Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(2); 2014 > Article
-
Original Article
Pleural Mesothelioma: An Institutional Experience of 66 Cases - Soomin Ahn, In Ho Choi, Joungho Han, Jhingook Kim1, Myung-Ju Ahn2
-
Korean Journal of Pathology 2014;48(2):91-99.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.2.91
Published online: April 28, 2014
Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
1Department of Thoracic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding Author: Joungho Han, M.D. Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 81 Irwon-ro, Gangnam-gu, Seoul 135-710, Korea. Tel: +82-2-3410-2765, Fax: +82-2-3410-0025, Joungho.han@samsung.com
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Malignant mesothelioma of the pleura is an aggressive tumor known to be associated with asbestos. Histological diagnosis of mesothelioma is challenging and is usually aided by immunohistochemical markers.
-
Methods
- During an 18-year period (1995-2012), 66 patients with pleural mesothelioma were diagnosed at the Samsung Medical Center in Seoul. We reviewed hematoxylin and eosin and immunohistochemical slides of pleural mesothelioma and evaluated their pathological and clinical features.
-
Results
- The male-to-female ratio was 1.75:1, and age of patients ranged from 28 to 80 years with an average age of 56.84 years. Twenty-two out of 66 patients underwent curative pneumonectomy. Follow-up data was available in 60 patients (90.9%), and 50 of them (83.3%) died from the disease. The average overall survival was 15.39 months. Histologically, the epithelioid type was the most common, followed by the sarcomatoid and the biphasic types. Epidemiologic information was not available in most cases, and only one patient was confirmed to have a history of asbestos exposure.
-
Conclusions
- Malignant mesothelioma of the pleura is a fatal tumor, and the therapeutic benefit of pneumonectomy remains unproven. The combination of calretinin, Wilms tumor 1, HMBE-1, and thyroid transcription factor-1 may provide high diagnostic accuracy in diagnosing mesothelioma.
- During an 18-year period (1995-2012), a total of 66 patients with pleural mesothelioma were diagnosed at the Samsung Medical Center in Seoul, South Korea. Only patients with a definite histological diagnoses were included in the present study. Initial histologic diagnoses varied among the 66 patients. Thirty-nine patients were diagnosed by needle biopsy; followed 23 patients by video-assisted thoracotomy biopsy, 3 patients by excisional biopsy, and one patient by pleural fluid cytology. For twenty-two patients, a pleuropneumonectomy was performed. Clinical and follow-up data were obtained from the patients' records. Clinical information included sex, age, history of exposure to asbestos, occupation, residential area, treatment, and follow-up visit dates. Adequate information was obtained for all patients. All patients were followed until September 2013. The median follow-up period was 14.1 months. We reviewed their hematoxylin and eosin and immunohistochemical slides and evaluated their histological features. Ten patients were diagnosed with slides from an outside hospital. Statistical analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The antibodies used in this study are as follows: thyroid transcription factor-1 (TTF-1; 1:100, Dako, Glostrup, Denmark), HBME-1 (1:400, Dako), calretinin (1:80, Novocastra, Newcastle upon Tyne, UK), vimentin (1:2,000, Dako), Wilms tumor 1 (WT-1; 1:50, Dako), carcinoembryonic antigen (CEA; 1:200, Dako), p53 (1:1,000, Invitrogen, Grand Island, NY, USA), and cytokeratin (CK [AE1/AE3]) (1:500, Dako). This study was approved by the Institutional Review Board of the Samsung Medical Center (SMC 2013-11-027-001).
MATERIALS AND METHODS
- Clinical features
- The male-to-female ratio was 1.75:1, and patient ages ranged from 28 to 80 years with an average age of 56.84 years. Most patients presented with advanced-stage disease. The initial presenting symptoms were described in 35 patients. The most common symptom was chest discomfort (16/35), followed by dyspnea (15/35) and cough (3/35). One patient had an incidentally found lesion on imaging work-up for another condition. Radiologic impressions were as follows: mesothelioma (42/66), advanced lung cancer (7/66), lymphoma (1/66), pneumonia (2/66), tuberculoid pleurisy (1/66), malignant effusion (11/66), and pleural effusion with undetermined character (2/66).
- Twenty-two patients underwent curative pleuropneumonectomy. After resection, adjuvant therapy was provided according to the protocol in seven patients. One patient underwent neoadjuvant chemotherapy before pleuropneumonectomy. Among the 44 patients without surgical intervention, 16 patients underwent chemotherapy and one patient underwent radiation therapy. Follow-up data was available in 60 patients (90.9%), and 50 (83.3%) patients died from the disease. The average survival period was 15.39 months. With regard to the treatment method, the average overall survival of surgically treated patients and non-surgically treated patients was 18.2 and 10.8 months, respectively. Surgery had no mortality benefit in patients with malignant mesothelioma, and it was statistically confirmed (p=.625).
- Gross and histological features
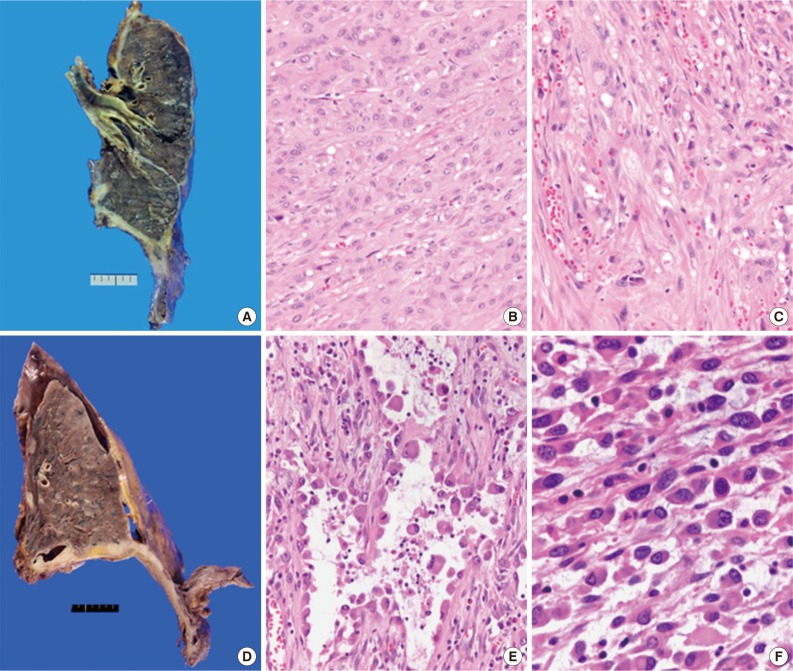
- We retrospectively reviewed the gross findings of the 22 patients who underwent an operation. Pleuropneumonectomy was performed in 21 patients, and one patient recieved right middle lobectomy. The tumor was more common on the right side (15/22) than the left side (7/22). Grossly, there was diffuse pleural thickening of the tumor (11/22), diffuse and nodular growth (8/22), or nodular growth (3/22). Regarding lymph nodal status, 54.5% (12/22) of patients had metastatic lymph nodes, and 40.9% (9/22) had no nodal metastasis.
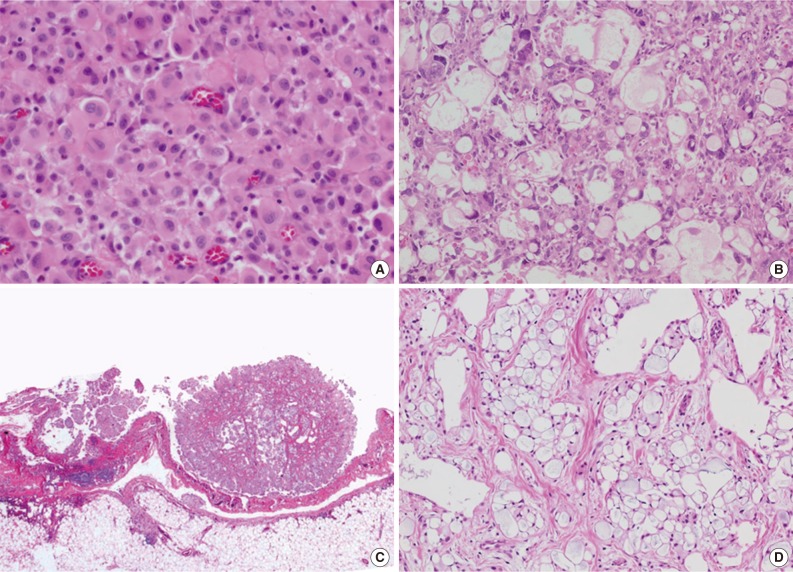
- Total 54 cases were availalbe for slide review. Fifty cases (92.6%) were epithelioid subtype with followed 3 cases of sarcomatoid subtype (5.6%) and one case of byphasic subtype (1.9%) (Fig. 1). There were three cases showing notably unique morphology. One epithelioid mesothelioma case exhibited a focal deciduoid feature, which showed diffuse proliferation of large neoplastic cells with well-defined borders and dense eosinophilic cytoplasm (Fig. 2A). The proportion of the deciduoid components ranged by approximately 30%. Another two cases revealed microcystic structures, with signet ring cell appearances (Fig. 2B-D).
- Histological subtype is a statistically significant prognostic factor in malignant mesothelioma of pleura by univariate analysis (p=.035). There were poorer outcomes in patients with sarcomatoid subtype (hazard ratio [HR], 3.973; confidence interval [CI], 0.854 to 18.451) and patients with biphasic subtype (HR, 10.777; CI, 1.182 to 98.216) compared to those with epithelioid subtype.
- Histological analysis of small biopsied specimens
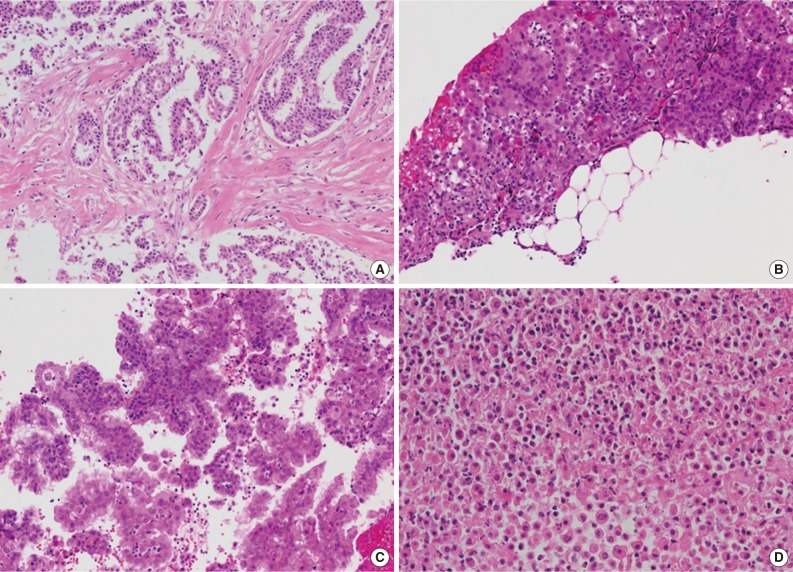
- Available small biopsy cases were histologically reviewed in order to evaluate the diagnostic features distinguishing mesothelioma from reactive mesothelial proliferation (Table 1, Fig. 3). Stromal components were identified in 26 out of 33 cases (78.8%), and stromal invasion was demonstrated in all stroma-included cases (Fig. 3A, B). Tumor cells displayed various growth patterns including complex papillary architecture (9/33), simple papillary architecture (1/33), and diffuse growth pattern (23/33) with or without desmoplastic reaction (Fig. 3C). Necrotic foci were identified in 11 out of 33 specimens (33.3%) (Fig. 3D). The degree of cellular atypia varied: mild (8/33), moderate (22/33), and severe (3/33).
- Immunohistochemistry
- With regard to immunohistochemistry, various markers including TTF-1, calretinin, WT-1, HMBE-1, vimentin, and CK (AE1/AE3) had been analyzed. Table 2 summarizes the immunohistochemical results. In most cases, two or three immunohistochemical markers were used for diagnosis. The choice of immunohistochemical markers varied according to the time and the pathologist. HBME-1, calretinin, and WT-1 were positive in 84.9%, 72.3%, and 80.9% of the cases studied, respectively (Fig. 4) when considering that positive results include diffuse positivity and focal positivity. TTF-1 was negative in 96.9% of the cases, except one case that had an unsatisfactory result. CK (AE1/AE3) was diffusely positive in 90% (9/10) of the cases and focally positive in 10% (1/10).
- Epidemiologic characteristics
- Epidemiologic information was not available in most cases, and only four patients had recorded information about asbestos exposure. Only one patient was confirmed to have a history of definite asbestos exposure.
- The occupational and residential information of patients varied. Information about occupation was not available in 38 out of the 66 patients. The occupations of the remaining patients were as follows: housewife (8/66), office worker (5/66), businessman (4/66), teacher (2/66), paint factory worker (1/66), electrical technician (1/66), sewage worker (1/66), automobile repair worker (1/66), fabric factory worker (1/66), brewery worker (1/66), farmer (1/66), law clerk (1/66), and stage director (1/66).
- Information about residential area was not obtained in four patients. The current residential areas of the remaining patients were as follows: Gyeonggi-do (12/66), Seoul (14/66), Gangwon-do (2/66), Gyeongsang-do (18/66), Incheon (1/66), Jeolla-do (8/66), Chungcheong-do (5/66), Jeju-do (1/66) of Korea, and Russia (1/66). Epidemiologic data is summarized in Table 3.
RESULTS
- Malignant mesothelioma is a rare malignant neoplasm arising from the serosal surfaces of the pleura, peritoneum, pericardium, and other body cavities.11 It is highly aggressive, with a mortality of nearly 100%.11 In the present study, we evaluated clinicopathologic and etiologic information of the patients with malignant mesothelioma at single institution over 18 years.
- We first focused on the histologic diagnosis of malignant mesothelioma. The diagnosis is challenging due to many tumors with similar histology.7 Therefore, the diagnosis is usually made with the aid of immunohistochemistry.6,8,9 To date, there is no single immunohistochemical marker that provides high sensitivity and specificity for the diagnosis of malignant mesothelioma.7,9,10 We reviewed the immunohistochemical panel used from 1995 to 2012 and discovered that there has been a shift in the combination of markers used for the diagnosis of malignant mesothelioma. Before introduction of calretinin and WT-1, various combinations of markers including CK (AE1/AE3), vimentin, bcl-2, and CD15 were used. In addition, electron microscopy often accompanied immunohistochemistry. In the 1990s, electron microscopy was performed in four cases and the final diagnosis was made with the aid of the characteristic long microvilli feature by electron microscopy.
- In general, a combination of two or more positive mesothelial markers with two or more negative epithelial markers is recommended under considerable findings of histology.6,8,10,11 Among positive mesothelioma markers, calretinin and WT-1 are usually recommended.6 It is known that virtually all mesothelioma are positive for calretinin with nuclear and cytoplasmic staining, and approximately 70% to 95% of mesotheliomas show nuclear positivity for WT-1.6 CK 5/6 and D2-40 are also useful.6 On the other hand, the value of HBME-1 in the diagnosis of malignant mesothelioma is still controversial due to low specificity.12,13 However, HBME-1 is commonly used in our institution because the thick membranous staining pattern of HBME-1 in malignant mesothelioma is helpful, in contrast to metastatic adenocarcinoma and reactive mesothelial cells which have a thin membrane and cytoplasmic pattern.12,13
- Regarding epithelial markers, at least two epithelial markers are recommended to rule out metastatic carcinoma.6 Markers useful in differentiating mesothelioma from metastatic pulmonary adenocarcinoma are MOC-31, BG8, CEA, B72.3, Ber-EP4, TTF-1, and Napsin A.6 In our institution, we usually used single marker, TTF-1, because TTF-1 shows high sensitivity and specificity for pulmonary adenocarcinoma.6
- Since 2002, the combination of HBME-1, calretinin, WT-1, and TTF-1 has been mainly used for the distinction between mesothelioma and metastatic adenocarcinoma in our institution. It seems that the combination of HBME-1, calretinin, WT-1, and TTF-1 enables a highly accurate and consistent diagnosis in the proper clinical context and hematoxylin and eosin morphology. In the present study, we could not compare sensitivity and specificity of individual markers because they were used in various combinations in different studies.
- The diagnostic distinction between reactive and neoplastic mesothelial proliferation is also challenging, particularly in small biopsied samples.6,8 Frank stromal invasion is considered the most significant discriminating diagnostic feature.6,14 We also concluded that histologic features including stromal invasion, the presence of necrosis, and cellular atypia are determining factors in the proper clinical context. In some difficult cases immunohistochemical markers could be helpful.6,14 In our institution, p53 immunostaining was performed in five of the small biopsied cases to rule out reactive conditions, and the results were positive in four out of the five cases. According to one study conducted by Attanoos et al.,14 malignant mesothelioma was reactive to p53 in 45% of mesothelioma cells in contrast to no reactivity of reactive mesothelial cells. We are in close agreement that p53 antibody may be of use as a second-line marker of neoplastic mesothelium within a standard immunohistochemical panel of antibodies and clinical settings.6,14 Other useful markers include desmin, epithelial membrane antigen, glucose transporter 1, and insulin-like growth factor-II mRNA-binding protein 3.6
- In terms of histological classification, there are various types of mesothelioma: epithelioid, sarcomatoid, desmoplastic, and biphasic types. Epithelioid subtype is the most common. While the subtype "desmoplastic mesothelioma" is generally accepted for a particular subtype of highly aggressive sarcomatoid mesothelioma, there is no agreement on the nomenclature of other subtypes.11 While several reports have suggested that patients with epithelioid subtype have a better prognosis than those with the sarcomatoid subtype, the other studies did not reveal prognostic differences among the different histologic subtypes.3,5,15 According to one study, patients with epithelioid histology had a more favorable survival than patients with non-epithelial histology. In the current study, histological subtype was a significant prognostic factor in a univariate analysis. Epithelioid subtype showed increased survival compared to the other types. On the other hand, high-grade deciduoid mesothelioma among epithelioid type is known to harbor a worse prognosis, and one patient showing deciduoid features also died shortly after the diagnosis.16
- There has been an ongoing debate regarding the optimal approach to malignant mesothelioma. The consensus among centers is that surgery, whether debulking surgery or radical resection, is best performed in combination with adjuvant chemotherapy, radiotherapy, immunotherapy, or other treatment.1,17,18,19 It is possible that some very early stage tumors have been cured by the so-called triple modality therapy, which includes extrapleural pneumonectomy followed by chemotherapy and radiation therapy; however, this finding remains inconclusive.11 There have been several studies supporting curative therapy including surgery rather than palliative treatment. Sugarbaker et al.4 reported that patients receiving any therapy survived longer than patients treated with supportive care only. In one recent trial, patients who underwent neo-adjuvant chemotherapy combined with pleuropneumonectomy and followed by radiotherapy showed an average three-year survival gain compared to patients with unimodal treatment.4 On the other hand, Takagi et al.5 reported that the survival and perioperative mortality rates of patients who had undergone pleuropneumonectomy or limited resection did not significantly differ. In our study, surgery provided no significant survival benefit in patients with malignant mesothelioma. Only one patient underwent neoadjuvant chemotherapy followed by pleuropneumonectomy. The overall survival of this patient was 24.9 months, approximately nine months longer than the average survival of 15.39 months. However, we could not statistically compare the therapeutic benefit of neoadjuvant therapy because of the limited sample size. In the present study, overall survival was 83.3%, which is a favorable result compared to the known survival rate. Most of alive patients in this study were recently diagnosed, possibly explaining this higher survival rate.
- Regarding tumor etiology, it is well established that mesothelioma is associated with asbestos exposure. In most industrialized countries, more than 90% of pleural mesotheliomas in men are related to prior asbestos exposure.11 On the other hand, only 25% of patients with malignant mesothelioma had a history of asbestos exposure in one study from Iran.20 In Korea, an average of 34 cases have been reported annually in the mesothelioma surveillance system data since 2001.21 It has been reported that about 60% of malignant mesothelioma patients in Korea have a history of asbestos exposure.22,23 Asbestos was commonly used among manufacturers and builders in the late 19th century because of its sound absorption, average tensile strength, and its resistance to fire and heat.24 Asbestos factories were mainly located in Gyeonggi-do and Gyeongsang-do, and many asbestos mines were located in Chungcheong-do of Korea.24 In our study, exposure history was not available in most cases. In addition, occupational and residential information included only current profession and geographic living area. Therefore, the data may not reflect an accurate history of past asbestos exposure. Regarding other etiologies, there has been a recent study investigating the relationship between Simian Virus 40 (SV40) and malignant mesothelioma in Korea. SV40 is a known cofactor in the carcinogenic effects of asbestos in malignant mesothelioma; however, its actual role is still controversial.25 According to one recent study, there was no association between SV40 and the development of malignant mesothelioma in Korea.25 As the epidemiologic background of malignant mesothelioma has not yet been determined, a more active epidemiologic study is warranted. There is a lack of specialized facilities and experts to diagnose and treat asbestos-related illnesses in Korea; therefore, nation-wide awareness and evaluation are needed.21
DISCUSSION
Acknowledgments
Acknowledgments
- 1. Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005; 353: 1591-1603. ArticlePubMed
- 2. Aigner C, Hoda MA, Lang G, Taghavi S, Marta G, Klepetko W. Outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2008; 34: 204-207. ArticlePubMed
- 3. Ceresoli GL, Locati LD, Ferreri AJ, et al. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer 2001; 34: 279-287. ArticlePubMed
- 4. Sugarbaker DJ, Garcia JP, Richards WG, et al. Extrapleural pneumonectomy in the multimodality therapy of malignant pleural mesothelioma: results in 120 consecutive patients. Ann Surg 1996; 224: 288-294. PubMedPMC
- 5. Takagi K, Tsuchiya R, Watanabe Y. Surgical approach to pleural diffuse mesothelioma in Japan. Lung Cancer 2001; 31: 57-65. ArticlePubMed
- 6. Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013; 137: 647-667. ArticlePubMed
- 7. Addis B, Roche H. Problems in mesothelioma diagnosis. Histopathology 2009; 54: 55-68. ArticlePubMed
- 8. Abutaily AS, Addis BJ, Roche WR. Immunohistochemistry in the distinction between malignant mesothelioma and pulmonary adenocarcinoma: a critical evaluation of new antibodies. J Clin Pathol 2002; 55: 662-668. ArticlePubMedPMC
- 9. Ordóñez NG. Immunohistochemical diagnosis of epithelioid mesothelioma: an update. Arch Pathol Lab Med 2005; 129: 1407-1414. ArticlePubMedPDF
- 10. Jung SH. Pathological diagnosis of malignant mesothelioma. J Korean Med Assoc 2009; 52: 456-464. Article
- 11. Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004.
- 12. Mocanu L, Cimpean AM, Raica M. Value of antimesothelioma HBME-1 in the diagnosis of inflammatory and malignant pleural effusions. Rom J Morphol Embryol 2006; 47: 351-355. PubMed
- 13. Oates J, Edwards C. HBME-1, MOC-31, WT1 and calretinin: an assessment of recently described markers for mesothelioma and adenocarcinoma. Histopathology 2000; 36: 341-347. ArticlePubMedPDF
- 14. Attanoos RL, Griffin A, Gibbs AR. The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium: a novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology 2003; 43: 231-238. ArticlePubMedPDF
- 15. Johansson L, Lindén CJ. Aspects of histopathologic subtype as a prognostic factor in 85 pleural mesotheliomas. Chest 1996; 109: 109-114. ArticlePubMed
- 16. Ordóñez NG. Deciduoid mesothelioma: report of 21 cases with review of the literature. Mod Pathol 2012; 25: 1481-1495. ArticlePubMedPDF
- 17. Waller DA. Malignant mesothelioma: British surgical strategies. Lung Cancer 2004; 45(Suppl 1):S81-S84. ArticlePubMed
- 18. Stewart DJ, Martin-Ucar A, Pilling JE, Edwards JG, O'Byrne KJ, Waller DA. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg 2004; 78: 245-252. ArticlePubMed
- 19. Janne PA. Chemotherapy for malignant pleural mesothelioma. Clin Lung Cancer 2003; 5: 98-106. ArticlePubMed
- 20. Bagheri R, Haghi SZ, Rahim MB, Attaran D, Toosi MS. Malignant pleural mesothelioma: clinicopathologic and survival characteristic in a consecutive series of 40 patients. Ann Thorac Cardiovasc Surg 2011; 17: 130-136. ArticlePubMed
- 21. Kim HR. Overview of asbestos issues in Korea. J Korean Med Sci 2009; 24: 363-367. ArticlePubMedPMC
- 22. Kim HR, Ahn YS, Jung SH. Epidemiologic characteristics of malignant mesothelioma in Korea. J Korean Med Assoc 2009; 52: 449-455. Article
- 23. Jung SH, Kim HR, Koh SB, et al. A decade of malignant mesothelioma surveillance in Korea. Am J Ind Med 2012; 55: 869-875. ArticlePubMed
- 24. Kang DM, Kim YK, Kim JE. Asbestos and environmental diseases. J Korean Med Assoc 2012; 55: 214-222. Article
- 25. Eom M, Abdul-Ghafar J, Park SM, et al. No detection of simian virus 40 in malignant mesothelioma in Korea. Korean J Pathol 2013; 47: 124-129. ArticlePubMedPMC
REFERENCES
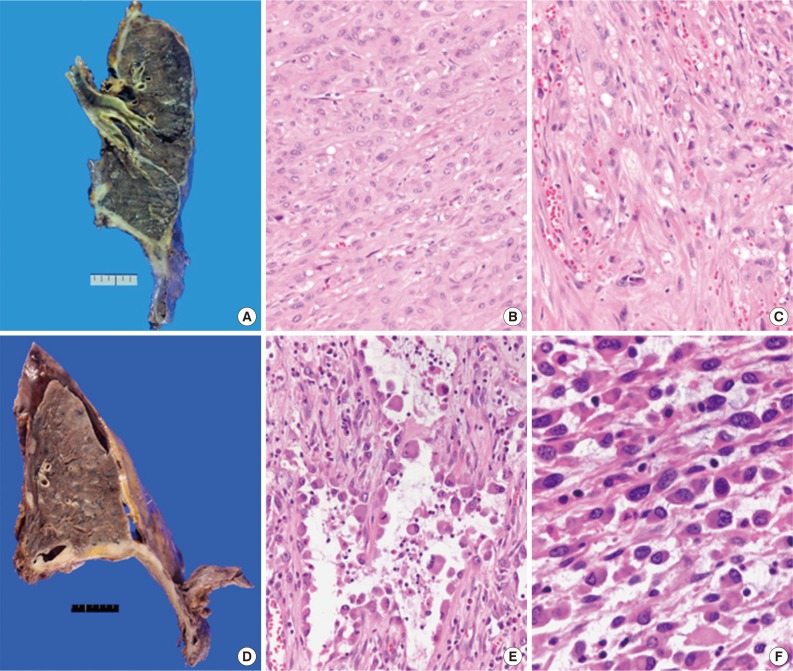
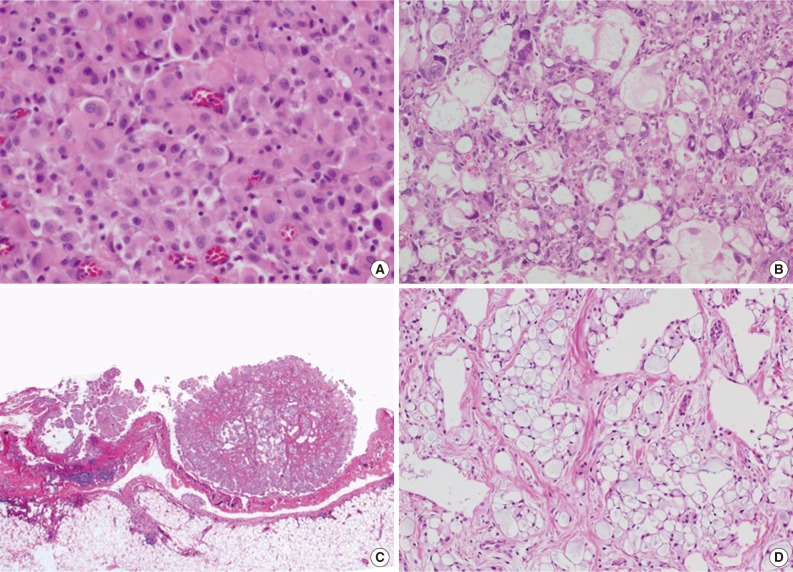
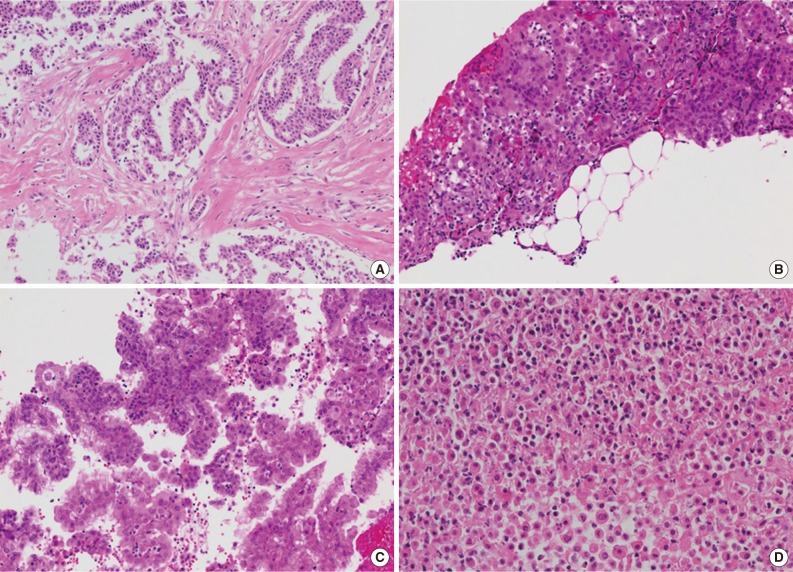
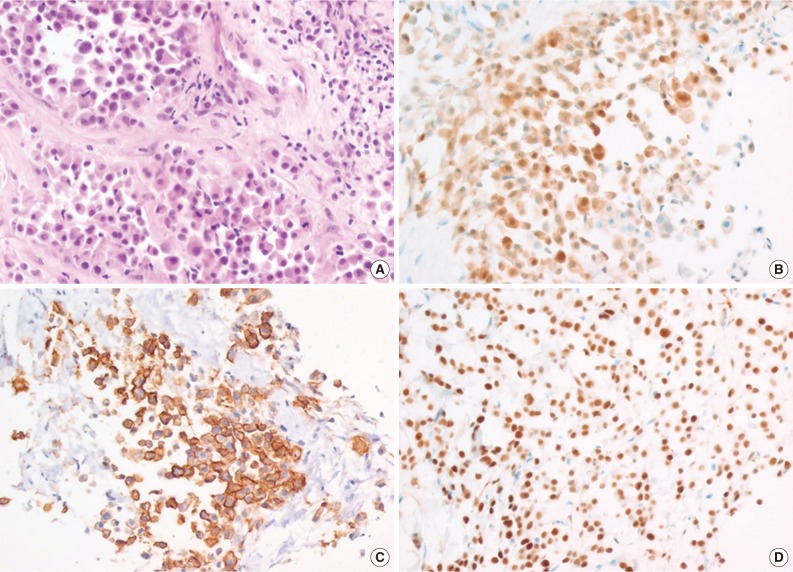
Figure & Data
References
Citations

- Expression of V-set immunoregulatory receptor in malignant mesothelioma
Yeon Seung Chung, Moonsik Kim, Yoon Jin Cha, Kyung A Kim, Hyo Sup Shim
Modern Pathology.2020; 33(2): 263. CrossRef - Is the pathology related to the amount of pleural thickening measured by thorax CT?
özgür katrancıoğlu, Tuba Sahinoglu, Kayhan Karakus, Ozan Kandemir, Semiha Urvay, Esra Aydın Karakaya, Nurkay Katrancioglu
Cumhuriyet Medical Journal.2018; 40(2): 157. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure




Fig. 1
Fig. 2
Fig. 3
Fig. 4
| No. of cases (%) | |
|---|---|
| Stroma | |
| Present | 26/33 (78.8) |
| Absent | 7/33 (21.2) |
| Stromal invasion | |
| Present | 26/33 (78.8) |
| Absent | 7/33 (21.2) |
| Cellularity | |
| High | 27/33 (81.8) |
| Moderate | 6/33 (18.2) |
| Structure | |
| Diffuse | 23/33 (69.7) |
| Complex papillae | 9/33 (27.3) |
| Simple papillae | 1/33 p.0) |
| Necrosis | |
| Present | 11/33 (33.3) |
| Absent | 22/33 (66.7) |
| Inflammation | |
| Mild | 28/33 (84.8) |
| Marked | 5/33 (15.2) |
| Atypia | |
| Mild | 8/33 (24.2) |
| Moderate | 22/33 (66.7) |
| Severe | 3/33 (9.1) |
| Mitosis | |
| Present | 12/33 (36.4) |
| Absent | 21/33 (63.6) |
| Positive (%) | Focal positive (%) | Negative (%) | Unsatisfactory (%) | |
|---|---|---|---|---|
| Positive markers | ||||
| Calretinin | 20/36 (55.6) | 6/36 (16.7) | 9/36 (25.0) | 1/36 (2.8) |
| WT-1 | 10/21 (47.6) | 7/21 (33.3) | 4/21 (19.0) | 0/21 (0) |
| HBME-1 | 23/33 (69.7) | 5/33 (15.2) | 4/33 (12.1) | 1/33 (3.0) |
| Vimentin | 9/9 (100) | 0/9 (0) | 0/9 (0) | 0/9 (0) |
| CK (AE1/AE3) | 9/10 (90) | 1/10 (10) | 0/10 (0) | 0/10 (0) |
| Negative markers | ||||
| TTF-1 | 0/32 (0) | 0/32 (0) | 31/32 (96.9) | 1/32 (3.1) |
| CEA | 0/16 (0) | 0/16 (0) | 16/16 (100) | 0/16 (0) |
| n | |
|---|---|
| Age (yr) | 28-80 (average, 56.84) |
| Sex | |
| Male | 42/66 |
| Female | 24/66 |
| Asbestos exposure | |
| Exposure | 1/66 |
| No exposure | 3/66 |
| Not available | 62/66 |
| Occupational information | |
| Housewife | 8/66 |
| Office worker | 5/66 |
| Businessman | 4/66 |
| Teacher | 2/66 |
| Etc. | 9/66 |
| Not available | 38/66 |
| Residential information | |
| Gyeongsang-do | 18/66 |
| Seoul | 14/66 |
| Gyeonggi-do | 12/66 |
| Jeolla-do | 8/66 |
| Chungcheong-do | 5/66 |
| Gangwon-do | 2/66 |
| Incheon | 1/66 |
| Jeju-do | 1/66 |
| Russia | 1/66 |
| Not available | 4/66 |
WT-1, Wilms tumor 1; CK, cytokeratin; TTF-1, thyroid transcription factor-1; CEA, carcinoembryonic antigen.

 E-submission
E-submission