Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(1); 2014 > Article
-
Case Study
Large Cell Calcifying Sertoli Cell Tumor of the Testis: A Case Study and Review of the Literature - Dae Hyun Song, Seong Muk Jeong, Jong Tak Park1, Gak Won Yun2, Byoung Kwon Kim3, Jong Sil Lee4
-
Korean Journal of Pathology 2014;48(1):50-53.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.1.50
Published online: February 25, 2014
Department of Pathology, The Armed Forces Capital Hospital, Seongnam, Korea.
1Department of Urology, The Armed Forces Capital Hospital, Seongnam, Korea.
2Department of Internal Medicine, The Armed Forces Capital Hospital, Seongnam, Korea.
3Department of Pathology and Human Genome Research Institute, Green Cross Reference Laboratory, Yongin, Korea.
4Department of Pathology, Gyeongsang National University School of Medicine, Jinju, Korea.
- Corresponding Author: Jong Sil Lee, M.D. Department of Pathology, Gyeongsang National University School of Medicine, 15 Jinju-daero 816beon-gil, Jinju 660-751, Korea. Tel: +82-55-751-8763, Fax: +82-55-759-7952, jongsil25@dreamwiz.com
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- A 24-year-old man was admitted due to an incidentally detected mass in his left testis, which showed radiopaque calcification on plain X-ray film. Left orchiectomy was performed, and the resected testis contained a well-demarcated, hard mass measuring 1.1 cm. Histological analysis revealed that the tumor was composed of neoplastic cells, fibrotic stroma, and laminated or irregularly shaped calcific bodies. The individual cells had abundant eosinophilic or clear cytoplasm with round nuclei, each of which contained one or two conspicuous nucleoli. They were arranged in cords, trabeculae, clusters, and diffuse sheets. There were several foci of intra-tubular growth patterns, with thickening of the basal lamina. Immunohistochemically, the neoplastic cells were positive for S-100 protein and vimentin, focally positive for inhibin alpha, and negative for cytokeratin, CD10, and Melan-A. In addition to reporting this rare case, we also review the relevant literature regarding large cell calcifying Sertoli cell tumors.
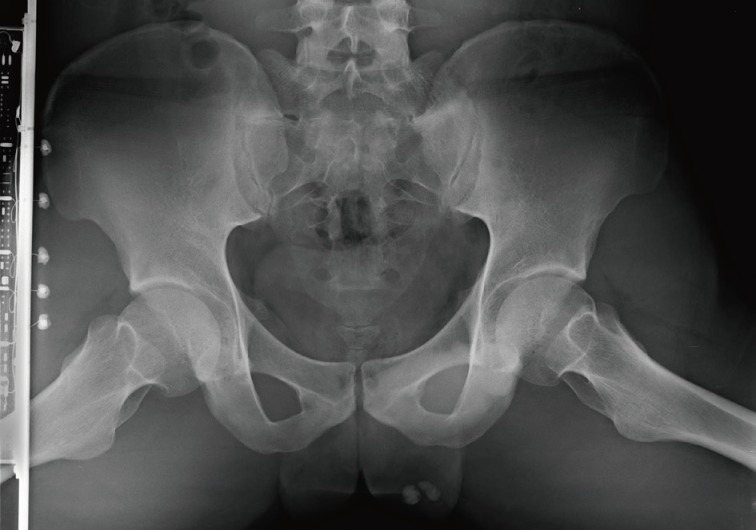
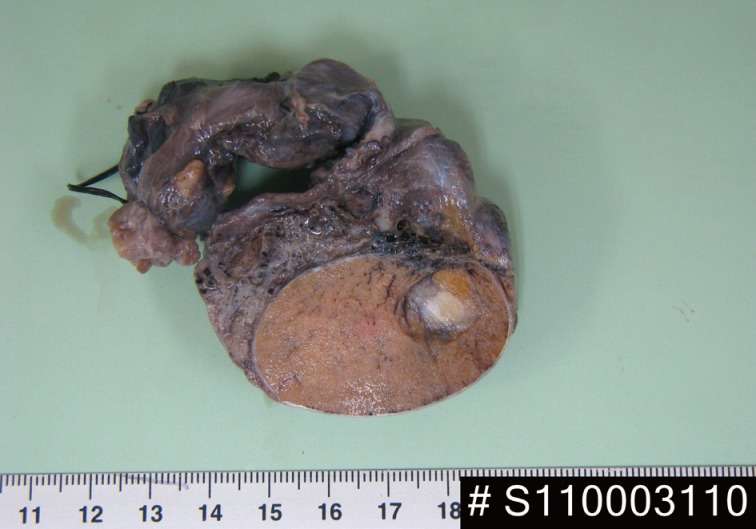
- A 24-year-old man was admitted to The Armed Forces Capital Hospital to investigate a left testicular mass, which had been incidentally detected in a plain radiograph taken for lower back pain (Fig. 1). There were no clinical signs of CNC or PJS. A left orchiectomy was performed, whereupon the testis was found to contain a 1.1-cm, well-demarcated, stony hard mass. On a cut section, this lesion had a grey-brown area with, whitish calcific changes and was confined to the testis (Fig. 2).
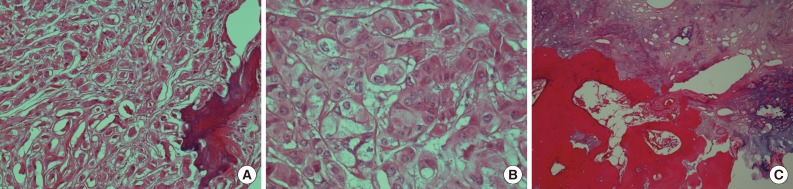
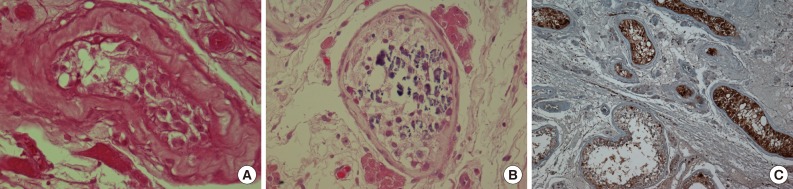
- Microscopically, the tumor was composed of neoplastic cells, fibrotic stroma, and laminated or irregularly shaped calcific bodies, containing focal ossification. The tumor cells had abundant eosinophilic or clear cytoplasm, and were arranged in cords, trabeculae, clusters, and diffuse sheets (Fig. 3). An intratubular growth pattern was also apparent, along with thickening of the basal lamina of these tubules, and intratubular microcalcification (IMC) at the periphery (Fig. 4). The nuclei were round with one or two conspicuous nucleoli, and showed moderate degree of nuclear pleomorphism. No mitotic figures, necrosis, Reinke crystalloids, or lipofuscin pigment were present, and the fibrotic stroma appeared benign.
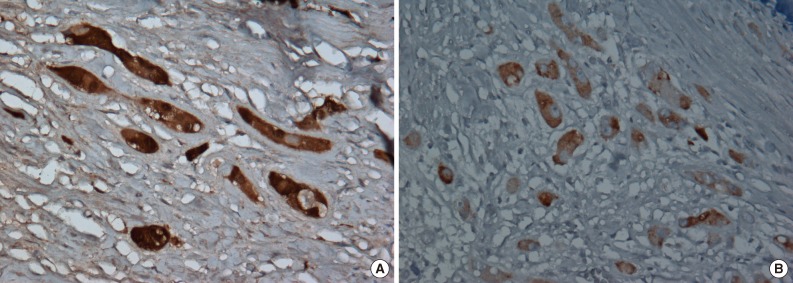
- Immunohistochemically, neoplastic cells were diffusely positive for S-100 protein (ready-to-use, anti-S-100 polyclonal antibody, Dako, Glostrup, Denmark), vimentin (ready-to-use, anti-vimentin monoclonal antibody, clone VIM 3B4, Dako), focally and weakly positive for inhibin alpha (ready-to-use, anti-inhibin alpha monoclonal antibody, clone R1, Dako) (Fig. 5) but negative for cytokeratin (ready-to-use, anti-cytokeratin monoclonal antibody, clone AE1/AE3, Dako), CD10 (ready-to-use, anti-CD10 monoclonal antibody, clone 56C6, Dako), and Melan-A (ready-to-use, anti-melan-A monoclonal antibody, clone A103, Dako).
CASE REPORT
- LCCSCTs originating from Sertoli cells are most commonly seen in patients younger than 20 years old. They are usually benign, especially when occurring concurrently with endocrine over-activity. These tumors are related to multiple neoplasia syndrome, and PJS and CNC are present in 40% of reported cases.3 PJS is a rare autosomal-dominant syndrome, presenting at a median age of 11 years, with multiple hamartomatous polyps in the gastrointestinal tract and mucocutaneous hyperpigmentation. About 50% of patients with this syndrome have a mutation in the LKB1/STK11 gene, but otherwise, the mechanism of LCCSCT pathogenesis in individuals with PJS remains unclear.
- CNC is also an autosomal-dominant mutation, with two or more clinical manifestations, including spotty skin pigmentation, cardiac myxoma, cutaneous or mucosal myxoma, myxomatosis of the breast, primary pigmented nodular adrenocortical disease, acromegaly with growth hormone-producing adenoma, LCCSCT, thyroid carcinoma, psammomatous melanotic schwannoma, blue nevus, ductal adenoma of breast, and osteochondromyxoma. Approximately 45% to 80% of individuals with CNC have inactivating mutations of the PRKAR1A gene. Around 50% of male patients with CNC develop a LCCSCT, but as with PJS, the mechanism of LCCSCT pathogenesis in men with CNC remains unclear.
- It has been reported that about 17% of LCCSCTs are malignant.4 Malignant LCCSCTs are more frequently present in older patients (with a reported range of 28 to 73 years), and are usually unifocal or unilateral lesions, greater than four cm in size, characterized by more than three mitoses per 10 high power fields, noteworthy nuclear atypia, necrosis, lymphovascular invasion and extratesticular growth.4,5 The present case was a unilateral tumor, with moderate nuclear pleomorphism, but tumor recurrence was only detected eight months postoperatively.
- An intratubular growth pattern, with hyalinization of the tubular basement membrane is a very useful diagnostic feature of LCCSCT, and is present in around 50% of all LCCSCTs,6 although it remains unclear whether the intratubular lesion is neoplastic or not.7 The tumor in the present case showed several foci of intratubular growth patterns, at the periphery of the tumor. Without this pattern, the differential diagnosis from Leydig cell tumor (LCT) may be problematic, since LCTs often have unusual features, including adipose differentiation, calcification with ossification, and spindle-shaped tumor cells.8 It may be impossible to differentiate LCCSCT, from LCT with calcification, without performing immunohistochemical studies.
- Sato et al.2 reported that LCCSCT is positive for CD10 and Melan-A, with a patchy pattern in the cytoplasm, whilst LCT shows a diffuse pattern. Furthermore, at an ultra-structural level the mitochondria often aggregate in the perinuclear area in a LCCSCT, whilst LCTs have mitochondria dispersed throughout the cytoplasm. The tumor, however, in this present case did not show any immunoreactivity for CD10 or Melan-A. Although this is unusual, it is not without precedent, as Petersson et al.9 have also described a CD10 and Melan-A negative LCCSCT. This discrepancy, however, should be addressed by a further study of more cases.
- Tanaka et al.10 reported that the S-100 beta isoform is an useful marker, as it is present in LCCSCT, but not in LCT. This is particularly noteworthy because S-100 is a calcium-binding protein and is potentially related to calcification.2 Thus, the immunoreactivity of S-100 beta in LCT with calcification needs to be further evaluated. The lesion in the present case also showed focal and weakly positive staining for inhibin alpha in tumor cells, but negative staining in normal Sertoli and Leydig cells, probably because of decalcification.
- The present case also showed several IMC foci in the tubules, which contained several germ cells (Fig. 4B), although there was no IMC in the tubules with intratubular Sertoli cell proliferation. Venara et al.7 reported that IMC was absent from most seminiferous tubules with intratubular Sertoli cell proliferation. The possible association between IMC and malignant tumors of the testis has been discussed previously by Furness et al.11 who suggested that IMC may be another sign of testicular dysfunction. Its apparent association with malignancy may be a result of selection bias.11 Thus, it remains unclear whether IMC is a premalignant condition; and therefore, long-term follow-up is required.
- In conclusion, our case of LCCSCT is a rare and distinctive entity and presents a diagnostic challenge for pathologists. We hope that the present report will help in future diagnostic and treatment decisions.
DISCUSSION
- 1. Proppe KH, Scully RE. Large-cell calcifying Sertoli cell tumor of the testis. Am J Clin Pathol 1980; 74: 607-619. ArticlePubMed
- 2. Sato K, Ueda Y, Sakurai A, et al. Large cell calcifying Sertoli cell tumor of the testis: comparative immunohistochemical study with Leydig cell tumor. Pathol Int 2005; 55: 366-371. ArticlePubMed
- 3. Gourgari E, Saloustros E, Stratakis CA. Large-cell calcifying Sertoli cell tumors of the testes in pediatrics. Curr Opin Pediatr 2012; 24: 518-522. ArticlePubMedPMC
- 4. Halat SK, Ponsky LE, MacLennan GT. Large cell calcifying Sertoli cell tumor of testis. J Urol 2007; 177: 2338.ArticlePubMed
- 5. De Raeve H, Schoonooghe P, Wibowo R, Van Marck E, Goossens A. Malignant large cell calcifying Sertoli cell tumor of the testis. Pathol Res Pract 2003; 199: 113-117. ArticlePubMed
- 6. Fletcher CD. Diagnostic histopathology of tumor. 3rd ed. Philadelphia: Elsevier, 2007; 836.
- 7. Venara M, Rey R, Bergadá I, Mendilaharzu H, Campo S, Chemes H. Sertoli cell proliferations of the infantile testis: an intratubular form of Sertoli cell tumor? Am J Surg Pathol 2001; 25: 1237-1244. PubMed
- 8. Ulbright TM, Srigley JR, Hatzianastassiou DK, Young RH. Leydig cell tumors of the testis with unusual features: adipose differentiation, calcification with ossification, and spindle-shaped tumor cells. Am J Surg Pathol 2002; 26: 1424-1433. PubMed
- 9. Petersson F, Bulimbasic S, Sima R, et al. Large cell calcifying Sertoli cell tumor: a clinicopathologic study of 1 malignant and 3 benign tumors using histomorphology, immunohistochemistry, ultrastructure, comparative genomic hybridization, and polymerase chain reaction analysis of the PRKAR1A gene. Hum Pathol 2010; 41: 552-559. ArticlePubMed
- 10. Tanaka Y, Carney JA, Ijiri R, et al. Utility of immunostaining for S-100 protein subunits in gonadal sex cord-stromal tumors, with emphasis on the large-cell calcifying Sertoli cell tumor of the testis. Hum Pathol 2002; 33: 285-289. ArticlePubMed
- 11. Furness PD 3rd, Husmann DA, Brock JW 3rd, et al. Multi-institutional study of testicular microlithiasis in childhood: a benign or premalignant condition? J Urol 1998; 160(3 Pt 2):1151-1154. ArticlePubMed
REFERENCES
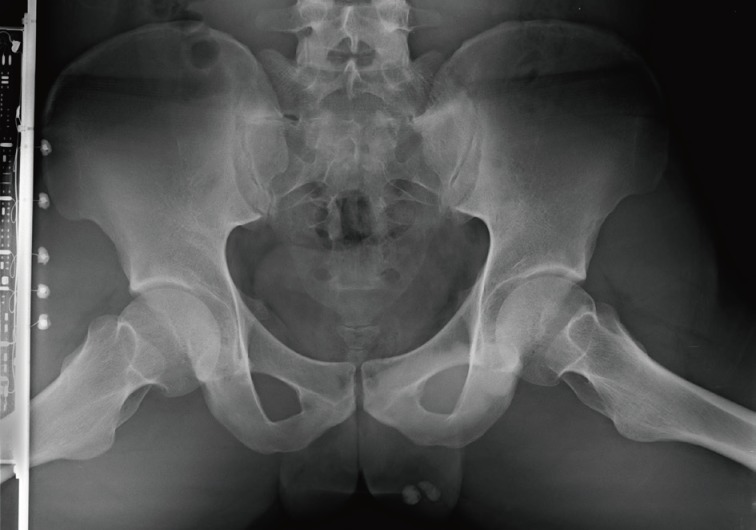
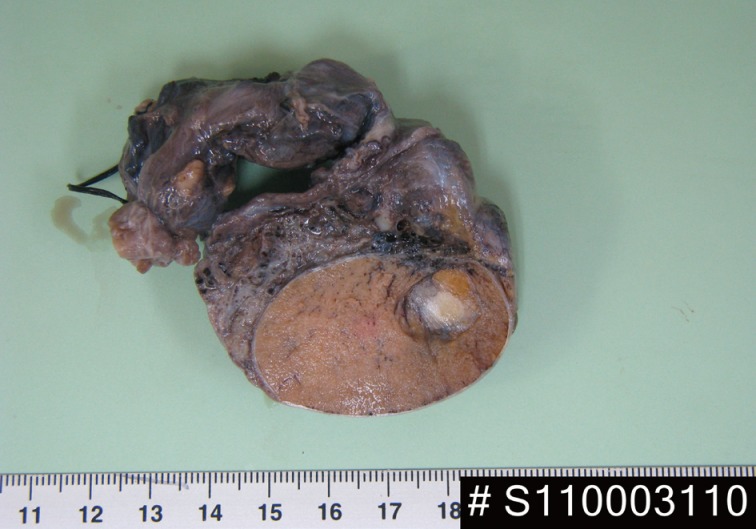
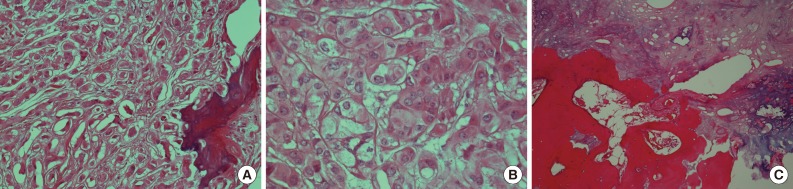
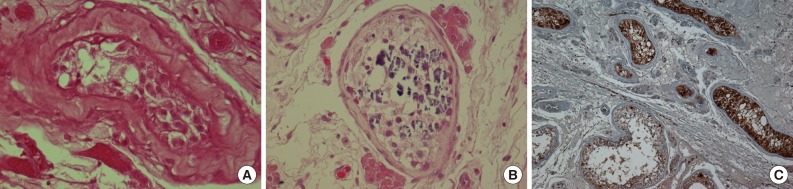
Figure & Data
References
Citations

- Extremely rare case of large cell calcifying Sertoli cell tumor in a young male
Mohammed Abdulrahman Alorini, Mohammed Abdullah Alnassar, Abdulrahman Suleiman Alghanem, Hasan Homod Alsughayer, Younes Abdulaziz Alothaim, Abdulrahman Abdulmohsen Alsultan, Saleh Mohammed Almuzaini, Faisal Abdulaziz Alhamed, Aseel Saleh Alnugaithan, Ali
International Journal of Health Sciences.2025; 19: 45. CrossRef - The First Sertoli Cell Tumor of the Adrenal Gland is Potentially Associated with Arterial Hypertension
Sara Ivanis, Milan Marinkovic, Milan Jovanovic, Matija Buzejic, Marija Milinkovic, Zlatibor Loncar, Vladan Zivaljevic, Branislav Rovcanin
International Journal of Endocrinology and Metabolism.2024;[Epub] CrossRef - The sclerosing sertoli cell tumor of the testis: a case report
Xueyao Tang, Yifan Hu, Hong Zhou, Yang Zhou
Diagnostic Pathology.2023;[Epub] CrossRef - Large Cell Calcifying Sertoli Cell Tumor
Khaleel I. Al-Obaidy, Muhammad T. Idrees, Eman Abdulfatah, Lakshmi P. Kunju, Angela Wu, Thomas M. Ulbright
American Journal of Surgical Pathology.2022; 46(5): 688. CrossRef - Intratubular large cell hyalinizing Sertoli cell tumor of the testis presenting with prepubertal gynecomastia: a case report
Hale Tuhan, Ayhan Abaci, Banu Sarsık, Tülay Öztürk, Mustafa Olguner, Gonul Catli, Ahmet Anik, Nur Olgun, Ece Bober
Acta Clinica Belgica.2017; 72(4): 254. CrossRef - A Comprehensive Review of Pediatric Tumors and Associated Cancer Predisposition Syndromes
Sarah Scollon, Amanda Knoth Anglin, Martha Thomas, Joyce T. Turner, Kami Wolfe Schneider
Journal of Genetic Counseling.2017; 26(3): 387. CrossRef - Medical and Surgical Management of Carney Complex
Juan A. Siordia
Journal of Cardiac Surgery.2015; 30(7): 560. CrossRef - Tumeurs testiculaires primitives à expression endocrine
J.-M. Kuhn
EMC - Endocrinologie - Nutrition.2015; 26(1): 1. CrossRef






 E-submission
E-submission
 PubReader
PubReader Cite this Article
Cite this Article