Articles
- Page Path
- HOME > J Pathol Transl Med > Volume 48(4); 2014 > Article
-
Original Article
Expression of HuR and Cyclooxygenase-2 in Nodular Fasciitis and Low-Grade Sarcoma: An Immunohistochemical Study - Hyun-Jin Son, Tae-Hwa Baek, Seung Yun Lee, Joo-Heon Kim, Dong-Wook Kang, Hye-Kyung Lee, Mee-Ja Park
-
Korean Journal of Pathology 2014;48(4):270-275.
DOI: https://doi.org/10.4132/KoreanJPathol.2014.48.4.270
Published online: August 26, 2014
Department of Pathology, Eulji University School of Medicine, Daejeon, Korea.
- Corresponding Author: Hyun-Jin Son, M.D. Department of Pathology, Eulji University Hospital, 95 Dunsanseo-ro, Seo-gu, Daejeon 302-799, Korea. Tel: +82-42-611-3451, Fax: +82-42-611-3459, shjpathol@eulji.ac.kr
• Received: May 14, 2014 • Revised: July 24, 2014 • Accepted: July 28, 2014
© 2014 The Korean Society of Pathologists/The Korean Society for Cytopathology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Nodular fasciitis is the most common reactive mesenchymal lesion to be misidentified as a type of sarcoma. HuR is an mRNA-binding protein that can stabilize cyclooxygenase-2 (COX-2) mRNA leading to COX-2 overexpression. The aim of this study is a comparison of the expressions of COX-2 and HuR and the relationships between their expressions and the clinicopathological parameters in nodular fasciitis and low-grade sarcoma.
-
Methods
- We measured the expression of HuR and COX-2 in 21 cases of nodular fasciitis and 37 cases of low-grade sarcoma using immunohistochemistry.
-
Results
- The frequency of cytoplasmic immunoreactivity for HuR was 5 of 21 cases of nodular fasciitis (23.8%) and 23 of 37 cases of low-grade sarcoma (62.1%) (p=.013). COX-2 expression was moderate or strong in nodular fasciitis (12/21, 57.1%) and in low-grade sarcoma (29/37, 78.4%) (p=.034). In addition, a significant difference existed between these two entities in terms of the relationship between moderate or strong COX-2 expression and HuR cytoplasmic immunoreactivity (p=.009). Moderate or strong COX-2 immunoreactivity correlated with nuclear (p=.016) or cytoplasmic HuR (p=.024) expression in low-grade sarcoma but not in nodular fasciitis.
-
Conclusions
- This study suggests that HuR and COX-2 expression may be useful to differentiate nodular fasciitis from low-grade sarcoma.
- Case selection and specimens
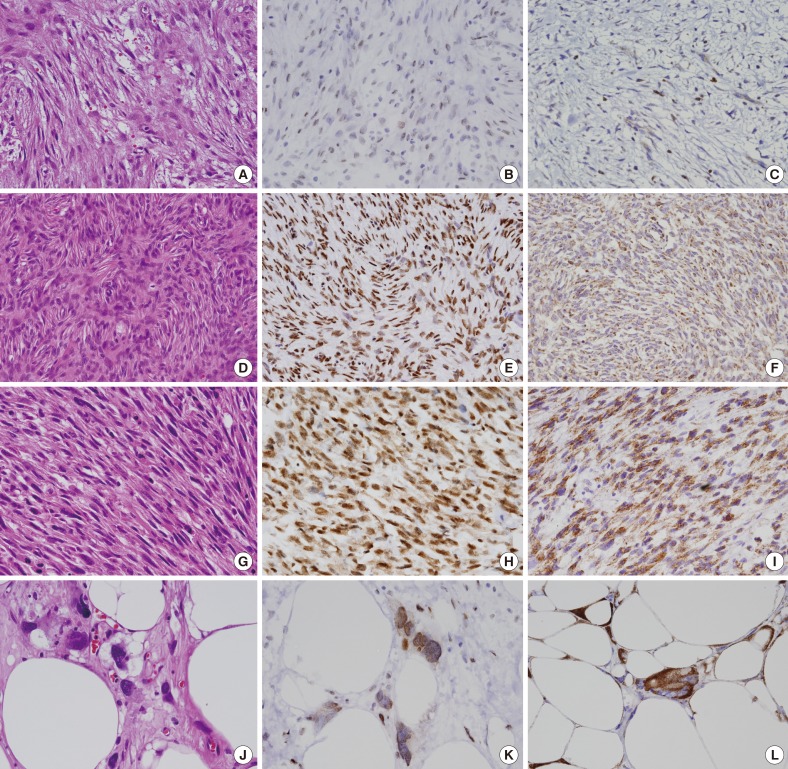
- Twenty-one cases of nodular fasciitis and 37 cases of low-grade sarcoma were chosen from the surgical specimens of Eulji University Hospital, Daejeon, between January 2000 and December 2009. All available clinical data were acquired from a review of the patients' medical records. The histopathological diagnosis was verified by an independent evaluation of all of the hematoxylin and eosin slides by two pathologists (Fig. 1A, D, G, J) and the histologic grade of the sarcoma was based on the Fédération Nationale de Centres de Lutte Contre le Cancer (FNCLCC) and National Cancer Institute (NCI) grading systems.
- Immunohistochemistry and scoring
- Immunohistochemical staining was performed using an EnVision Detection Kit System (Dako, Glostrup, Denmark). Briefly, 4-µm-thick sections were cut and mounted onto silanized slides, dewaxed in xylene, and rehydrated with a graded ethanol series. The sections were treated in a 0.1 M citrate buffer (pH 6.0) for 10 minutes using a microwave oven antigen-retrieval procedure. The endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 5 minutes at room temperature. The tissue sections were incubated for 1 hour at 37℃ with a COX-2 specific monoclonal antibody (1:100, Cayman Chemical, Ann Arbor, MI, USA), or with a mouse HuR monoclonal antibody (1:100, Zymed, South San Francisco, CA, USA). The sections were then incubated with the secondary antibody for 30 minutes at room temperature, visualized using diaminobenzidine and counter-stained with Mayer's hematoxylin. The negative control was performed with the antibody diluent as a substitute for the primary antibody.
- Two pathologists scored the frequency and intensity of COX-2 and HuR staining in a blinded procedure and all specimens with discordant scores were reevaluated and the consensus score was obtained and used for statistical analysis. The expression of COX-2 was scored into the following four groups: 0, no staining; 1, weak diffuse cytoplasmic staining (may contain a stronger intensity in less than 10% of the cells); 2, moderate granular cytoplasmic staining in more than 10% of the cells; and 3, strong granular cytoplasmic staining in more than 50% of cells. In HuR scoring, the nuclear and cytoplasmic staining were scored independently into the following four groups: 0, no staining; 1, weak and/or focal (≤5% of the cells) staining; 2, moderate or strong staining (≤50% of the cells); and 3, moderate or strong staining (≥50% of the cells). COX-2 scores 0 and 1 were combined and were considered weakly staining in order to represent low COX-2 expression. HuR scores 2 and 3 were combined and were considered strong staining.
- Statistical analysis
- The correlations between the COX-2 and HuR staining intensity and clinicopathological parameters were evaluated using a chi-square (χ2) test. A p<.05 was considered statistically significant.
MATERIALS AND METHODS
- Clinicopathologic characteristics of nodular fasciitis and low-grade sarcoma
- In the 21 nodular fasciitis cases, the age of the patients ranged from 9 to 51 years (mean age, 31 years). Twelve patients were male and 9 were female. The greatest diameter of the 21 nodular fasciitis cases ranged from 0.4 to 4.2 cm (mean, 1.6 cm). In the 37 low-grade sarcoma cases, the age of the patients ranged from 3 to 79 years (mean age, 46 years). Twenty patients were male and 17 were female. The greatest diameter of the 37 low-grade sarcoma cases ranged from 1.2 to 21.0 cm (mean, 5.6 cm). The specific pathologic entities of low-grade sarcoma were dermatofibrosarcoma protuberans (DFSP; n=22), well-differentiated or myxoid liposarcoma (n=9), and leiomyosarcoma (n=6). According to the FNCLCC and NCI grading systems, the histologic grade of the 37 low-grade sarcoma cases was grade 1. Of the 37 low-grade sarcoma cases, 4 cases showed recurrences and 1 case showed metastasis.
- Nuclear and cytoplasmic expression of HuR and its correlation with clinical parameters
- In the nodular fasciitis cases, the tumor cells did not express HuR in the nucleus or cytoplasm in 23.8% of the cases (5/21), in the nucleus only in 52.4% of the cases (11/21), and in both the cytoplasm and nucleus in 23.8% of the cases (5/21) (Fig. 1B). Among the 5 nodular fasciitis cases, 4 cases showed weak or focal staining, and only 1 case showed moderate cytoplasmic expression (Table 1). In the low-grade sarcoma cases, the tumor cells did not express HuR in the nucleus or cytoplasm in 16.2% of the cases (6/37), in the nucleus only in 21.6% of the cases (8/37), and in both the cytoplasm and nucleus in 62.2% of the cases (23/37) (Fig. 1E, H, K). In DFSP, the tumor cells did not express HuR in the nucleus and cytoplasm in 13.6% of the cases (3/22), in the nucleus only in 13.6% of the cases (3/22), and in both the cytoplasm and nucleus in 72.7% of the cases (16/22). Among the 23 low-grade sarcoma cases, 12 cases showed weak or focal staining, and 11 cases showed moderate or strong cytoplasmic expression (Table 1). The frequency of cytoplasmic positivity for HuR was higher in the low-grade sarcoma cases than in the nodular fasciitis cases and this was statistically significant (p=.013) (Table 1). No correlation was identified between nuclear or cytoplasmic HuR expression and age, gender, or tumor size in the nodular fasciitis and low-grade sarcoma cases. No correlation was seen between nuclear or cytoplasmic HuR expression and specific pathologic entities of low-grade sarcoma and recurrence or metastasis.
- Expression of COX-2 and its correlation with clinical parameters
- Moderate or strong COX-2 expression was observed in 12 (57.1%) of the 21 nodular fasciitis cases (Fig. 1C). Moderate or strong COX-2 expression was observed in 29 of the 37 low-grade sarcoma cases (78.4%) and in 17 of 22 DFSP cases (77.3%) (Fig. 1F, I, L). The frequency of moderate or strong COX-2 expression was higher in low-grade sarcoma than in nodular fasciitis and was statistically significant (p=.034) (Table 1). No correlation was identified between COX-2 expression and age, gender, and tumor size in nodular fasciitis and low-grade sarcoma cases. No correlation was seen between COX-2 expression and specific pathologic entities of low-grade sarcoma and recurrence or metastasis as well.
- Nuclear and cytoplasmic expression of HuR and its correlation with COX-2 expression
- Moderate or strong COX-2 immunoreactivity correlated with nuclear (p=.016) or cytoplasmic HuR (p=.024) expression in low-grade sarcoma (Table 2). However, in nodular fasciitis, no correlation was seen between moderate or strong COX-2 immunoreactivity and either nuclear or cytoplasmic HuR expression. Among the 12 cases of nodular fasciitis showing moderate or strong COX-2 immunoreactivity, 11 cases were negative or weakly positive for cytoplasmic HuR expression and the remaining 1 case showed only strong positive cytoplasmic HuR expression. In contrast to the nodular fasciitis cases, among the 29 cases of low-grade sarcoma showing moderate or strong COX-2 expression, 22 cases showed weak or strong (11 cases, weak; 11 cases, strong) cytoplasmic HuR immunoreactivity, and 7 cases were negative. Among the 17 cases of DFSP showing moderate or strong COX-2 expression, 15 cases showed weak or strong (6 cases, weak; 9 cases, strong) cytoplasmic HuR immunoreactivity and 2 cases were negative. In addition, there was a significant difference between these two entities (nodular fasciitis and low-grade sarcoma) in terms of the relationship between moderate or strong COX-2 expression and cytoplasmic HuR immunoreactivity (p=.009) (Table 1).
RESULTS
- The modulation of gene expression by the post-transcriptional change of mRNA stability is an important process in the control of cellular growth.12 HuR, which is one of the essential ARE binding stabilizing factors for mRNA, is up-regulated in several cancers.13,14,15,16 This suggests that a dysregulation of mRNA stability might be involved in the development, growth and acquisition of malignant behaviors of these cancers. In the normal condition, COX-2 expression is tightly controlled and the alteration of HuR expression accelerates the expression of COX-2. The overexpression of COX-2 in several carcinomas and some sarcomatous lesions suggests its roles in tumorigenesis.1,2,3,4,5,6,7,8 Lee et al.6 suggest that COX-2 is directly associated with cell proliferation, migration and invasion in human osteosarcoma cells. Endo et al.7 suggest that COX-2 overexpression in chondrosarcoma is a negative prognostic factor that may be strongly associated with histologic grade. There have been some reports showing that the constitutive overexpression of COX-2 is the consequence of HuR overexpression in ovarian, colon, laryngeal, and salivary cancers, and that the relationship between HuR and COX-2 plays a significant role in the carcinogenesis of several carcinomas, but there are only a few reports for mesenchymal lesions.10,11,17,18,19 Our study investigates the expression and correlation of HuR and COX-2 expression in sarcomas and nodular fasciitis, a benign pseudosarcomatous lesion. Dixon et al.10 reported that mRNAs of COX-2, vascular endothelial growth factor (VEGF), and interleukin-8 (IL-8) were constitutively trasnscribed and turned over slowly in colon cancer cells showing augmented tumor growth. They concluded that the observed mRNA stabilization is the consequence of the faulty recognition of class II-type AREs present within COX-2, VEGF, and IL-8, as well as the dysregulatory overexpression of HuR. Several studies have recently reported that the cytoplasmic expression of HuR is correlated with COX-2 over-expression and a poor outcome. HuR binds to certain mRNAs in the nucleus, and this HuR-mRNAs complex is then transported to the cytoplasm.20 Because the nucleocytoplasmic translocation of HuR is essentially needed for its activity and its cytoplasmic presence is found in several types of carcinomas, it was hypothetically considered that cytoplasmic expression of HuR could be a prognostic marker in several cancers.2,3,4,14,17,21 In our study, there was a correlation between moderate or strong COX-2 immunoreactivity and cytoplasmic or nuclear HuR expression in low-grade sarcomas, but not in nodular fasciitis. Therefore, we think that HuR expression is needed for COX-2 overexpression, which might even be partly associated with the tumorigenesis of low-grade sarcoma in addition to several carcinomas.
- In our study, the COX-2 protein was detected in 12 of the 21 nodular fasciitis cases (57.1%). Among them, COX-2 expression showed strong immunoreactivity in only two cases. On the other hand, 29 of the 37 low-grade sarcoma cases showed COX-2 immunoreactivity (78.4%) and among them, 15 cases showed strong immunoreactivity. There was a considerable difference in the incidence of COX-2 overexpression between cases of nodular fasciitis and low-grade sarcoma (p=.034). Cytoplasmic HuR expression was lower in the nodular fasciitis than in low-grade sarcoma (p=.013). Moreover, cytoplasmic HuR expression was strong in only 1 out of 12 cases showing moderate or strong COX-2 expression in nodular fasciitis (8.3%) and in 11 of 29 cases showing moderate or strong COX-2 expression in low-grade sarcoma (37.9%) and this difference was statistically significant (p=.009). This suggests that HuR and COX-2 expression may be useful in differentiating nodular fasciitis from low-grade sarcoma and may be used to determine the unknown biologic behavior of proliferative spindle cell lesions, particularly in those of a borderline nature. In our study, there was no correlation found between COX-2 overexpression and cytoplasmic or nuclear HuR immunoreactivity in nodular fasciitis. This result suggests a possibility that COX-2 mRNA stability is modulated by more complex mechanisms.22
DISCUSSION
- 1. Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med 2002; 53: 35-57. ArticlePubMed
- 2. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860-867. ArticlePubMedPMCPDF
- 3. Aoki T, Tsukinoki K, Karakida K, Ota Y, Otsuru M, Kaneko A. Expression of cyclooxygenase-2, Bcl-2 and Ki-67 in pleomorphic adenoma with special reference to tumor proliferation and apoptosis. Oral Oncol 2004; 40: 954-959. ArticlePubMed
- 4. Leung WK, To KF, Go MY, et al. Cyclooxygenase-2 upregulates vascular endothelial growth factor expression and angiogenesis in human gastric carcinoma. Int J Oncol 2003; 23: 1317-1322. ArticlePubMed
- 5. Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. FASEB J 2003; 17: 1640-1647. ArticlePubMedPDF
- 6. Lee EJ, Choi EM, Kim SR, et al. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Exp Mol Med 2007; 39: 469-476. ArticlePubMedPDF
- 7. Endo M, Matsumura T, Yamaguchi T, et al. Cyclooxygenase-2 overexpression associated with a poor prognosis in chondrosarcomas. Hum Pathol 2006; 37: 471-476. ArticlePubMed
- 8. Sutton KM, Wright M, Fondren G, Towle CA, Mankin HJ. Cyclooxygenase-2 expression in chondrosarcoma. Oncology 2004; 66: 275-280. ArticlePubMedPDF
- 9. Guhaniyogi J, Brewer G. Regulation of mRNA stability in mammalian cells. Gene 2001; 265: 11-23. ArticlePubMed
- 10. Dixon DA, Tolley ND, King PH, et al. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J Clin Invest 2001; 108: 1657-1665. ArticlePubMedPMC
- 11. Do SI, Araujo ES, Kalil RK, et al. Expression of embryonic lethal abnormal vision (ELAV)-like protein HuR and cyclooxygenase-2 (COX-2) in Ewing sarcoma. Tumori 2008; 94: 347-350. ArticlePubMedPDF
- 12. Sachs AB. Messenger RNA degradation in eukaryotes. Cell 1993; 74: 413-421. ArticlePubMed
- 13. Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine-and uridine-rich elements within the 3' untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res 2001; 61: 2154-2161. PubMed
- 14. Denkert C, Weichert W, Pest S, et al. Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Res 2004; 64: 189-195. ArticlePubMedPDF
- 15. Heinonen M, Bono P, Narko K, et al. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res 2005; 65: 2157-2161. ArticlePubMedPDF
- 16. Lopez de Silanes I, Fan J, Yang X, et al. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene 2003; 22: 7146-7154. ArticlePubMedPDF
- 17. Erkinheimo TL, Lassus H, Sivula A, et al. Cytoplasmic HuR expression correlates with poor outcome and with cyclooxygenase 2 expression in serous ovarian carcinoma. Cancer Res 2003; 63: 7591-7594. PubMed
- 18. Cho NP, Han HS, Soh Y, Lee KY, Son HJ. Cytoplasmic HuR over-expression is associated with increased cyclooxygenase-2 expression in laryngeal squamous cell carcinomas. Pathology 2007; 39: 545-550. ArticlePubMed
- 19. Cho NP, Han HS, Soh Y, Son HJ. Overexpression of cyclooxygenase-2 correlates with cytoplasmic HuR expression in salivary mucoepidermoid carcinoma but not in pleomorphic adenoma. J Oral Pathol Med 2007; 36: 297-303. ArticlePubMed
- 20. Ristimaki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res 1997; 57: 1276-1280. PubMed
- 21. Erkinheimo TL, Sivula A, Lassus H, et al. Cytoplasmic HuR expression correlates with epithelial cancer cell but not with stromal cell cyclooxygenase-2 expression in mucinous ovarian carcinoma. Gynecol Oncol 2005; 99: 14-19. ArticlePubMed
- 22. Sully G, Dean JL, Wait R, et al. Structural and functional dissection of a conserved destabilizing element of cyclo-oxygenase-2 mRNA: evidence against the involvement of AUF-1 [AU-rich element/poly(U)-binding/degradation factor-1], AUF-2, tristetraprolin, HuR (Hu antigen R) or FBP1 (far-upstream-sequence-element-binding protein 1). Biochem J 2004; 377(Pt 3): 629-639. ArticlePubMedPMCPDF
REFERENCES
Fig. 1Representative microphotographs of hematoxylin and eosin (H&E) and immunohistochemical stainings. In nodular fasciitis (A, H&E; B, HuR; C, COX-2), the tumor cells show weak or focal HuR staining in the nucleus (B) and weak COX-2 staining in the cytoplasm (C). In dermatofibrosarcoma protuberans (D, H&E; E, HuR; F, COX-2), the tumor cells show strong HuR staining in the nucleus (E) and moderate COX-2 staining in the cytoplasm (F). In leiomyosarcoma (G, H&E; H, HuR; I, COX-2), the tumor cells show moderate or strong HuR staining in the nucleus and cytoplasm (H) and strong COX-2 staining in the cytoplasm (I). In liposarcoma (J, H&E; K, HuR; L, COX-2), the tumor cells show moderate or strong HuR staining in the nucleus and cytoplasm (K) and strong COX-2 staining in the cytoplasm (L).
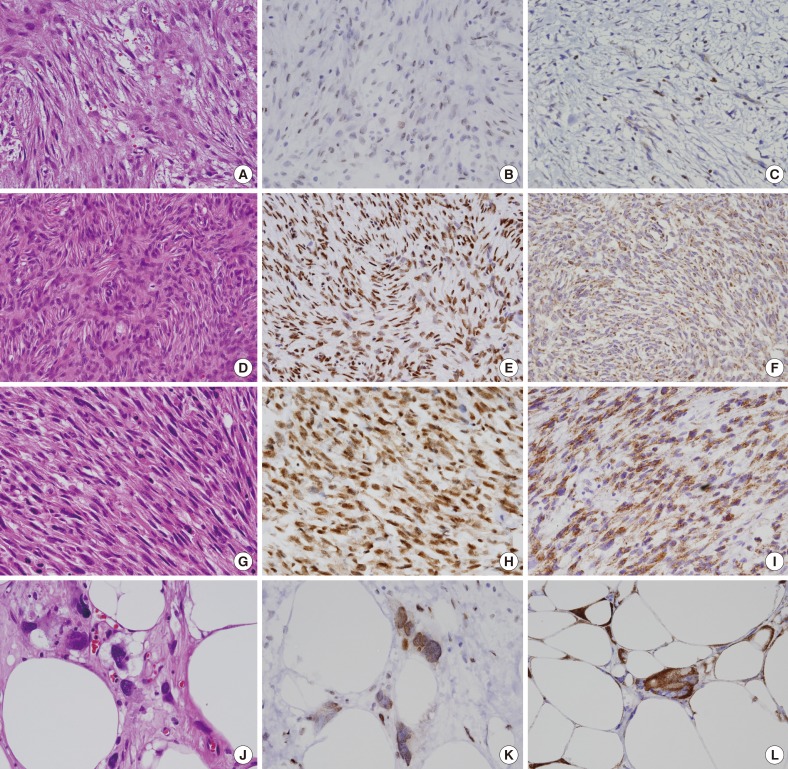

Table 1.Comparison of nodular fasciitis and low-grade sarcoma according to HuR and COX-2 expression
Table 2.Association between nuclear and cytoplasmic HuR immunoreactivity and COX-2 expression in nodular fasciitis and low-grade sarcoma
Figure & Data
References
Citations
Citations to this article as recorded by 

- Nodular Fasciitis of the Cubital Fossa in a Young Female Mimicking a Neurogenic Tumor
Hyung-Joon Lee, Ji-Kang Park, Seok-Won Kim, Min-Boo Kim
Journal of the Korean Orthopaedic Association.2023; 58(2): 179. CrossRef - Nodular fasciitis of the anterior chest wall mimicking myxofibrosarcoma: A case report and literature review
Antonino Cattafi, Mariarosaria Galeano, Pietro Pitrone, Carmelo Sofia, Maria Adele Marino, Giorgio Ascenti, Maria Lentini, Antonio Ieni, Roberta Cardia, Alfio Luca Costa, Dario Familiari, Mario Barone, Francesco Monaco, Michele Rosario Colonna
Radiology Case Reports.2021; 16(6): 1557. CrossRef
 PubReader
PubReader ePub Link
ePub Link-
 Cite this Article
Cite this Article
- Cite this Article
-
- Close
- Download Citation
- Close
- Figure
Expression of HuR and Cyclooxygenase-2 in Nodular Fasciitis and Low-Grade Sarcoma: An Immunohistochemical Study

Fig. 1 Representative microphotographs of hematoxylin and eosin (H&E) and immunohistochemical stainings. In nodular fasciitis (A, H&E; B, HuR; C, COX-2), the tumor cells show weak or focal HuR staining in the nucleus (B) and weak COX-2 staining in the cytoplasm (C). In dermatofibrosarcoma protuberans (D, H&E; E, HuR; F, COX-2), the tumor cells show strong HuR staining in the nucleus (E) and moderate COX-2 staining in the cytoplasm (F). In leiomyosarcoma (G, H&E; H, HuR; I, COX-2), the tumor cells show moderate or strong HuR staining in the nucleus and cytoplasm (H) and strong COX-2 staining in the cytoplasm (I). In liposarcoma (J, H&E; K, HuR; L, COX-2), the tumor cells show moderate or strong HuR staining in the nucleus and cytoplasm (K) and strong COX-2 staining in the cytoplasm (L).
Fig. 1
Expression of HuR and Cyclooxygenase-2 in Nodular Fasciitis and Low-Grade Sarcoma: An Immunohistochemical Study
| Immunohistochemical reactivity | Nodular fasciitis (n = 21) | Low-grade sarcoma (n = 37) | p-value (chi-square test) |
|---|---|---|---|
| Nuclear HuR | |||
| Negative | 5 | 6 | .393 |
| Weakly | 8 | 10 | |
| Strong | 8 | 21 | |
| Cytoplasmic HuR | |||
| Negative | 16 | 14 | .013 |
| Weakly | 4 | 12 | |
| Strong | 1 | 11 | |
| COX-2 | |||
| Weakly | 9 | 8 | .034 |
| Moderate | 10 | 14 | |
| Strong | 2 | 15 | |
| Cytoplasmic HuR/Moderate or strong COX-2 | 12 | 29 | |
| Negative | 9 | 7 | .009 |
| Weakly | 2 | 11 | |
| Strong | 1 | 11 |
| HuR immunoreactivity | Nodular fasciitis (n=21), COX-2 positive |
p-value (chi-square test) | Low-grade sarcoma (n=37), COX-2 positive |
p-value (chi-square test) | ||||
|---|---|---|---|---|---|---|---|---|
| Weakly | Moderate | Strong | Weakly | Moderate | Strong | |||
| Nuclear | ||||||||
| Negative or weakly | 5 | 6 | 2 | .497 | 7 | 5 | 4 | .016 |
| Strong | 4 | 4 | 0 | 1 | 10 | 10 | ||
| Cytoplasmic | ||||||||
| Negative | 7 | 7 | 2 | .784 | 7 | 4 | 3 | .024 |
| Weakly | 2 | 2 | 0 | 1 | 5 | 6 | ||
| Strong | 0 | 1 | 0 | 0 | 6 | 5 | ||
Table 1. Comparison of nodular fasciitis and low-grade sarcoma according to HuR and COX-2 expression
COX-2, cyclooxygenase-2.
Table 2. Association between nuclear and cytoplasmic HuR immunoreactivity and COX-2 expression in nodular fasciitis and low-grade sarcoma
COX-2, cyclooxygenase-2.

 E-submission
E-submission