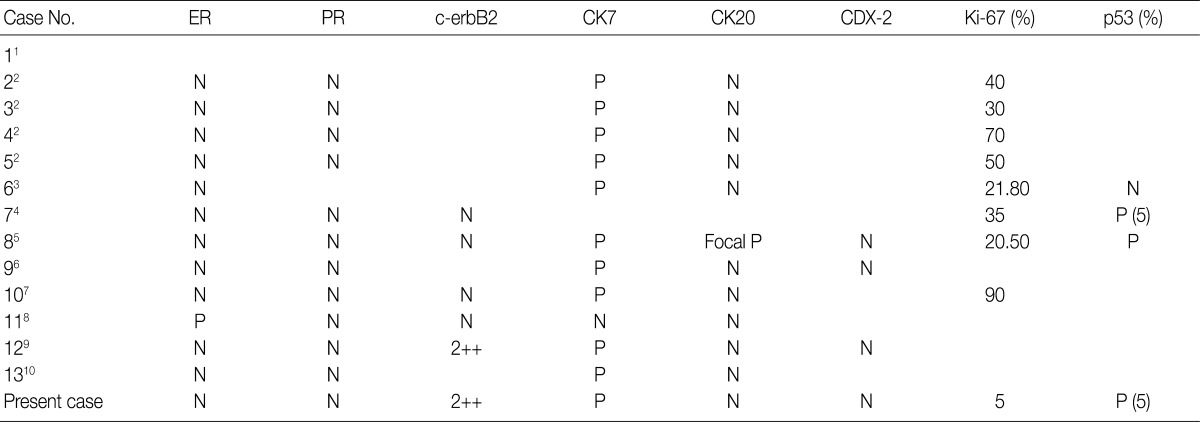
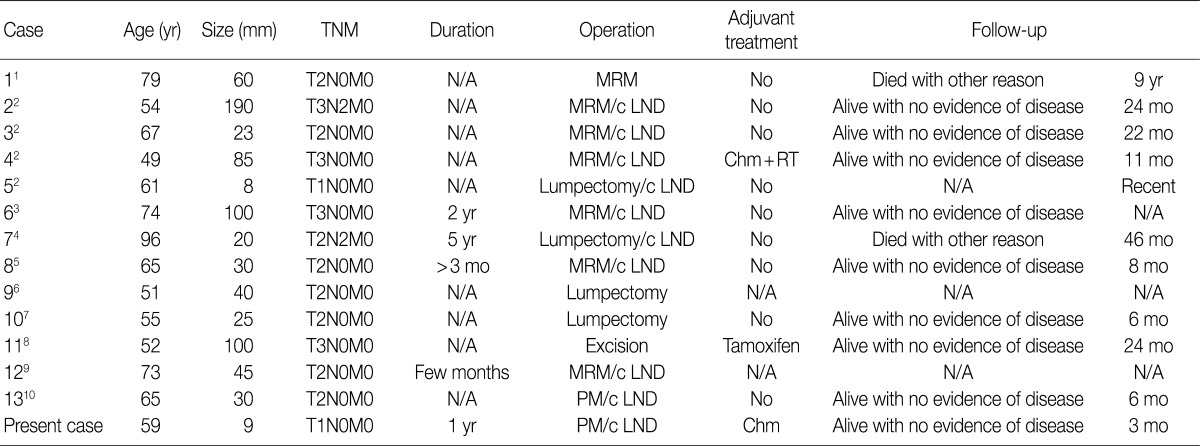
TNM, tumor-node-metastasis; N/A, not acquired; MRM, modified radical mastectomy; c LND, with lymph node dissection; Chm+RT, chromatography+radiation therapy; PM, partial mastectomy.
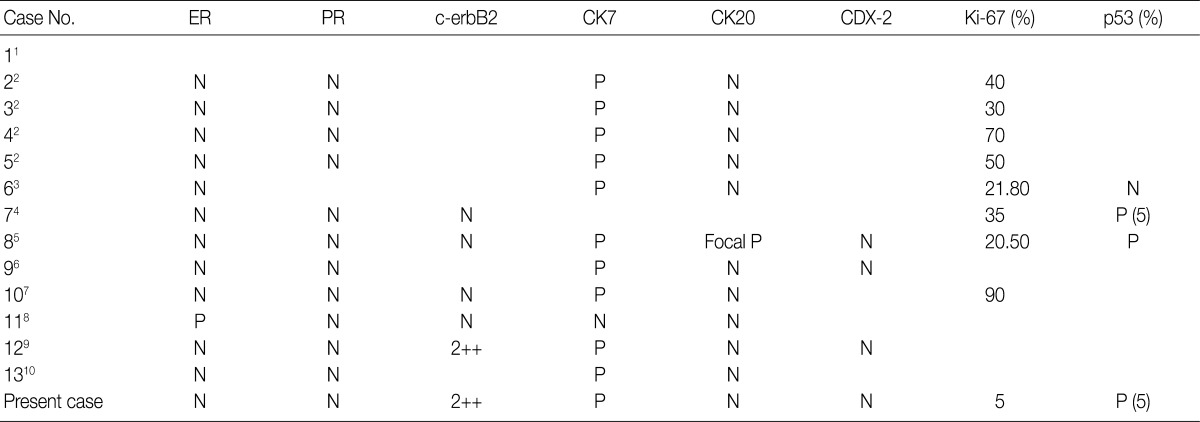
ER, estrogen receptor; PR, progesterone receptor; CK7, cytokeratin 7; CK20, cytokeratin 20; N, negative; P, positive.
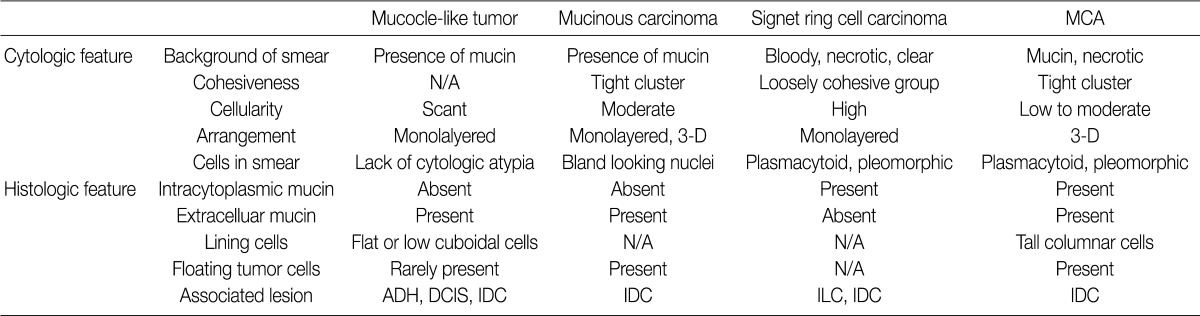
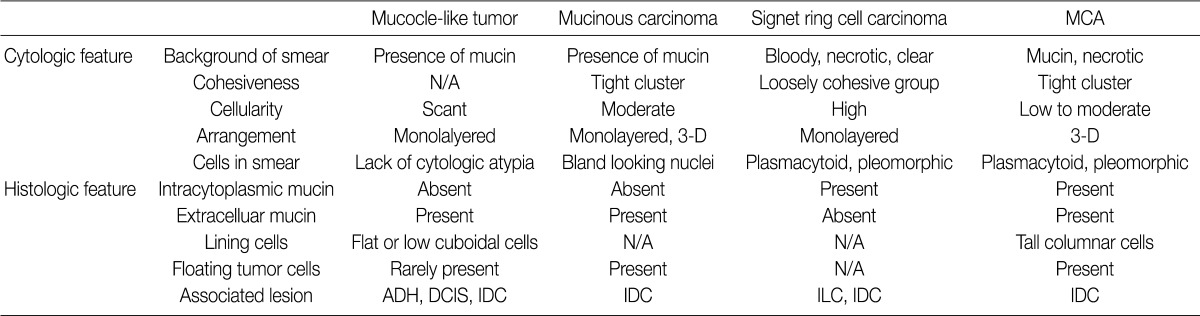
MCA, mucinous cystadenocarcinoma; N/A, not availale; ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma

 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article