Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(2); 2013 > Article
-
Original Article
Human Papillomavirus Prevalence and Cell Cycle Related Protein Expression in Tonsillar Squamous Cell Carcinomas of Korean Patients with Clinicopathologic Analysis - Miji Lee, Sung Bae Kim1, Sang-wook Lee2, Jong-Lyel Roh3, Seung-Ho Choi3, Soon Yuhl Nam3, Sang Yoon Kim3, Kyung-Ja Cho
-
Korean Journal of Pathology 2013;47(2):148-157.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.2.148
Published online: April 24, 2013
Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
1Department of Medical Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
2Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
3Department of Head and Neck Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- Corresponding Author: Kyung-Ja Cho, M.D. Department of Pathology, University of Ulsan College of Medicine, Asan Medical Center, 88 Olympic-ro 43-gil, Songpa-gu, Seoul 138-736, Korea. Tel: +82-2-3010-4545, Fax: +82-2-472-7898, kjc@amc.seoul.kr
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Human papillomavirus (HPV)-related tonsillar squamous cell carcinoma (TSCC) has recently been characterized as a distinct subset with a favorable prognosis. The prevalence and clinicopathologic significance of HPV-related TSCC in Koreans are not well known.
-
Methods
- HPV in situ hybridization (ISH) accompanied by p53, p16, pRb, and cyclin D1 immunohistochemical staining were performed on 89 resection cases of TSCC from 2000 through 2010.
-
Results
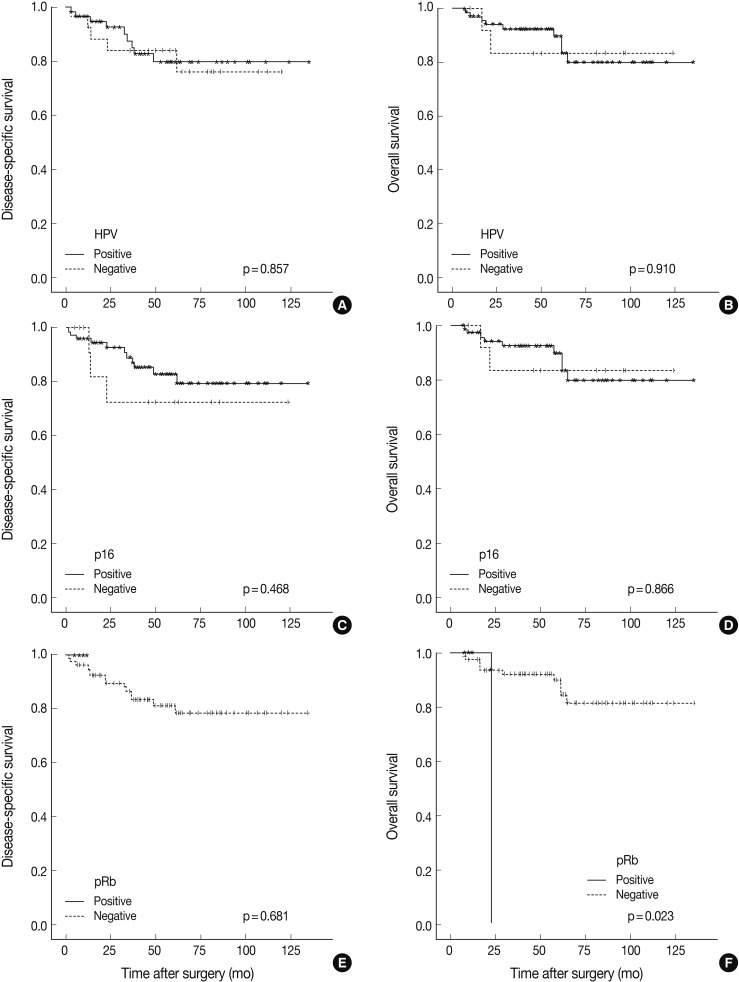
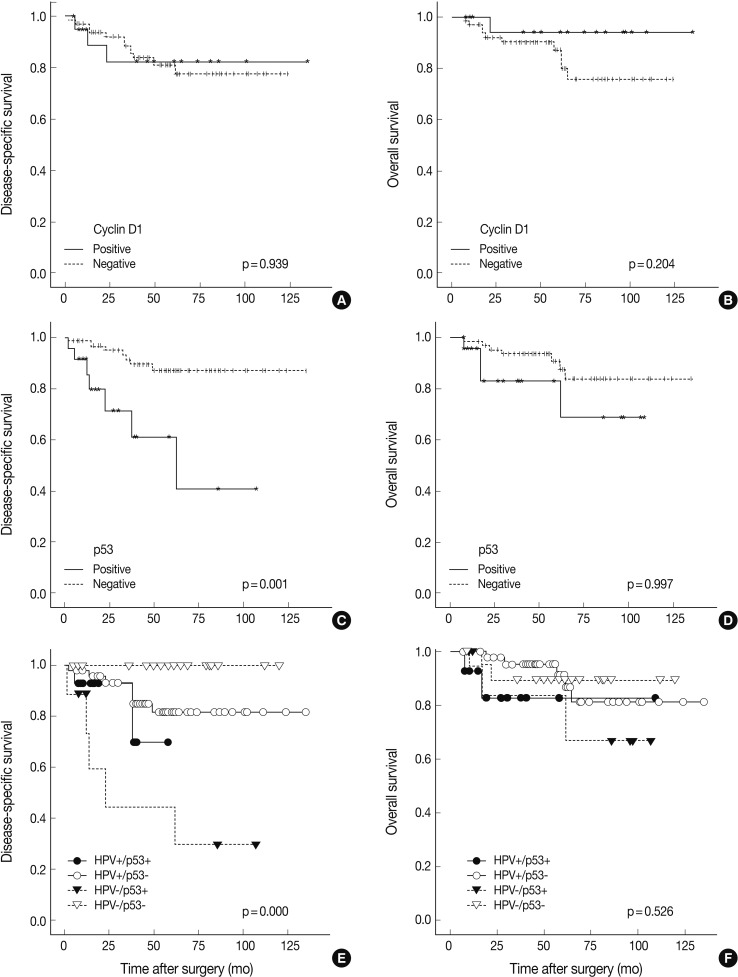
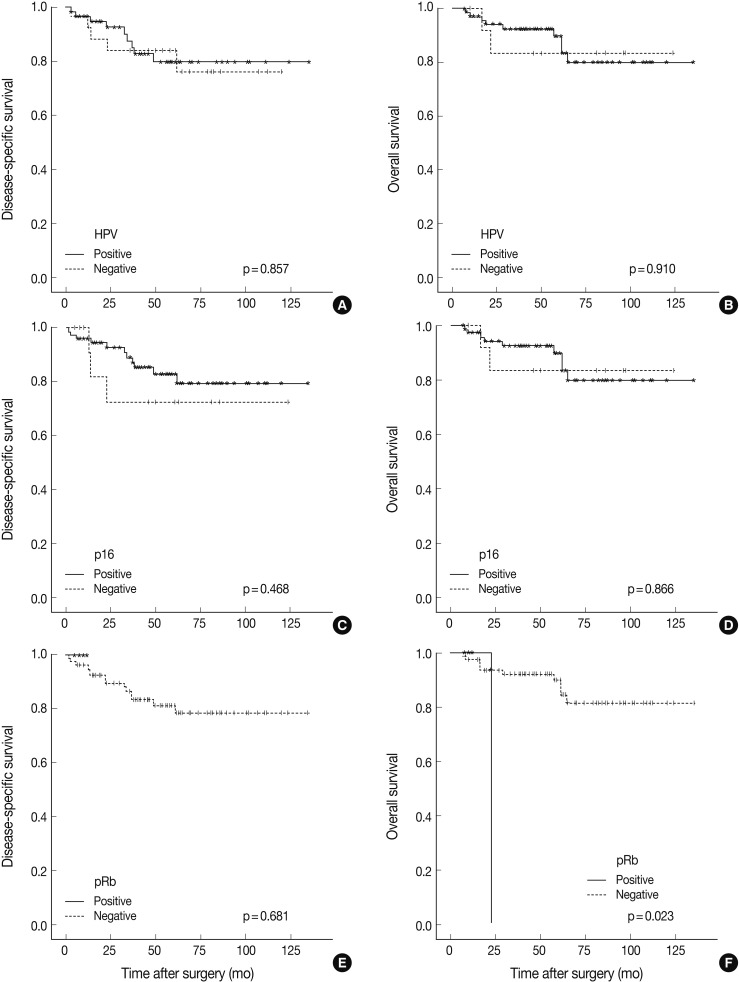
- HPV was detected by ISH in 59 of 89 cases (66.3%). HPV-positive TSCCs were more common in younger ages (p=0.005), and tumor sizes were smaller in the HPV-positive compared to the HPV-negative group (p=0.040). Positive HPV staining was significantly correlated with p16 expression (p<0.001), pRb inactivation (p=0.003), and cyclin D1 down-regulation (p<0.001) but not with p53 expression (p=0.334). Seventeen cases that showed p16-immunopositivity with HPV-negativity by ISH were retested by HPV typing; HPV DNA was not detected in all cases. There was no significant difference between HPV-positive and HPV-negative patients either in the disease-specific survival (DSS, p=0.857) or overall survival (p=0.910). Furthermore, pRb-inactivated cases showed better DSS (p=0.023), and p53-positive cases showed worse DSS (p=0.001).
-
Conclusions
- Although high HPV prevalence was noted, it was not correlated with histopathologic findings or survival benefit. In addition to p53 expression, pRb inactivation along with p16 overexpression and down-regulation of cyclin D1 are thought to be important pathogenetic steps for developing TSCCs.
- Patients and tumor materials
- Formalin-fixed, paraffin-embedded tissue blocks were obtained from 89 patients diagnosed with TSCC who were treated with surgery and neoadjuvant and/or postoperative adjuvant chemoradiotherapy during the period from January 2000 to December 2010 at Asan Medical Center, Seoul, Korea. Clinical information including age, gender, histories of smoking and alcohol consumption, tumor-node-metastasis (TNM) stage, and survival were abstracted from the electronic medical records. Tumors were staged according to the 7th edition of the TNM classification scheme from the American Joint Committee on Cancer 2010. Smoking history was measured in pack-years and classified into three categories: non-smoker, former smoker that quit smoking more than ten years ago, or current smoker. Alcohol consumption was defined as having no history of alcohol use, having three or fewer drinks per day, or having more than three drinks per day.
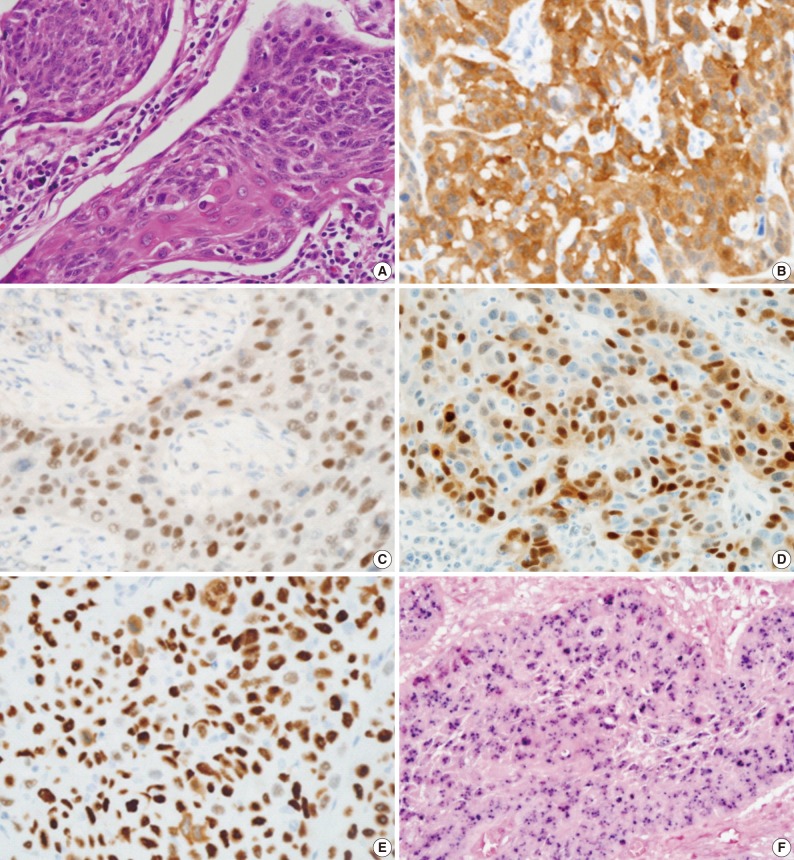
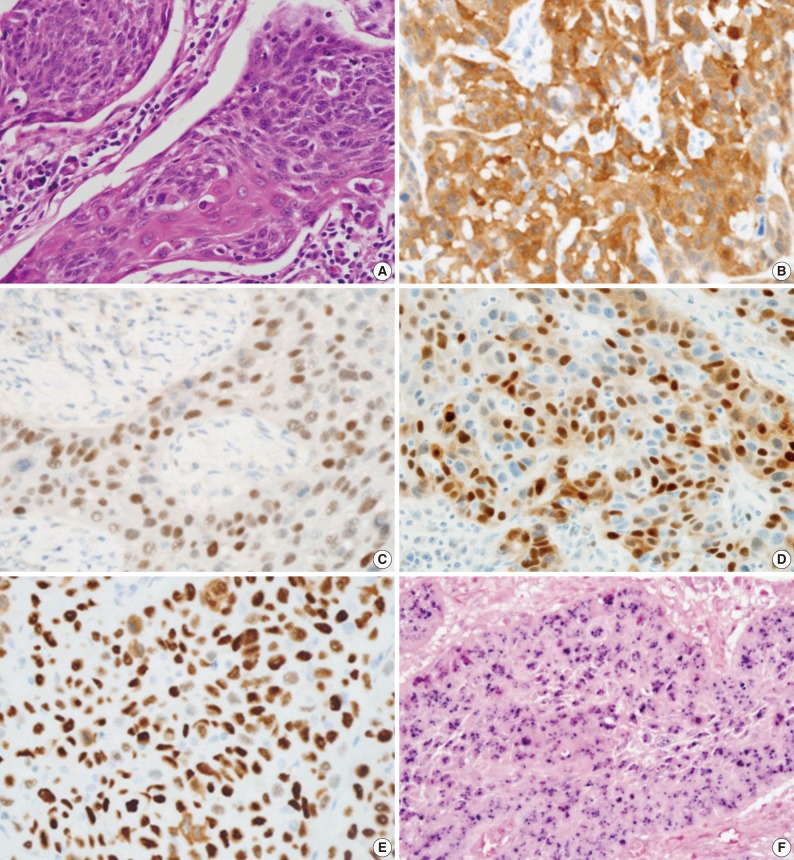
- All hematoxylin and eosin-stained sections were reviewed for reconfirmation of the diagnosis. Tumor differentiation was further sub-classified into three categories according to the World Health Organization (WHO) classification: well differentiated, moderately differentiated, and poorly differentiated squamous cell carcinoma. Tumor origin was divided into crypt or surface according to the location and growth direction of tumors. Tumors were also examined to determine whether keratinization was present. Duplicated 2 mm tissue cores were arrayed to decrease sampling errors and minimize tissue loss during processing. Tissue microarray sections were evaluated by HPV in situ hybridization (ISH), p16, pRb, cyclin D1, and p53 immunohistochemistry.
- HPV ISH
- An INFORM HPV III Family 16 Probe (B) was used in conjunction with ISH iView Blue Plus detection kit (Ventana Medical System Inc., Tucson, AZ, USA). The INFORM HPV III Family 16 Probe (B) detects the following high risk HPV genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 66. Using light microscopy, any blue nuclear dots in the tumor cells were regarded as positive staining. All cases were classified in a binary scheme as either positive or negative.
- Immunohistochemistry
- Immunoperoxidase staining was performed on 4-micrometer tissue microarray sections using a Ventana autostainer and ultraView DAB detection kit (Ventana Medical System Inc.) according to the manufacturer's instructions. The following antibodies were used: monoclonal p16INK4 (1:10, Pharmingen, Franklin Lakes, NJ, USA), monoclonal p53 (1:3,000, DAKO-M7001, DAKO, Glostrup, Denmark), monoclonal pRb (1:40, DAKO-M7131), and monoclonal cyclin D1 (1:100, Neomarkers, Fremont, CA, USA). p16INK4 expression was regarded as positive if strongly and diffusely stained in nuclei and/or cytoplasm in ≥70% of the tumor cells. p53, pRb, and cyclin D1 staining were scored as positive if strong and diffuse nuclear staining was present in ≥20% of the tumor cells.
- DNA extraction
- HPV-negative, p16-positive cases were retested by HPV DNA chip. DNA was extracted from formalin-fixed paraffin-embedded tissue using a LaboPass Tissue Mini DNA Purification Kit (Cosmo Genetech, Seoul, Korea). Paraffin-embedded tumor tissues were cut into 20 µm-thick sections, using disposable microtome blades, and three consequent sections were collected using microcentrifuge tubes. Then, two extractions were mixed with 1.2 mL of xylene, and excess xylene was removed by two 1.2 mL 100% ethanol washes. Dried tissue samples were incubated with lysis buffer and proteinase K at 56℃ for 30 minutes. Subsequently, the mixture was applied to the spin column and centrifuged into a collection tube according to the manufacturer's protocol. The purified DNA was used directly for polymerase chain reaction (PCR).
- DNA amplification and HPV genotyping
- A commercially available HPV DNA chip (Goodgene, Seoul, Korea) was used. The HPV DNA chip contained 40 type-specific probes: 21 types of high-risk type HPV (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 67, 68, 69, 70, 73, and 82) and 19 types of low-risk type HPV (6, 11, 30, 32, 34, 40, 41, 42, 43, 44, 54, 55, 61, 62, 72, 81, 83, 84, and 90). Briefly, DNA amplification was performed in a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA) by PCR with primer sets, which target L1 and L2 regions of HPV DNA. As a control gene, the human β-globin gene was also amplified. The PCR products from all samples were detected by electrophoresis using 2% agarose gels, and the HPV DNA product size was 185 base pairs. Hybridized HPV DNA was visualized using a DNA chip scanner (GeneScan, Goodgene). To avoid contamination that may yield a false positive result, all PCR-related work was performed in specialized zones within a PCR laboratory.
- Statistical analysis
- Two-tailed Fisher's exact test and/or χ2 test were used to analyze the correlation between the clinicopathologic variables and HPV status. For continuous variables (e.g., tumor size), Student's t-test was chosen to evaluate the difference between HPV-negative and -positive groups. Survival analysis was performed based on Kaplan-Meier method and compared by log-rank test. All potential prognostic factors with a p-value<0.05 from the univariate analysis were incorporated into the multivariate analysis. The univariate and multivariate analyses were performed using the Cox proportional hazard regression model. All statistical analyses were conducted using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). A p-value<0.05 was considered to be significant.
MATERIALS AND METHODS
- The patients were comprised of 81 (91%) men and eight (9%) women with a mean age of 55 years, ranging from 25 to 78. HPV was detected in 59 (66.3%) cases by ISH. The mean age of patients was significantly younger in the HPV-positive group (53.7±8.76) relative to the HPV-negative group (59.0±10.93, p=0.020). HPV positivity was not associated with gender (p=1.000), smoking history (p=0.490), or alcohol consumption (p=0.907). HPV-positive tumors were smaller in size (2.34±1.00 cm) than HPV-negative tumors (2.96±1.42 cm, p=0.040). However, anatomic stage, T stage, N stage, and M stage were not associated with HPV status (Table 1).
- Comparison of immunohistochemistry with HPV status is demonstrated in Table 2 and Fig. 1. In total, 83.1% (74/89) of TSCCs were p16-positive, 5.6% (5/89) pRb-positive, 23.6% (21/89) cyclin D1-positive, and 27.0% (24/89) p53-positive. Strong positive correlation between p16 expression and HPV status (p<0.001) and an inverse correlation between expressions of pRb and cyclin D1 and HPV positivity (p=0.003 and p<0.001, respectively) were noted. HPV positivity was noted in 77% (57/74) and 13.3% (2/15) of p16-positive and -negative tumors, respectively. None of the pRb-positive tumors were HPV-positive (0/5), whereas 59 (70%) of 84 pRb-negative tumors were HPV-positive. While 28.6% (6/21) of cyclin D1-overexpressing tumors were HPV-positive, 77.9% (53/68) of cyclin D1-negative tumors showed HPV-positivity. There was no significant correlation between HPV status and p53 expression (p=0.334).
- HPV genotyping using DNA chip was performed on the 17 cases that showed positive p16-staining but negative HPV staining by ISH. No HPV DNA was detected by genotyping in any of the 17 cases.
- Upon histopathologic analysis, positive HPV results were higher in the crypt origin TSCCs (46/64, 72%) compared to surface origin TSCCs (13/25, 52%), but this difference was not statistically significant (p=0.075). In addition, there were no significant correlations between tumor differentiation, keratinization, cystic cervical lymph node metastasis, and HPV status (Table 3).
- Follow-up periods ranged from 7 to 135 months with a mean follow-up of 55 months. The recurrence rate was 15.7% (14/89), with 15.2% (9/59) in the HPV-positive group and 16.6% (5/30) in the HPV-negative group (p=0.430). Our findings revealed no significant survival benefit by HPV status not only in overall survival (OS, p=0.910), but also in disease-specific survival (DSS, p=0.857) (Fig. 2). pRb positivity was significantly related to a worse OS (p=0.023) but was not related to DSS (p=0.681) (Fig. 2). The patients who showed high p53 expression had remarkably worse DSS (p=0.001) without affecting OS (p=0.997) (Fig. 3). Other cell cycle-related proteins, p16 and cyclin D1, did not show significant correlation with OS (p16, p=0.866; cyclin D1, p=0.204) or DSS (p16, p=0.468; cyclin D1, p=0.939). We performed survival analysis with combinations of HPV ISH status and the expression status of cell cyclerelated proteins. Each group was divided to four subgroups (i.e., HPV-positive/p16-positive; HPV-positive/p16-negative; HPV-negative/p16-positive; HPV-negative/p16-negative). Any HPV status with p16, pRb, or cyclin D1 expression status did not influence OS and DSS. However, the HPV-negative/p53-positive subgroup showed worse DSS than the other HPV-positive/p53-positive, HPV-positive/p53-negative, and HPV-negative/p53-negative subgroups (p=0.000) (Fig. 3). In univariate and multivariate Cox proportional analyses, T stage (T1-T2 vs T3-T4; hazard ratio, 5.444; 95% confidence interval [CI], 1.641 to 18.062; p=0.006) and pRb expression status (positive vs negative; hazard ratio, 0.109; 95% CI, 0.010 to 1.153; p=0.066) were identified as independent factors for OS in TSCC patients. M stage (M0 vs M1; hazard ratio, 8.215; 95% CI, 2.641 to 25.553; p=0.0003) and p53 expression status (positive vs negative; hazard ratio, 0.185; 95% CI, 0.063 to 0.547; p=0.002) were revealed as independent predictors for DSS.
RESULTS
- In prior studies, HPV infection rates in head and neck squamous cell carcinomas varied considerably, ranging from 0% to 100%.5,6 In our study, HPV was detected in 59/89 (66.3%) of TSCC patients by in ISH. The differences in HPV prevalence among the studies might be explained by the HPV detection methodology, sample size, and changes in lifestyle with time, such as tobacco use, alcohol consumption, and sexual behavior. Although high risk sexual habits such as oral sex may not be common in Korea, the considerably high prevalence of HPV could mean that other infection routes than sexual transmission are important for Koreans.
- For HPV detection, ISH and PCR have been most widely used, and both methods have merits and limitations. ISH can visualize the virus in nuclei of tumor cells, but it is less sensitive than PCR depending on the copy number of viral genome, and it cannot demonstrate oncogenic transcription. On the other hand, PCR is a more sensitive method but may detect the virus present in non-tumor cells that are not clinically relevant or virus from the laboratory environment or other specimens, leading to false-positive results.14 HPV genotyping, another method of HPV detection, has the advantage of simultaneous detection of multiple HPV subtypes with high sensitivity, but its cost is high. Considering these problems, the difficulty lies in using ISH, PCR, or HPV genotyping as screening tests for HPV detection. Therefore, p16 immunoreactivity has been proposed as a surrogate marker for HPV-positive tumors of the anogenital tract, as well as the upper aerodigestive tract.15
- p16 is a tumor suppressor gene that prevents progression of the S-phase of cell cycle, through blocking cyclin D/CDK2-mediated phosphorylation of pRb, thereby keeping the pRb checkpoint in place. In HPV-positive tumors, p16 overexpression is observed due to loss of negative feedback by inactivation of pRb by HPV oncoprotein E7.16 In this study, HPV-positive tumors showed high p16 overexpression (98%, 58/59) and low pRb immunoreactivity (0%, 0/59), and these results were in agreement with previous reports.17,18 However, 17 cases of HPV-negative tumors also showed p16 overexpression. These p16-positive/HPV-negative tumors were retested by HPV genotyping using DNA chip, and none of them were shown to harbor any of the 21 types of high-risk or 19 types of low-risk HPV. This result confirmed the accuracy of HPV ISH applied in our study. On the contrary, Lewis et al.19 found 42% of p16-positive/HPV-negative cases as positive for HPV on repeated PCR. We share a view with Perrone et al.20 that the false positive rate of PCR cannot be ignored. The mechanisms of p16-overexpression in HPV-negative tumors have not been ascertained. One can conjecture that p16 expression might be innate, and the tumor could develop independently regardless of HPV infection. HPV might be shed to tumors with genetic progression, resulting in pRb gene deletion or suppressed pRb gene expression independent of active viral protein expression.19 Also, other pRb pathway disturbances, regardless of HPV status such as mutational inactivation of RB protein, can be considered.21 Methodologically, there may be yet unidentified HPV genotypes that current HPV-specific tests cannot detect and do not contain the consensus DNA sequences detectable by ISH, PCR, or genotyping.19 Robinson et al.22 found 5% of p16-positive/HPV ISH-negative results in six studies examining 496 oropharyngeal squamous cell carcinomas, while Song et al.9 reported a greater number of p16-positive/HPV ISH-negative cases at 27.6% (13/47) in Koreans. The high proportion of p16-positive/HPV ISH-negative cases in our results (19.1%, 17/89) together with the result of Song et al.9 suggest that this considerably high incidence of p16-positive/HPV ISH-negative cases may be a distinguishing feature of TSCC in Koreans. Furthermore, in light of these results, there are limitations in using p16 as a surrogate marker for judging HPV infection status.
- In HPV-infected tumor cells with p16 expression, its downstream regulators such as pRb or cyclin D1, may exhibit diverse expression profiles. Several studies have reported the rate of cyclin D1 overexpression in head and neck cancers as 17-80%.23,24 Overexpression of cyclin D1 might result from gene amplification, gene rearrangement, or post-translational modification. In oral cavity cancers, prognostic significance of cyclin D1 or pRb and the relationship between expression profiles of these proteins and HPV status have been diverse in previous studies.18,25 Our results showed an inverse correlation between expression of cyclin D1 and pRb with HPV ISH status (cyclin D1, p<0.001; pRb, p=0.003). While cyclin D1 overexpression had no effects on overall or DSS outcome, pRb overexpression was significantly correlated with worse OS (p=0.023), and pRb expression was identified as an independent predictor for OS by univariate and multivariate analyses.
- In HPV-related tumors, wild-type p53 undergoes ubiquitination and degradation by HPV oncoprotein E6, resulting in less immunopositive protein.7,26 Thus, focal positive or negative p53 immunoreactivity has been regarded as a molecular signature for HPV-induced tumors. However, E6 degradation of p53 protein is not functionally equivalent to p53 mutation. Although HPV-infected cells express E6 oncoprotein, endogenous wild-type p53 can activate some target genes.27 In this study, p53 immunopositive rates were not significantly different between HPV-positive and -negative groups (24% vs 30%). However, p53-positive TSCC showed worse DSS (p=0.001). Although we did not perform p53 mutation analysis, 14 HPV-positive/p53-immunoreactive cases in this study might have harbored non-disruptive p53 mutations, according to Westra et al.,28 and may be related to smoking habits. Our results showed that p53-immunopositivity was related to a worse DSS (p=0.001), and p53 was an independent prognostic factor for DSS by univariate and multivariate analyses. Furthermore, upon combination analysis of HPV status and p53 immunoreactivity status, the HPV-negative/p53-positive subgroup showed the worst DSS. From these results, p53 status seems to be a single important factor for predicting DSS.
- According to our results, no survival benefits were observed in HPV-positive TSCC patients in comparison with HPV-negative patients. This phenomenon might be attributed to higher smoking or drinking rates in Korea than in developed countries. According to the records of the Ministry of the Health and Welfare of Korea for 2008, the smoking rates were 40.9% in males and 4.1% in females, with a mean of 22.3%. In contrast, the U.S. Centers for Disease Control announced the smoking rates as 23% in males and 18% in female, and a mean of 20.6%. In light of these differences, it can be presumed that the higher smoking rates in males account for a majority of TSCC in Korea and thereby have a greater effect on survival than does HPV status, resulting in no survival benefits as analyzed by HPV infection. Hafkamp et al.10 reported marked differences in survival rate between smokers and non-smokers with HPV 16-associated tonsillar carcinomas. Interestingly, past results of Western studies parallel ours with respect to relationships of HPV infection and survival outcomes.29,30 There is a possibility that, with smoking rates reducing gradually in Western countries, the subset of HPV-related tumors has become outstanding. Clinical implication of HPV-positive oropharyngeal cancer may yet vary depending on sociocultural circumstances.
- Routine HPV assessment is a rising issue in the standard pathologic evaluation of HNSCCs to assess cancer risk, predict patient outcomes, determine the site of tumor origin for metastatic tumors, and guide therapeutic strategies. Although high HPV prevalence was noted and epidemic HPV infection is suspected in Korea, we failed to segregate the HPV-positive TSCC cases as a clinically or pathologically distinct subset. The prognostic value of HPV and related proteins in HNSCC remains to be studied more elaborately.
- In conclusion, high HPV prevalence in TSCCs (66.3%) is noted in Koreans. In addition to p53 expression, pRb inactivation along with p16 overexpression and down-regulation of cyclin D1 are thought to be important pathogenetic steps for developing TSCCs. In the present study, HPV status was not correlated with histopathologic features or survival benefits.
DISCUSSION
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893-2917. ArticlePubMed
- 2. D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007; 356: 1944-1956. ArticlePubMed
- 3. Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 2007; 110: 1429-1435. ArticlePubMed
- 4. Ministry for Health and Welfare. Korea Central Cancer Registry. National Cancer Center. Annual report of cancer statistics in Korea in 2009. 2011; Seoul: Ministry for Health and Welfare, 23.
- 5. Li W, Thompson CH, Xin D, et al. Absence of human papillomavirus in tonsillar squamous cell carcinomas from Chinese patients. Am J Pathol 2003; 163: 2185-2189. ArticlePubMedPMC
- 6. Suarez PA, Adler-Storthz K, Luna MA, El-Naggar AK, Abdul-Karim FW, Batsakis JG. Papillary squamous cell carcinomas of the upper aerodigestive tract: a clinicopathologic and molecular study. Head Neck 2000; 22: 360-368. ArticlePubMed
- 7. El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil, and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol 2003; 27: 1463-1470. PubMed
- 8. Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck 2008; 30: 898-903. ArticlePubMed
- 9. Song JS, Kim MS, Park JW, Lee YS, Kang CS. Expression of human papillomavirus-related proteins and its clinical implication in tonsillar squamous cell carcinoma. Korean J Pathol 2012; 46: 177-186. ArticlePubMedPMC
- 10. Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer 2008; 122: 2656-2664. ArticlePubMed
- 11. Kuo KT, Hsiao CH, Lin CH, Kuo LT, Huang SH, Lin MC. The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and predicting favorable outcome. Mod Pathol 2008; 21: 376-386. ArticlePubMedPDF
- 12. Chernock RD, Lewis JS Jr, Zhang Q, El-Mofty SK. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum Pathol 2010; 41: 1016-1023. ArticlePubMed
- 13. Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer 2007; 120: 1418-1425. ArticlePubMed
- 14. Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol 2009; 27: 6213-6221. ArticlePubMed
- 15. Klussmann JP, Gültekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 2003; 162: 747-753. ArticlePubMedPMC
- 16. Helbig M, Andl T, Kahn T, et al. The role of oncogenic human papillomaviruses in tonsillar squamous cell carcinomas with functional inactivation of the retinoblastoma protein. HNO 1999; 47: 796-803. ArticlePubMedPDF
- 17. Charfi L, Jouffroy T, de Cremoux P, et al. Two types of squamous cell carcinoma of the palatine tonsil characterized by distinct etiology, molecular features and outcome. Cancer Lett 2008; 260: 72-78. ArticlePubMed
- 18. Li W, Thompson CH, Cossart YE, et al. The expression of key cell cycle markers and presence of human papillomavirus in squamous cell carcinoma of the tonsil. Head Neck 2004; 26: 1-9. ArticlePubMed
- 19. Lewis JS Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma: an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol 2010; 34: 1088-1096. ArticlePubMed
- 20. Perrone F, Gloghini A, Cortelazzi B, Bossi P, Licitra L, Pilotti S. Isolating p16-positive/HPV-negative oropharyngeal cancer: an effort worth making. Am J Surg Pathol 2011; 35: 774-777.
- 21. Marur S, D'Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 2010; 11: 781-789. ArticlePubMedPMC
- 22. Robinson M, Sloan P, Shaw R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol 2010; 46: 492-496. ArticlePubMed
- 23. Bova RJ, Quinn DI, Nankervis JS, et al. Cyclin D1 and p16INK4A expression predict reduced survival in carcinoma of the anterior tongue. Clin Cancer Res 1999; 5: 2810-2819. PubMed
- 24. Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 2007; 6: 24.ArticlePubMedPMC
- 25. Almadori G, Galli J, Cadoni G, Bussu F, Maurizi M. Human papillomavirus infection and cyclin D1 gene amplification in laryngeal squamous cell carcinoma: biologic function and clinical significance. Head Neck 2002; 24: 597-604. ArticlePubMed
- 26. Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006; 24: 736-747. ArticlePubMed
- 27. Butz K, Whitaker N, Denk C, Ullmann A, Geisen C, Hoppe-Seyler F. Induction of the p53-target gene GADD45 in HPV-positive cancer cells. Oncogene 1999; 18: 2381-2386. ArticlePubMedPDF
- 28. Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res 2008; 14: 366-369. ArticlePubMedPDF
- 29. Pintos J, Franco EL, Black MJ, Bergeron J, Arella M. Human papillomavirus and prognoses of patients with cancers of the upper aerodigestive tract. Cancer 1999; 85: 1903-1909. ArticlePubMed
- 30. Paz IB, Cook N, Odom-Maryon T, Xie Y, Wilczynski SP. Human papillomavirus (HPV) in head and neck cancer: an association of HPV 16 with squamous cell carcinoma of Waldeyer's tonsillar ring. Cancer 1997; 79: 595-604. ArticlePubMed
REFERENCES

| Variables | Total | HPV-positive | HPV-negative | p-value |
|---|---|---|---|---|
| Gender | 1.000 | |||
| Male | 81 (91) | 54 (60.7) | 27 (30.3) | |
| Female | 8 (9) | 5 (5.6) | 3 (3.4) | |
| Age (yr) | 0.005* | |||
| ≤ 60 | 64 (72) | 48 (54) | 16 (18) | |
| > 60 | 25 (28) | 11 (12.3) | 14 (15.7) | |
| Tobacco use | 0.490 | |||
| Non-smoker | 28 (31.5) | 21 (23.6) | 7 (7.9) | |
| Former smoker | 8 (9) | 5 (5.6) | 3 (3.4) | |
| Current smoker | 53 (59.5) | 33 (37.1) | 20 (22.4) | |
| Alcohol consumption | 0.907 | |||
| No history | 27 (30.3) | 18 (20.2) | 9 (10.1) | |
| ≤ 3 drinks per day | 26 (29.2) | 18 (20.2) | 8 (9) | |
| > 3 drinks per day | 36 (40.5) | 23 (25.9) | 13 (14.6) | |
| Tumor size (cm) | 0.039* | |||
| ≤ 4 | 78 (87.6) | 55 (61.8) | 23 (25.8) | |
| > 4 | 11 (12.4) | 4 (4.5) | 7 (7.9) | |
| T stage | 0.156 | |||
| T1 and T2 | 70 (78.7) | 49 (55.1) | 21 (23.6) | |
| T3 and T4 | 19 (21.3) | 10 (11.2) | 9 (10.1) | |
| N stage | 0.247 | |||
| N0 | 10 (11.2) | 5 (5.6) | 5 (5.6) | |
| N1, N2, and N3 | 79 (88.8) | 54 (60.7) | 25 (28.1) | |
| M stage | 0.659 | |||
| M0 | 83 (93.2) | 54 (60.7) | 29 (32.6) | |
| M1 | 6 (6.8) | 5 (5.6) | 1 (1.1) | |
| Anatomic stage | 0.970 | |||
| I and II | 18 (20.2) | 12 (13.5) | 6 (6.7) | |
| III and IV | 71 (79.8) | 47 (52.8) | 24 (27.0) |
| Variables | Total | HPV-positive | HPV-negative | p-value |
|---|---|---|---|---|
| p16 | < 0.001* | |||
| Positive | 74 (83.1) | 57 (64.0) | 17 (19.1) | |
| Negative | 15 (16.9) | 2 (2.3) | 13 (14.6) | |
| pRb | 0.003* | |||
| Positive | 5 (5.6) | 0 (0) | 5 (5.6) | |
| Negative | 84 (94.4) | 59 (66.3) | 25 (28.1) | |
| Cyclin D1 | < 0.001* | |||
| Positive | 21 (23.6) | 6 (6.7) | 15 (16.9) | |
| Negative | 68 (76.4) | 53 (59.5) | 15 (16.9) | |
| p53 | 0.334 | |||
| Positive | 24 (27.0) | 14 (15.7) | 10 (11.3) | |
| Negative | 65 (73.0) | 45 (50.5) | 20 (22.5) |
Figure & Data
References
Citations

- Assessment of the Mutation Profile of Tonsillar Squamous Cell Carcinomas Using Targeted Next-Generation Sequencing
Ha Young Park, Joong Seob Lee, Jee Hye Wee, Jeong Wook Kang, Eun Soo Kim, Taeryool Koo, Hee Sung Hwang, Hyo Jung Kim, Ho Suk Kang, Hyun Lim, Nan Young Kim, Eun Sook Nam, Seong Jin Cho, Mi Jung Kwon
Biomedicines.2023; 11(3): 851. CrossRef - Clinicopathologic characterization of cervical metastasis from an unknown primary tumor: a multicenter study in Korea
Miseon Lee, Uiree Jo, Joon Seon Song, Youn Soo Lee, Chang Gok Woo, Dong-Hoon Kim, Jung Yeon Kim, Sun Och Yoon, Kyung-Ja Cho
Journal of Pathology and Translational Medicine.2023; 57(3): 166. CrossRef - Negative Prognostic Implication of TERT Promoter Mutations in Human Papillomavirus–Negative Tonsillar Squamous Cell Carcinoma Under the New 8th AJCC Staging System
Hyunchul Kim, Mi Jung Kwon, Bumjung Park, Hyo Geun Choi, Eun Sook Nam, Seong Jin Cho, Kyueng-Whan Min, Eun Soo Kim, Hee Sung Hwang, Mineui Hong, Taeryool Koo, Hyo Jung Kim
Indian Journal of Surgical Oncology.2021; 12(S1): 134. CrossRef - Prevalence of high-risk human papillomavirus and its genotype distribution in head and neck squamous cell carcinomas
Yuil Kim, Young-Hoon Joo, Min-Sik Kim, Youn Soo Lee
Journal of Pathology and Translational Medicine.2020; 54(5): 411. CrossRef - Frequent hepatocyte growth factor overexpression and low frequency of c-Met gene amplification in human papillomavirus–negative tonsillar squamous cell carcinoma and their prognostic significances
Mi Jung Kwon, Dong Hoon Kim, Hye-Rim Park, Hyung Sik Shin, Ji Hyun Kwon, Dong Jin Lee, Jin Hwan Kim, Seong Jin Cho, Eun Sook Nam
Human Pathology.2014; 45(7): 1327. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure



Fig. 1
Fig. 2
Fig. 3
| Variables | Total | HPV-positive | HPV-negative | p-value |
|---|---|---|---|---|
| Gender | 1.000 | |||
| Male | 81 (91) | 54 (60.7) | 27 (30.3) | |
| Female | 8 (9) | 5 (5.6) | 3 (3.4) | |
| Age (yr) | 0.005 |
|||
| ≤ 60 | 64 (72) | 48 (54) | 16 (18) | |
| > 60 | 25 (28) | 11 (12.3) | 14 (15.7) | |
| Tobacco use | 0.490 | |||
| Non-smoker | 28 (31.5) | 21 (23.6) | 7 (7.9) | |
| Former smoker | 8 (9) | 5 (5.6) | 3 (3.4) | |
| Current smoker | 53 (59.5) | 33 (37.1) | 20 (22.4) | |
| Alcohol consumption | 0.907 | |||
| No history | 27 (30.3) | 18 (20.2) | 9 (10.1) | |
| ≤ 3 drinks per day | 26 (29.2) | 18 (20.2) | 8 (9) | |
| > 3 drinks per day | 36 (40.5) | 23 (25.9) | 13 (14.6) | |
| Tumor size (cm) | 0.039 |
|||
| ≤ 4 | 78 (87.6) | 55 (61.8) | 23 (25.8) | |
| > 4 | 11 (12.4) | 4 (4.5) | 7 (7.9) | |
| T stage | 0.156 | |||
| T1 and T2 | 70 (78.7) | 49 (55.1) | 21 (23.6) | |
| T3 and T4 | 19 (21.3) | 10 (11.2) | 9 (10.1) | |
| N stage | 0.247 | |||
| N0 | 10 (11.2) | 5 (5.6) | 5 (5.6) | |
| N1, N2, and N3 | 79 (88.8) | 54 (60.7) | 25 (28.1) | |
| M stage | 0.659 | |||
| M0 | 83 (93.2) | 54 (60.7) | 29 (32.6) | |
| M1 | 6 (6.8) | 5 (5.6) | 1 (1.1) | |
| Anatomic stage | 0.970 | |||
| I and II | 18 (20.2) | 12 (13.5) | 6 (6.7) | |
| III and IV | 71 (79.8) | 47 (52.8) | 24 (27.0) |
| Variables | Total | HPV-positive | HPV-negative | p-value |
|---|---|---|---|---|
| p16 | < 0.001 |
|||
| Positive | 74 (83.1) | 57 (64.0) | 17 (19.1) | |
| Negative | 15 (16.9) | 2 (2.3) | 13 (14.6) | |
| pRb | 0.003 |
|||
| Positive | 5 (5.6) | 0 (0) | 5 (5.6) | |
| Negative | 84 (94.4) | 59 (66.3) | 25 (28.1) | |
| Cyclin D1 | < 0.001 |
|||
| Positive | 21 (23.6) | 6 (6.7) | 15 (16.9) | |
| Negative | 68 (76.4) | 53 (59.5) | 15 (16.9) | |
| p53 | 0.334 | |||
| Positive | 24 (27.0) | 14 (15.7) | 10 (11.3) | |
| Negative | 65 (73.0) | 45 (50.5) | 20 (22.5) |
| Variables | Total | HPV-positive | HPV-negative | p-value |
|---|---|---|---|---|
| Tumor origin | 0.075 | |||
| Crypt | 64 (71.9) | 46 (51.7) | 18 (20.2) | |
| Surface | 25 (28.1) | 13 (14.6) | 12 (13.5) | |
| Differentiation | 0.128 | |||
| WD | 16 (18.0) | 8 (9.0) | 8 (9.0) | |
| MD and PD | 73 (82.0) | 51 (57.3) | 22 (24.7) | |
| Keratinization | 0.832 | |||
| Keratinizing | 31 (34.8) | 21 (23.6) | 10 (11.2) | |
| Non-keratinizing | 58 (65.2) | 38 (42.7) | 20 (22.5) | |
| LN metastasis | 0.367 | |||
| Cystic | 37 (41.5) | 27 (30.3) | 10 (11.2) | |
| Non-cystic | 52 (58.5) | 32 (36.0) | 20 (22.5) |
Values are presented as number (%). HPV, human papillomavirus; TSCCs, tonsillar squamous cell carcinomas. Statistically significant.
Values are presented as number (%). HPV, human papillomavirus; TSCCs, tonsillar squamous cell carcinomas. Statistically significant.
Values are presented as number (%). HPV, human papillomavirus; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; LN, lymph node.

 E-submission
E-submission