Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 47(5); 2013 > Article
-
Case Study
Fine-Needle Aspiration Cytology of Low-Grade Cribriform Cystadenocarcinoma with Many Psammoma Bodies of the Salivary Gland - Ji Yun Jeong, Dongbin Ahn1, Ji Young Park
-
Korean Journal of Pathology 2013;47(5):481-485.
DOI: https://doi.org/10.4132/KoreanJPathol.2013.47.5.481
Published online: October 25, 2013
Department of Pathology, Neck Surgery, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
1Department of Otorhinolaryngology-Head, Neck Surgery, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea.
- Corresponding Author: Ji Young Park, M.D. Department of Pathology, Kyungpook National University Hospital, Kyungpook National University School of Medicine, 130 Dongdeok-ro, Jung-gu, Daegu 700-721, Korea. Tel: +82-53-420-5247, Fax: +82-53-426-1525, jyparkmd@knu.ac.kr
• Received: August 7, 2013 • Revised: September 3, 2013 • Accepted: September 4, 2013
© 2013 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Low-grade cribriform cystadenocarcinoma (LGCCC) is a rare salivary gland tumor that was recently defined as a variant of cystadenocarcinoma by the 2005 World Health Orgazniation (WHO) classification system. We report cytologic findings of an unusual case of LGCCC with many psammoma bodies. A 90-year-old man presented a palpable mass on his left parotid gland. Fine-needle aspiration (FNA) cytology showed tumor cells that were arranged in clusters and dispersed individually. The tumor cells showed mild atypia and had clear or dense cytoplasm with some vacuoles. Numerous psammoma bodies were noted. After surgical resection, the histologic examination revealed a mixed solid and cystic mass showing intraductal growth with focal stromal invasion. The S-100 protein expressed in the tumor cells, but smooth muscle actin and p63 were positive only in myoepithelial cells. Although LGCCCs resemble other salivary gland tumors, differentiating LGCCC during preoperative FNA is important to avoid unnecessary overtreatment.
- A 90-year-old man presented with a palpable mass on his left postauricular region that had been present for three years. The mass was hard, fixed, and recently increased in size. These symptoms were not accompanied by pain, tenderness, local hotness, or recent weight loss. The patient was healthy for his age and had an unremarkable medical history.
- Magnetic resonance imaging revealed a well-enhanced 5 cm solid mass including a cystic component in the left parotid gland. The margin of the mass was partly ill-defined. Neither regional nor distant metastasis was found on positron emission tomography-computed tomography scan. The initial diagnosis from clinical and radiologic evidence was a metastatic lymph node or a malignant tumor of the parotid gland. FNA of the tumor was performed.
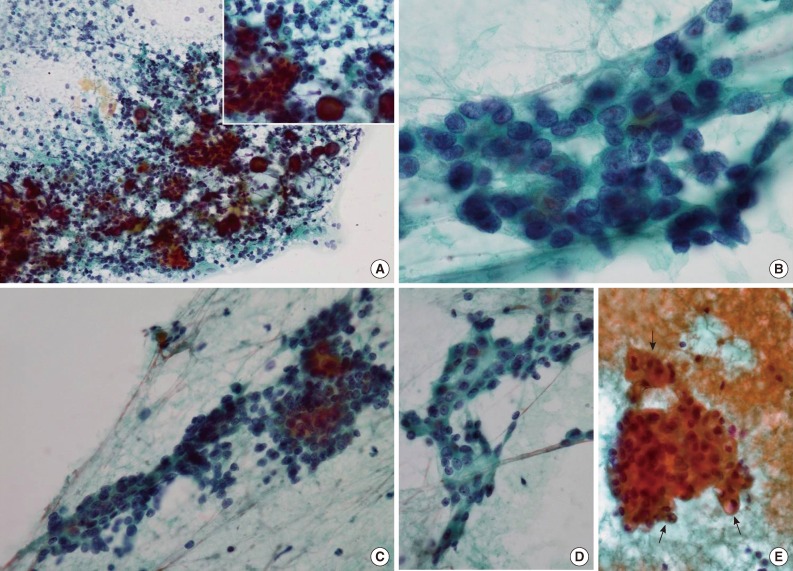
- Papanicolaou-stained smears revealed that tumor cells were arranged both in tight clusters and individually dispersed (Fig. 1A). The tumor cells had round or oval nuclei with mild atypia. Most of the tumor cells had fine chromatin without prominent nucleoli, but some had inconspicuous small nucleoli (Fig. 1B). The cytoplasm was scant in most of the tumor cells, and numerous naked cells were identified (Fig. 1C). Some tumor cells had a polygonal shape with dense opaque cytoplasm, which suggested squamoid differentiation even though no obvious keratin material was noted (Fig. 1D), and a few had cytoplasmic vacuoles of various sizes (Fig. 1E). Cytoplasmic granules were not found. Inflammatory cells, including lymphocytes, neutrophils, and some macrophages were scattered across the background, and characteristically, numerous psammoma bodies were identified throughout the smears. Most psammoma bodies were present in the background and not connected to the tumor cells, but some of them were surrounded by tumor cells (Fig. 1A, inset). Necrosis, mucin, and myxoid stromal components were not found in the background, and only one mitotic figure was noted. In view of these cytologic findings, we considered the possibility of either a malignant tumor of the parotid gland or a metastatic tumor of some other organs, most likely the thyroid gland. The possibility of a metastatic thyroid carcinoma could not be excluded because of the presence of many psammoma bodies, which are unusual findings in FNA of salivary glands. No other findings suggesting a specific type of thyroid carcinoma were identified, and no intra-thyroidal lesion was found in radiological examination. Therefore, we diagnosed a low-grade malignancy of the parotid gland and recommended complete excision of the mass.
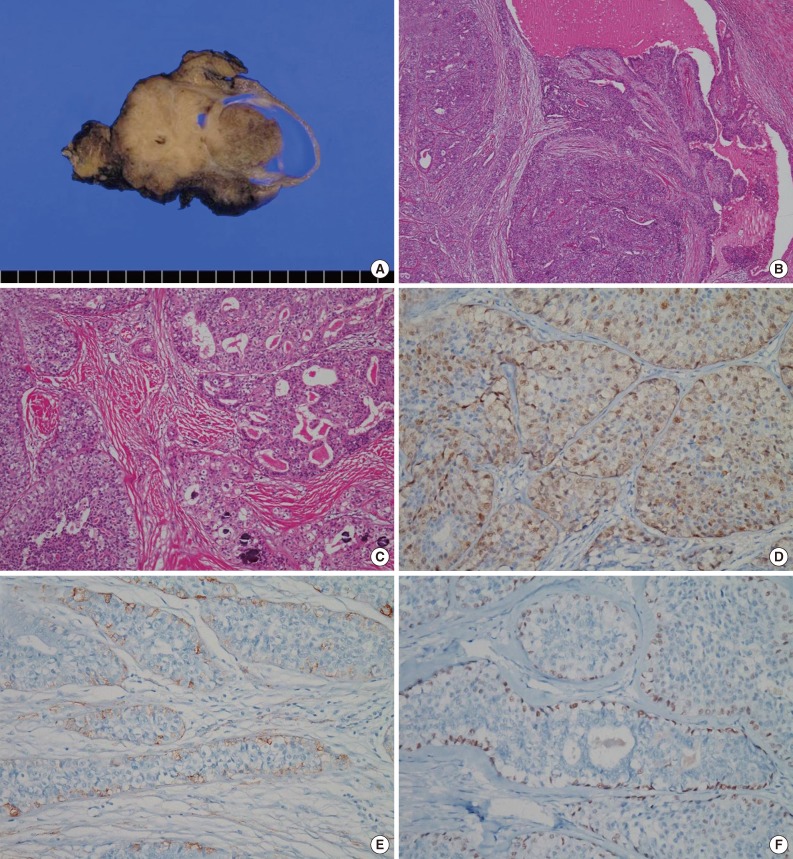
- We surgically removed the parotid gland, which measured 8.5 cm×6 cm×5 cm. On a cut section, a tan to gray solid tumor with a cystic area measuring 5.3 cm×4.5 cm×4 cm was seen. It was relatively well demarcated from the surrounding parotid gland but was nonencapsulated. In the cystic area of the tumor, an intracystic papillary-growing pattern was noted (Fig. 2A). Microscopically, the tumor was composed of multilocular cystic lumens in the periphery and solid or papillary proliferation of tumor cells with fibrous stroma in the cystic area (Fig. 2B). The cystic lumens were lined by two or more bland tumor cells. In the intracystic solid area, the tumor cells were arranged in papillary, cribriform, and solid patterns (Fig. 2C). The cystic lumens were filled with pink secretory materials, including some macrophages. The tumor cells had round to oval nuclei with inconspicuous nuclear atypia and eosinophilic or clear cytoplasm. Mitotic figures were very rare (<1/10 high power fields), and necrosis was absent. Cellular pleomorphisms and prominent nucleoli were not observed, but some small nucleoli were present. Neither perineural nor vascular invasion were present, but focal stromal invasion of the tumor cells into the fibrotic stroma was noted. Numerous psammoma bodies were observed in fibrotic stroma and between the tumor cells (Fig. 2C). All these microscopic findings in the surgical specimen were similar to the cytologic findings from preoperative FNA. Immunohistochemically, most tumor cells were positive for S-100 protein (Fig. 2D). Smooth muscle actin (SMA) (Fig. 2E) and p63 (Fig. 2F) were positive only in the periphery of the cystic lumens and the rims of papillary, cribriform, and solid tumor cell nests, which suggests intracystic or intraductal growth. The Ki-67 proliferation index was less than 5%. Thyroid transcription factor-1 (TTF-1) and thyroglobulin were negative.
CASE REPORT
- LGCCC is a rare salivary gland tumor, and only 38 cases have been reported to date.2,3 In 1996, Delgado et al.4 were the first to define this tumor as a low-grade variant of salivary ductal carcinoma (SDC). They reported 10 cases of SDC showing low-grade histology and prominent intraductal growth and developed the term low-grade salivary ductal carcinoma (LGSDC) for this tumor. All 10 cases in the Delgado et al.4 report were tumors that presented in the parotid gland, and none of the patients died from the disease during the follow-up period. Brandwein-Gensler et al.5 reported 16 more cases of LGSDC in 2004, and all patients in their report had a favorable prognosis as well.
- LGCCC came to be regarded as a variant of cystadenocarcinoma by the 2005 WHO classification system1 and was characterized by a distinct histologic presentation and biological behavior. Most cases in that report were also from tumors that presented in the parotid gland, with the exception of one case that presented in the submandibular gland. Because of its favorable outcome, differentiating LGCCC from other salivary gland tumors at the point of preoperative FNA diagnostics is critical for appropriate treatment decisions. However, it is very difficult to make a differential diagnosis by cytologic findings in the salivary gland, and only two reports, by Nakazawa et al.6 and Obokata et al.3 have described the cytologic features of LGCCC.
- LGCCC has characteristic histologic features that consist of single or multiple cysts with adjacent intraductal proliferation. The tumor cells are bland and are typically arranged in a cribriform or papillary pattern. Focal invasion of the tumor cells into the surrounding stroma can be seen, but perineural invasion, vascular invasion, nuclear pleomorphisms, mitotic figures, and necrosis are usually absent. The tumor cells are diffusely positive for S-100 proteins, and myoepithelial markers are positive only along the rim of cystic spaces.1,2
- Based on the histologic findings, LGCCC shares some similar features with several salivary gland tumors including conventional SDC, mucoepidermoid carcinoma (MC), papillary cystic variant of acinic cell carcinoma (PCV-ACC), conventional cystadenocarcinoma, mammary analogue secretory carcinoma (MASC), and polymorphous low-grade adenocarcioma (PLGA). Conventional SDC could be easily excluded from the cytologic diagnosis in the present case, because there was neither a lack of moderate or marked nuclear pleomorphisms nor a necrotic background. MC was also easily ruled out because, despite the fact that the present case exhibited some squamoid cells and cytoplasmic vacuoles, there were no signs of definite intracytoplasmic mucin, intermediate cells, or a mucinous background. Thus, we focused on PCV-ACC, conventional cystadenocarcinoma, MASC, PLGA, and LGCCC during the main differential diagnosis, which was based on the initial FNA. PCV-ACC resembles LGCCC in that PCV-ACC can show papillary-cystic growth patterns and vacuolated cells with clear, cytoplasmic vacuoles. In contrast with the present case, PCV-ACC mostly occurs in young people and is usually negative for S-100 protein.1 While the sizes of cytoplasmic vacuoles in the present case were variable, those of PCV-ACC are typically more uniform.2,6 Conventional cystadenocarcinoma also has a cystic growth pattern with papillary proliferation, but it tends to be more invasive and does not resemble ADH or LGDCIS of the breast.1 MASC is a newly described tumor of the salivary gland that is associated with ETV6-NTRK3 genetic translocation.7 Similar to LGCCC, MASC also has papillary and cystic architectural patterns, cytoplasmic vacuoles, and positivity for S-100 protein on immunohistochemical stain.7,8 Although we did not perform the appropriate molecular studies to confirm this, the presence of myoepithelial cells around tumor cell nests, which suggests intracystic or intraductal growth, is a feature of LGCCC that helped to distinguish this case from a case of MASC. PLGAs have some similar features to LGCCC, such as cytologic uniformity, papillary-cystic or cribriform growth pattern, and positivity for S-100 protein.1 However, in contrast to the intracystic or intraductal growth that we observed in the present case, PLGA is characterized by an infiltrative growth pattern with mainly lobular, trabecular, or ductal structures. Papillary-cystic or cribriform growth patterns are typically present in focal areas. The present case can be distinguished from PLGA by its intracystic or intraductal growth which has been shown through immunohistochemical staining to be positive for SMA and p63, to have prominent papillary-cystic and cribriform growth patterns that resemble ADH and LGDCIS of the breast, to have an absence of infiltrative lobular or trabecular tumor cell nests, and to have an absence of perineural invasion, which is common in PLGA.
- The presence of numerous psammoma bodies that were widely spread in the tissue smears made the initial diagnosis difficult. Exuberant psammoma bodies are infrequent findings in salivary gland tumors, thus common conditions such as cystic papillary thyroid carcinomas should be ruled out in such circumstances. Negahban et al.9 reported many psammoma bodies in the FNA of a salivary gland tumor that was diagnosed as PCV-ACC; based on a description of the resected specimen, it was described as a so-called psammoma body-rich papillary cystic acinic cell carcinoma. One report3 described psammoma bodies as the cytologic findings for a LGCCC diagnosis, but only a few tumor cell clusters in that case contained psammoma bodies. In the present case, numerous psammoma bodies, which were found in the fibrotic stroma and between the tumor cells of the resected specimen, were among the striking features of the FNA smears. No other findings that could suggest a specific type of thyroid carcinoma, such as nuclear clearing, nuclear grooves, intranuclear pseudoinclusions, obvious follicular patterns, or colloid in the background, were identified, and no intra-thyroidal lesion were found. Therefore, for the initial diagnosis, we favored primary malignancy of the salivary gland rather than metastatic thyroid carcinoma. The possibility of metastatic thyroid carcinoma could be completely eliminated by histologic and immunohistochemical findings because we found total negativity for TTF-1 and thyroglobulin after resection of the tumor.
- In summary, we report the cytologic findings of an extremely unusual case of LGCCC showing many psammoma bodies in the parotid gland. Differentiating LGCCC from the other salivary gland tumors through preoperative FNA is difficult, but pathologists should consider LGCCC in their differential diagnoses, especially when they encounter low-grade cystic lesions. The recognition of LGCCC through comprehensive differential diagnosis by FNA is important for appropriate treatment and for predicting patient prognosis.
DISCUSSION
- 1. Brandwein-Gensler MS, Gnepp DR. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Evenson JW, Reichart P, Sidransky D, eds. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press, 2005; 233.
- 2. Wang L, Liu Y, Lin X, et al. Low-grade cribriform cystadenocarcinoma of salivary glands: report of two cases and review of the literature. Diagn Pathol 2013; 8: 28.ArticlePubMedPMCPDF
- 3. Obokata A, Sakurai S, Hirato J, Sakamoto K, Takekoshi T, Aoki J. Cytologic features of low-grade cribriform cystadenocarcinoma of the submandibular gland: a case report. Acta Cytol 2013; 57: 207-212. ArticlePubMedPDF
- 4. Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma: a distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer 1996; 78: 958-967. ArticlePubMed
- 5. Brandwein-Gensler M, Hille J, Wang BY, et al. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol 2004; 28: 1040-1044. PubMed
- 6. Nakazawa T, Kondo T, Yuminomochi T, et al. Fine-needle aspiration biopsy of low-grade cribriform cystadenocarcinoma of the salivary gland. Diagn Cytopathol 2011; 39: 218-222. ArticlePubMed
- 7. Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010; 34: 599-608. ArticlePubMed
- 8. Bishop JA, Yonescu R, Batista DA, Westra WH, Ali SZ. Cytopathologic features of mammary analogue secretory carcinoma. Cancer Cytopathol 2013; 121: 228-233. ArticlePubMed
- 9. Negahban S, Daneshbod Y, Khademi B, Seif I. Papillary cystic acinic cell carcinoma with many psammoma bodies, so-called psammoma body-rich papillary cystic acinic cell carcinoma: report of a case with fine needle aspiration findings. Acta Cytol 2009; 53: 440-444. PubMed
REFERENCES
Fig. 1Cytologic findings of the tumor using fine-needle aspiration. (A) Tumor cell clusters and individually dispersed tumor cells with numerous psammoma bodies (inset) are identifiable in this image. (B) The tumor cells have round to oval bland nuclei, and show fine chromatin with occasional small nucleoli. (C) The tumor cells have a small amount of dense or clear cytoplasm with indistinct borders. (D) Some tumor cells show squamoid differentiation, and are polygonal to spindle shaped with dense cytoplasm. (E) Cytoplasmic vacuoles of various sizes are present in a few tumor cells (arrows).
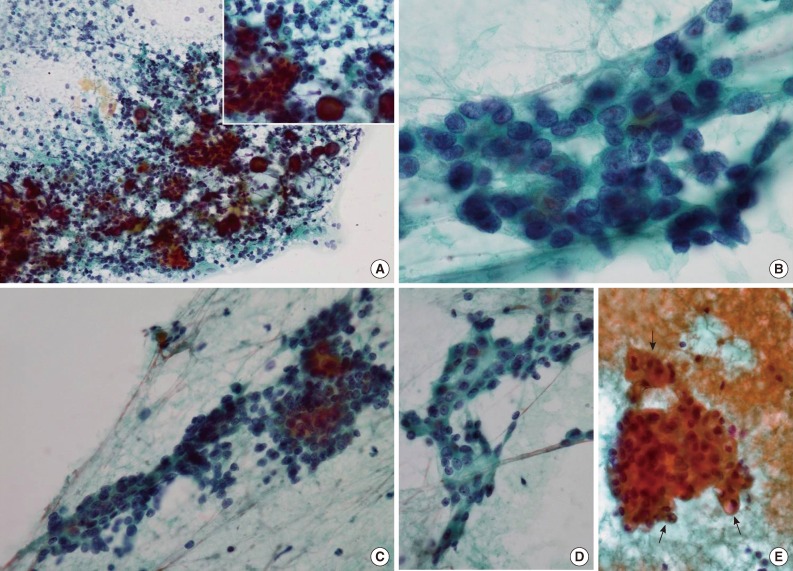

Fig. 2Macroscopic and microscopic findings of the resected tumor. (A) The tumor is composed of papillary and solid proliferative components and has a focally multilocular cystic pattern. (B) Proliferation of the tumor cells within the cystic lumens is noted. (C) The tumor cells are arranged in cribriform, papillary, and solid patterns, and many psammoma bodies are observed. (D) Most tumor cells are positive for S-100 protein. (E) Smooth muscle actin and (F) p63 are positive only in myoepithelial cells along the periphery of tumor nests.
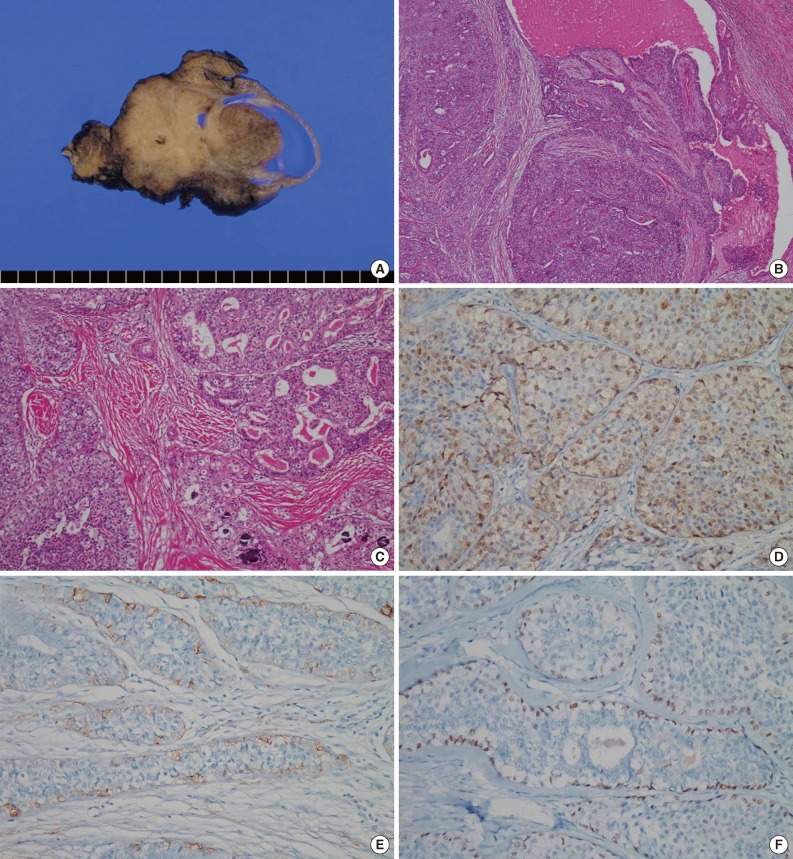

Figure & Data
References
Citations
Citations to this article as recorded by 

- Salivary Gland Intraductal Carcinoma: How Do 183 Reported Cases Fit Into a Developing Classification
Lester D.R. Thompson, Justin A. Bishop
Advances in Anatomic Pathology.2023; 30(2): 112. CrossRef - Duct tales of a parotid gland swelling
Swati Raj, Monika Singh, Mamta Gupta, Naveen Thapliyal
Cytojournal.2023; 20: 22. CrossRef - Intraductal carcinoma of the parotid gland
Yukiya HIRATA, Kayoko HIGUCHI, Toshitaka NAGAO, Yoko ZUKERAN, Takao KINJO, Naoki WADA
The Journal of the Japanese Society of Clinical Cytology.2022; 61(6): 431. CrossRef - Intraductal carcinoma of the retromolar trigone found with elevated serum CEA and CA19-9 levels: a case report
Mao KAWAKAMI, Nobuhiro UEDA, Yuka TAKAHASHI, Sho ARIKAWA, Nobuhiro YAMAKAWA, Tadaaki KIRITA
Japanese Journal of Oral and Maxillofacial Surgery.2021; 67(5): 292. CrossRef - Endoscopic trans‐pterygoid resection of a low‐grade cribriform cystadenocarcinoma of the infratemporal fossa
Vikram G. Ramjee, Landon J. Massoth, John P. Richards, Kibwei A. McKinney
World Journal of Otorhinolaryngology - Head and Neck Surgery.2020; 6(2): 115. CrossRef - Psammoma Bodies in a Large Myoepithelioma
Marcela Pessoa de Melo, Diego Filipe Bezerra Silva, Rodrigo Alves Ribeiro, Tony Santos Peixoto, Daliana Queiroga de Castro Gomes, Pollianna Muniz Alves, Cassiano Francisco Weege Nonaka, Bárbara Vanessa de Brito Monteiro
Journal of Craniofacial Surgery.2020; 31(4): e326. CrossRef - Low-grade intraductal carcinoma of salivary glands: A systematic review of this rare entity
Francesco Giovacchini, Caterina Bensi, Stefano Belli, Maria Elena Laurenti, Martina Mandarano, Daniele Paradiso, Michele Giansanti, Antonio Tullio
Journal of Oral Biology and Craniofacial Research.2019; 9(1): 96. CrossRef - What is your diagnosis? Submandibular mass in a dog
Julie Allen, Ashley M. Talley, Carol B. Grindem, Jennifer A. Neel
Veterinary Clinical Pathology.2018; 47(4): 676. CrossRef - Primary acinic cell carcinoma of the lung with psammoma bodies: A case report and review of literature
Xiu-Peng Zhang, Gui-Yang Jiang, Qing-Fu Zhang, Hong-Tao Xu, Qing-Chang Li, En-Hua Wang
Pathology - Research and Practice.2017; 213(4): 405. CrossRef - Cytology of low‐grade cribriform cystadenocarcinoma in salivary glands: Cytological and immunohistochemical distinctions from other salivary gland neoplasms
Yoshiki Ohta, Yuko Hirota, Yohko Kohno, Koji Kishimoto, Tomoko Norose, Nobuyuki Ohike, Masafumi Takimoto, Akira Shiokawa, Hidekazu Ota
Diagnostic Cytopathology.2016; 44(3): 241. CrossRef - Low-grade cribriform cystadenocarcinoma arising from a minor salivary gland: a case report
Masashi Kimura, Shinji Mii, Shinichi Sugimoto, Kosuke Saida, Shojiroh Morinaga, Masahiro Umemura
Journal of Oral Science.2016; 58(1): 145. CrossRef - A Case of Cystadenocarcinoma Arising from Parotid Gland
Jong Chul Hong, Tae Kyoung Koh, Min Gyoung Pak, Heon Soo Park
Korean Journal of Otorhinolaryngology-Head and Neck Surgery.2016; 59(4): 300. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Fine-Needle Aspiration Cytology of Low-Grade Cribriform Cystadenocarcinoma with Many Psammoma Bodies of the Salivary Gland


Fig. 1 Cytologic findings of the tumor using fine-needle aspiration. (A) Tumor cell clusters and individually dispersed tumor cells with numerous psammoma bodies (inset) are identifiable in this image. (B) The tumor cells have round to oval bland nuclei, and show fine chromatin with occasional small nucleoli. (C) The tumor cells have a small amount of dense or clear cytoplasm with indistinct borders. (D) Some tumor cells show squamoid differentiation, and are polygonal to spindle shaped with dense cytoplasm. (E) Cytoplasmic vacuoles of various sizes are present in a few tumor cells (arrows).
Fig. 2 Macroscopic and microscopic findings of the resected tumor. (A) The tumor is composed of papillary and solid proliferative components and has a focally multilocular cystic pattern. (B) Proliferation of the tumor cells within the cystic lumens is noted. (C) The tumor cells are arranged in cribriform, papillary, and solid patterns, and many psammoma bodies are observed. (D) Most tumor cells are positive for S-100 protein. (E) Smooth muscle actin and (F) p63 are positive only in myoepithelial cells along the periphery of tumor nests.
Fig. 1
Fig. 2
Fine-Needle Aspiration Cytology of Low-Grade Cribriform Cystadenocarcinoma with Many Psammoma Bodies of the Salivary Gland

 E-submission
E-submission